 About Authors:
About Authors:
Ratnadeep V. Ghadage (M. Pharmacy)
Department of Pharmaceutical Chemistry,
Appasaheb Birnale College of Pharmacy,
South Shivaji Nagar, Sangli-416416,
Maharashtra
ABSTRACT:
Quinoxaline derivatives have emerged as an important class of benzoheterocycles because of their diverse pharmacological and biological properties, which make them privileged structures in combinatorial drug discovery libraries. Also Quinoxaline derivatives constitute useful intermediates in organic synthesis. The pharmaco-logical importance of quinoxalines and their utility as building blocks in organic synthesis have directed considerable research activities toward the synthesis of suitably substituted quinoxaline rings. Extensive researches have generated numerous synthetic approaches for the construction of the skeleton of such heterocycles. Quinoxalines are, in general, comparatively easy to prepare, and numerous derivatives have been designed and prepared for potential use as biologically active materials. Oxidation of both nitrogen of the quinoxaline ring dramatically increases the diversity of certain biological properties.
Quinoxalines, including their fused-ring derivatives, display diverse pharmacological activities.A number of synthetic strategies have been developed for the preparation of substituted quinoxalines. The classical synthesis of quinoxalines involves the condensation of an aromatic 1, 2-diamine with a 1, 2-dicarbonyl compound. The reaction is facile and is the most widely used synthetic method for both quinoxaline itself and its derivatives. Despite remarkable efforts,the development of an effective method for the synthesis of quinoxalines is still an important challenge.
Refrence Id: PHARMATUTOR-ART-1152
[adsense:336x280:8701650588]
INTRODUCTION:-
The heterocycles are those cyclic organic compounds in which O, N or S elements replaced by one or more these hetero atoms of the carbon ring atoms. The sulpher and nitrogen atoms are important components of functional materials since heteroatoms presents in their rings stabilize ion radical species and extended ?-conjugation facillate in decreasing columbic repulsion [1].
Heterocyclic compounds represent an important class of biological active molecules. Specifically those containing quinoxaline derivatives have evoked considerable attention in recent years as these are endowed. Quinoxalines are a versatile class of nitrogen containing heterocyclic compounds and they constitute useful intermediates in organic synthesis. Quinoxaline, also called a benzopyrazine, in organic chemistry, is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring and they are isomeric with cinnolenes, phthalazinesand quinazolines[2]. There are a number of processes available to generate quinoxaline but generally, they are synthesized by the condensation of 1, 2-dicarbonylswith 1,2 diamines in in precence of suitable catalyst using various solvent systems.
They possess well known biological activities including AMPA/GlyN receptor antagonis[3],antihistaminic agents[4], anti-trypanosomal activity[5], anti-herps[6], antiplasmodial activity[7],Ca uptake/ Release inhibitor[8], inhibit vascular smooth muscle cell proliferation[9]. Quinoxaline derivatives constitute the basis of many insecticides, fungicides, herbicides, as well as being important in human health and as receptor antagonists. Although rarely described in nature, syntheticquinoxaline moiety is a part of number of antibiotics such as echinomycin, levomycin and actinomycin which are known to inhibit the growth of Gram-positive bacteria and also active against various transplantable tumours. [10,11] In addition, quinoxaline derivatives are reported for their application in dyes, efficient electroluminescent materials, organic semiconductors and DNA cleaving agents[12]. These are useful as intermediates for many target molecules in organic synthesis and also as synthons.
Numerous methods are available for the synthesis of quinoxaline derivatives which Extensive researches have generated numerous synthetic approaches for the construction of the skeleton of such heterocycles. Among these methods, the most widely used one relies on the condensation of aryl-1,2- diamines with aryl ketones, usually α-dicarbonyl compounds or their equivalents. Recent improvements on these conditions were reported via solid-phase [13]. Improved methods have been reported via a condensation process catalyzed by various catalyst systems which are reported in Table No.1. Recently, a number of catalysts have been reported for the synthesis of quinoxalines. Considering the significant applications in the fields of medicinal, industrial andsynthetic organic chemistry, there has been tremendous interest in developing efficient methods for the synthesis of quinoxalines.
[adsense:468x15:2204050025]
SYNTHESIS OF QUINOXALINE NUCLEUS:
1. By three-component condensation reaction[14]:
The three-component condensation reaction of O-phenylenediamine, aromatic aldehydes, and cyclohexyl isocyanide to afford the corresponding N-cyclohexyl-3-aryl-quinoxaline- 2-amines in good yields. (Figure No.1)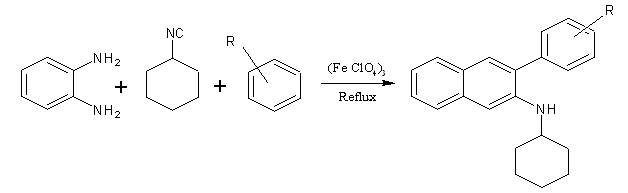
Where, R = Benzaldehyde, 3- nitro benzaldehyde, 4-chloro benzaldehyde,4- nitro benzaldehyde,4-methoxy benzaldehyde.
Figure No.1 Synthesis of N-cyclohexyl-3-aryl-quinoxaline- 2-amines
2. By oxidation of Alkynes[15]:
synthesis of substituted quinoxalinesin this Alkynes were oxidized efficiently using the catalytic amount of PdCl2 and CuCl2 in PEG-400 in the presence of water, providing excellent yields of the corresponding 1, 2-diketones. A variety of alkynes were well-suited substrates for the oxidation under the described conditions. Further, the optimized conditions were successfully utilized for the one-pot synthesis of 2, 3 disubstituted quinoxaline derivatives. (Figure No.2)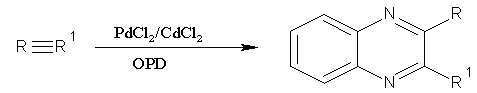
Figure No.2synthesis of 2, 3 disubstituted quinoxaline derivatives
3. From oxidative couplingof epoxides[16]:
Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1,2-diamines by a Bi-catalyzed oxidative coupling, by using Bi (5 mo%) as catalyst in presence of DMSO solvent. (Figure No.3)
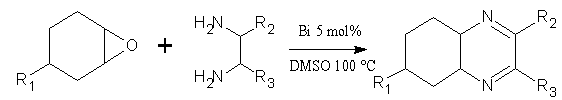
Figure No. 3 synthesis of quinoxaline derivatives from epoxides
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4. By hexulose derivatives[17]:
A novel and efficient method for the synthesis of quinoxaline derivatives. 2, 3-O-isopropylidene- 4-chloro-4-deoxy-hex-5-ulose di-methyl acetal that is hexulose reacted with O-phenylenediamines under neutral conditions in presences of dichloro methane to afford quinoxaline derivatives in reasonable yields. The in vitro cytotoxic activities of these quinoxaline derivatives were investigated. (Figure No.4)
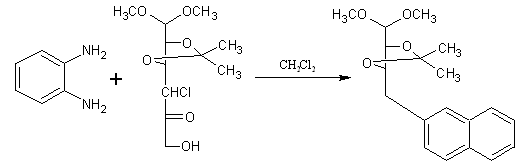
Figure no.4 synthesis of quinoxaline derivatives by hexulose derivatives
5. By reactions of α-bromoketones[18]:
A facile and convenient synthesis method for substituted quinoxalines from the reactions of α-bromoketones with benzene-1,2-diamine and 2-aminophenol, which were catalyzed by tetrabutyl ammonium bromide(TBAB) in aqueous basic media. This method proved to be easy, economic safe, and time consuming. (Figure No.5)
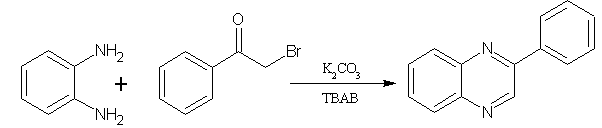
Figure No.5 synthesis for substituted quinoxalines and from the reactions of α-bromoketones
6. From Ni-nanoparticles[19]:
Synthesis of biologically active quinoxalines in excellent yields and in less reaction time using inexpensive, monodispersed And easily recyclable Ni-nanoparticles. In order to elucidate the role of the Ni-nanoparticles, a control reaction was conducted using glyoxal and o-phenylenediamine in acetonitrile in the absence of Ni-nanoparticles. (Figure No.6)
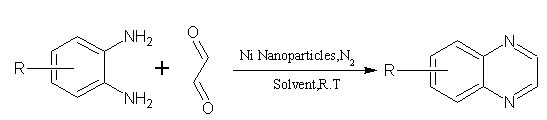
Where; R= H, NO2, Cl, CF3
Figure no.6 synthesis method for substituted quinoxalines by Ni-nanoparticles
7. By using [TMPSA]. HSO4 as a catalyst[20]:
A cheap and recyclable task-specific ionic liquid N, N, N-trimethyl-N-propanesulfic acid ammonium hydrogen sulfate [TMPSA]. HSO4 was used as the catalyst for the synthesis of quinoxaline derivatives. The reaction could be accomplished in water as well as organic solvent, and the satisfactory results were obtained under the mild conditions. (Figure No.7)
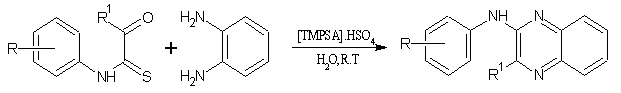
Where; R= H, NO2, Cl, CF3 & R1=H, NO2, CH3
Figure No.7 synthesis of quinoxaline derivatives by [TMPSA]. HSO4 as a catalyst
8. By using different the catalyst systems:
For a one-pot synthesis of quinoxaline derivatives at room temperature is reported by using different catalysts systems. The procedure presented is operationally simple, practical and green. (Figure No.8)
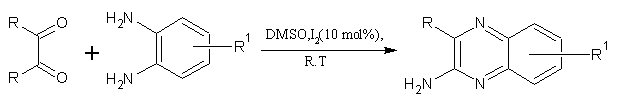
Where; R= H, Me, F, OCH3 & R1=H,NO2,CH3
Figure No.8 one-pot synthesis of quinoxaline derivatives by using different catalyst
Table No. 1 List of different catalyst system used for synthesis of quinoxaline derivatives
|
Sr. No. |
Scientists |
Catalyst used |
|
1. |
Heravi MM et al[21] |
CuSO4.5H2O |
|
2. |
Heravi MM et al[22] |
IBX |
|
3. |
Kotharkar SA et al [23] |
Lead Oxide (PbO) |
|
4. |
Ajaikumar S et al[24] |
ZrO2 |
|
5. |
Bandyopadhyay D et al[25] |
Iodine |
|
6. |
Wallace JM et al[26] |
Palladium |
|
7. |
Rao V J et al[27] |
Polyaniline sulphate |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
9. From other hetero-monocyclic substrates/synthons[28]:
Furans as Substrates/Synthons:
1,2-Benzenediamine and 3-bromomethyl-4-methyl-2,5-dihydro-2,5-furandione (2-bromomethyl-3-methylmaleic anhydride) gave 3-(1-carboxyvinyl)- 3-methyl-3,4-dihydro-2(1H)-quinoxalinone with loss of hydrogen bromide. (Figure No.9)
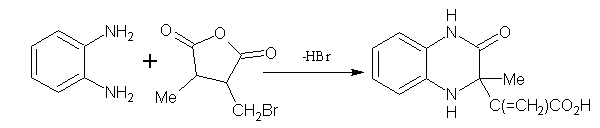
Figure No.9 Furans as Substrates/Synthons-
Thiophenes as Substrates/Synthons:
This category is represented in the facile reaction of o-phenylenediamine with 4-benzoyl-5-phenyl-2,3-dihydro-2,3-thiophenedione (in toluene at 20 0C for 30 min) to afford 3-(a-benzoyl-b-mercaptostyryl)-2(1H)-quinoxalinone in 98% yield. (Figure No.10)
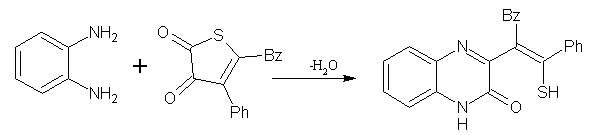
Figure No.10 Thiophene as Substrates/Synthons-
Pyridines as Substrates/Synthons-
The sole recent example in this category is the condensation (in hot aqueous ethanolic sodium hydrogen carbonate) of o-phenylenediamine with tri-tertbutyl 2-hydroxy-3-oxo-1, 2, 6- to give tert-butyl3-[3-(tert-butoxycarbonyl)-3-(tert-butoxycarbonylamino)propyl]-2-quinoxalinecarboxylate in 87% yield. (Figure No.11)
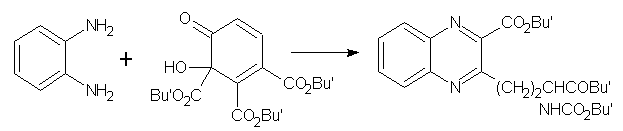
Figure No.11 Pyridines as Substrates/Synthons-
Pyrimidines as Substrates/Synthons-
The unlikely transformation of a pyrimidine into a quinoxaline has, indeed, been reported. Thus 4,5-dimethyl-1,2 benzenediamine and alloxan under acidic conditions gave 6,7-dimethyl-3-ureidocarbonyl-2(1H)-quinoxalinone. (Figure No.12)
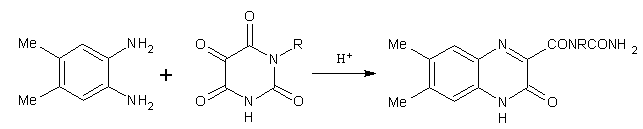
Figure No.12 pyrimidine as Substrates/Synthons-
Pyridazines as Substrates/Synthons:
Treatment of o-phenylenediamine with 3,4,5,6-tetrachloropyridazine in N-methylpyrrolidine at 1150C for 17 hr gave a separable mixture of products, one of which was 2,3-bis(benzimidazol-2-yl) quinoxaline. (Figure No.13)
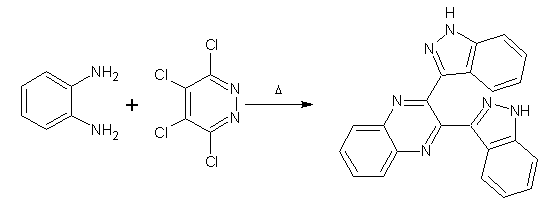
Figure No.13 Pyridazines as Substrates/Synthons-
Oxazoles as Substrates/Synthons:
Only one procedure in this category emerged from the present survey. Thus treatment of 1,2-benzenediamine with 3,3-bis(trifluoromethyl)-5-oxazolinone in ethyl acetate containing a trace of acetic acid at room temperature for a short time afforded 2(1H)-quinoxalinone. (Figure No. 14)
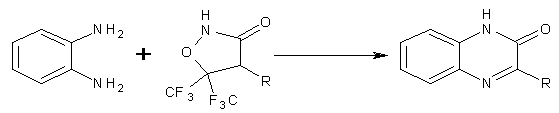
Figure No.14 Oxazoles as Substrates/Synthons-
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
10. From heterobicyclic substrates/synthons[28]:
There is an extensive literature on the use of 2, 1, 3-benzoxadiazole 1-oxide [often called benzofuroxan(e) (BFO) as a substrate for the primary synthesis of quinoxaline 1,4-dioxides and occasionally quinoxaline mono-N-oxides or even simple quinoxalines. Very few substituted derivatives of the parent substrate have been employed in recent years. The general mechanism clearly involves fission (usually amine-catalyzed) of the oxadiazole ring followed by reaction with an ancillary synthon. The following examples are divided according to the type of synthon employed.
Benzoxadiazoles as Substrates/Synthons-
1,3-Benzoxadiazole 1-oxide gave either 2-phenylquinoxaline 4-oxide or 2-acetyl-3-phenylquinoxaline 4-oxidein presence of piperidine and BuNH2 change in regioselectivity and preservation from deacylation may perhaps be explained by the possibility for Schiff base formation of the synthon and product, respectively, in the presence of the primary amine plausible reason for the formation of mono- instead of di-N-oxides was advanced. (Figure No.15).
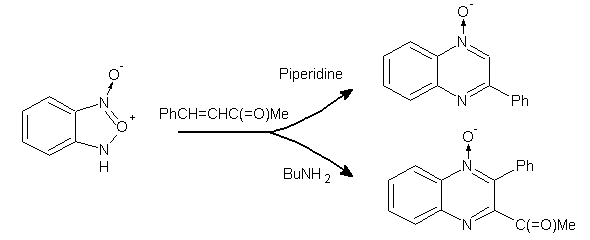
Figure No.15 Benzoxadiazole as Substrates/Synthons-
Benzopyrans (Chromenes) as Substrates/Synthons-
1,2-Benzenediamine and 3,4-dihydro-2H-1-benzopyran-2,3-dione gave 3-o-hydroxybenzyl-2(1H)-quinoxalinone reaction conditionsare as follows. (1M NaOH, 1000C, 15 min, 84%; EtOH, reflux, 1 hr). (Figure No.16)
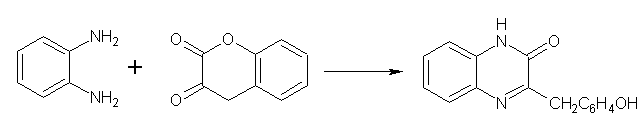
Figure No.16 Benzopyrans as Substrates/Synthons-
Benzimidazoles as Substrates/Synthons:
The ring expansion of benzimidazoles to afford qunoxalines has been done in several ways, mostly of little preparative value. They are illustrated in the following examples. Benzimidazole and chloroform gave a separable 9:1 mixture of 2-chloroquinoxaline and 1,2-benzenedicarbonitrile. (Figure No.17)
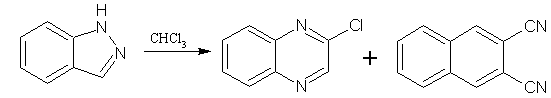
Figure No.17 Benzimidazoles as Substrates/Synthons-
Indoles as Substrates/Synthons-
3-Azido-3-methyl-2-indolinone gave 3-methyl-2(1H)-quinoxalinone (reaction conditions are-xylene, reflux, 8 hr, 95%). (Figure No.18)
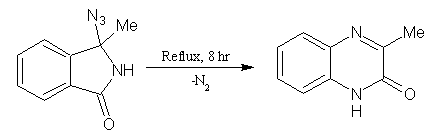
Figure No. 18 Indoles as Substrates/Synthons
Pyrrolo [3, 4-b]pyrazines as Substrates/Synthons:
One interesting example of this type of synthesis has been reported. 6-Phenyl- 5H-5,7(6H)-pyrrolo[3,4-b]pyrazine underwent electrolytic reduction in the presence of chlorotrimethylsilane to give the intermediate substrate that reacted with methyl acrylate to afford a mixture of methyl 8-anilino-5-oxo-1,5-dihydro-6-quinoxalinecarboxylate and methyl 5-anilino- 8-oxo-4,8-dihydro-6-quinoxalinecarboxylate. (Figure No.19)
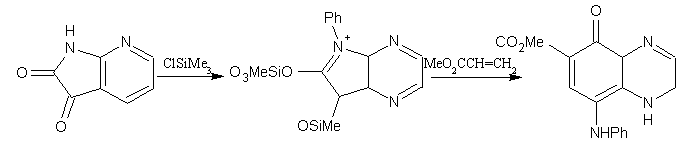
Figure No.19 Pyrrolo [3, 4-b]pyrazines as Substrates/Synthons
CONCLUSION:
In conclusion a various routes of synthesis of quinoxaline nucleus have been described. In medical chemistry the chemist attempts to design and synthesize a medicine or a pharmaceutical agent which will benefit humanity. In the last century, the classical drugs were primarily discovered either by alteration of natural substances or by chemical synthesis. Amongst wide ranges of heterocyclic compounds quinoxalines are most popular compounds. Heterocyclic compounds represent an important class of biological active molecules. Specifically those containing quinoxaline derivatives have evoked considerable attention in recent years as these are endowed with wide range of pharmaceutical activities. Quinoxaline derivatives have emerged as an important class of benzo-heterocycles because of their diverse pharmacological and biological properties, which make them privileged structures in combinatorial drug discovery libraries. The pharmaco-logical importance of quinoxalines and their utility as building blocks in organic synthesis have directed considerable research activities toward the synthesis of suitably substituted quinoxaline rings. Extensive researches have generated numerous synthetic approaches for the construction of the skeleton of such heterocycles. By far, the most common method relies on the condensation of an aryl 1,2-diamine with a 1,2-dicarbonyl compound Although the reported methods provide good isolated yields,these methods suffer from tedious work-up, longer reactiontime, use of metal catalyst and narrow scope of substrates.Moreover, some of the methods have drawbacks likeyields, expensive and detrimental metal reagents. In view of this, there is still need to develop ageneral, efficient and catalyst-free method for the synthesis ofmore functionalized quinoxaline derivatives.
REFERENCES:
1. Misskire, Yohannes, Synthesis and characterization of metal complexes of a new heterocyclic ligands, Addis Ababa University, (2006)
2. http://wikipedia.org/wiki/ quinoxalines
3. Nikam SS., Cordon J.J., Ortwine DF., Design and synthesis of novel quinoxaline-2,3-dione AMPA/GlyN receptor antagonis , Journal of Medicinal Chemistry, (1999), 42: 2266-71.
4. Sridevi CH., Balaji K., Naidu A., Antimicrobial Evaluation and Synthesis of Some Phenylpyrazolo benzothiazoloquinoxaline Derivatives, E-Journal of Chemistry, (2010),7(1) : 234-238.
5. Urquiola C. Vieites M., Aguirre G., Improving anti-trypanosomal activity of 3-aminoquinoxaline- 2-carbonitrile N1, N4-dioxide derivatives by complexation with vanadium, Bioorganic & Medicinal Chemistry, (2006), 14: 5503–5509.
6. Harmenberg J., Wahren B., Antiherpes virus Activity and Mechanism of Action of Indolo-(2,3-b)Quinoxaline and Analogs, Antimicrobial Agents and Chemotherapy, (1988),32: 1720-1724.
7. Zarranz B., Jaso M., Lima LM., Antiplasmodial activity of 3-trifluoromethyl-2-carbonylquinoxaline di-N-oxide derivatives, Rev. Bras. Cienc. Farm., (2006), 42 : 55-67
8. Xia H., Wang F., Yu K., Novel cyclophilin D inhibitors derived from quinoxaline exhibit highly inhibitory activity against rat mitochondrial swelling and Ca2+ uptake/ release, Acta Pharmacologica Sinica, (2005),26 (10): 1201–1211.
9. Chung HJ., Jung OJ., Synthesis and biological evaluation of quinoxaline-5, 8-diones that inhibit vascular smooth muscle cell proliferation, Bioorganic & Medicinal Chemistry Letters, (2005), 15: 3380–3384.
10. Bailly C., Echepare S., Gago F., Recognition elements that determine affinity and sequence-specific binding to DNA of 2QN, a biosynthetic bis-quinoline analogue of echinomycin Jornal of Anti-Cancer Drug Des., (1999),15: 291.
11. Sato S., Shirator O., Katagiri K., Mode of action of quinoxaline antibiotics: Interaction of quinomycin A with deoxyribonucleic acid. Antibiot J., (1967), 20: 270.
12. Srinivas C., Sesha C. Kumar S., Efficient, convenient and reusable polyaniline-sulfate salt catalyst for the synthesis of quinoxaline derivatives, Journal of Molecular Catalysis, (2007): 227–230.
13. Jeon MK., Hyun DS., Gong YD., Solid-phase synthesis of quinoxaline derivatives using 6-amino-2,3-dichloroquinoxaline loaded on AMEBA resin, Tetrahedron Letters, (2005), 46: 4979–4983.
14. Heravi MM, Baghernejad B., Oskooie HA, A novel three-component reaction for the synthesis of N-cyclohexyl-3-aryl-quinoxaline-2-amines, Tetrahedron Letters, 50, (2009), 767–769.
15. Chandrasekhar S.,Reddy N., Praveen Kumar V., Oxidation of alkynes using PdCl2/CuCl2 in PEG as a recyclable catalytic system: one-pot synthesis of quinoxalines, Tetrahedron Letters 51, (2010), 3623–3625.
16. Antoniottia S., and Duach E., Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1,2-diamines, Tetrahedron Letters 43, (2002), 3971–3973.
17. Yan L., Liu F., Dai G. and Liu H. , An efficient synthesis of quinoxaline derivatives from 4-chloro-4-deoxy-a-D-galactose and their cytotoxic activities, Bioorganic & Medicinal Chemistry Letters, 17 (2007), 609–612.
18. Sherman D., Kawakami J., He HY, Dhun F., Weitao Pan, Xu YJ, Labelle M., Synthesis of unsymmetrical and regio-defined 2,3,6-quinoxaline Synthesis of unsymmetrical and regio-defined 2,3,6-quinoxaline, Tetrahedron Letters 48, (2007), 8943–8946.
19. Ajeet Kumar , Santosh kumar , Saxena A., Mozumdar S., Ni-nanoparticles: An efficient catalyst for the synthesis of quinoxalines, Catalysis Communications, 9, (2008), 778–784.
20. Dong F., Kai G., Zhenghao F., Xinli Z., Zuliang L., A practical and efficient synthesis of quinoxaline derivative catalyzed by task-specific ionic liquid, Catalysis Communications, 9, (2008), 317–320.
21. Heravi MM, Taheri S., Bakhtiari K., Oskooie H., On Water: A practical and efficient synthesis of quinoxaline derivatives catalyzed by CuSO4. 5H2O, Catalysis Communications, 8, (2007), 211–214.
22. Heravi MM, Taheri S., Bakhtiari K., Oskooie H., Javadi NM, Facile synthesis of quinoxaline derivatives using o-iodoxybenzoic acid (IBX) at room temperature, ARKIVOC, (2006), 16, 16-22.
23. Kotharkar SA and Shinde DB., Lead Oxide (PbO) Mediated Synthesis of Quinoxaline, Journal of the Iranian Chemical Society, 3,(2006), 267-271.
24. Ajaikumar S., Pandurangan A., Efficient synthesis of quinoxaline derivatives over ZrO2/MxOy (M = Al, Ga, In and La) mixed metal oxides supported on MCM-41 mesoporous molecular sieves, Applied Catalysis A: General 357 (2009) 184–192.
25. Bandyopadhyay D., Mukherjee S., Rodriguez RR, Banik BK, An Effective Microwave-Induced Iodine-Catalyzed Method for the Synthesis of Quinoxalines via Condensation of 1,2-Diamines with 1,2-Dicarbonyl Compounds, Molecules, (2010), 15, 4207-4212.
26. Wallace JM., Bjorn CG., Tamariz J., Akhmedov NG, Hurley MT., Palladium-catalyzed synthesis of quinoxaline derivatives, Tetrahedron, 64, (2008), 9675–9684.
27. Rao VJ., Palaniappan S., Efficient, convenient and reusable polyaniline-sulfate salt catalyst for the synthesis of quinoxaline derivatives, Journal of Molecular Catalysis A: Chemical, 265, (2007), 227–230.
28. Brown DJ., The Chemistry of Heterocyclic Compounds; Quinoxalines: Supplement II, John Wiley & Sons, INC., New York, 2004, 1-92.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









