About Author:
Kunal Jain
Indo Soviet Friendship College of Pharmacy,
Moga, Punjab
jainkunal87@gmail.com
1 Introduction-
1.1 Colon Targeting-
The oral route is considered to be most convenient for administration of drugs to patients. Oral administration of conventional dosage forms normally dissolves in the stomach fluid or intestinal fluid and absorb from these regions of the GIT depends upon the physicochemical properties of the drug. It is a serious drawback in conditions where localized delivery of the drugs in the colon is required or in conditions where a drug needs to be protected from the hostile environment of upper GIT. Dosage forms that deliver drugs into the colon rather than upper GIT offers number of advantages.
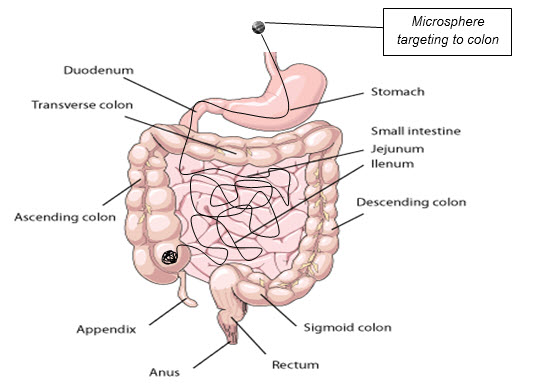
Fig.1.1
In order to develop a reliable colonic drug delivery system, the transit time of dosage forms through the gastrointestinal (GI) tract and impact of disease on the delivery system needs to be understood very well.
REFERENCE ID: PHARMATUTOR-ART-1751
Transit time of dosage forms-
The transit of per orally administered formulation through the GI tract is highly variable and depends on various factors. For example factors like disease state of the lumen (diarrhoea, diabetes, peptic ulcer etc), concomitant administration of other drugs (domperidone, cisapride, metoclopromide etc), body posture (vertical or supine) and food type (fat and protein content) can influence the gastric emptying rate.
Impact of disease on the delivery system-
The luminal pH of the distal intestine in patients with inflammatory bowel disease (IBD) can be lower than that seen in healthy volunteers. Intestinal transit is found to vary in patients with IBD on account of mucosal inflammation and diarrhoea.
1.2 Advantages of colon targeting-
1) Oral delivery of drugs to the colon is valuable in the treatment of diseases of colon (ulcerative colitis, Chron's disease, carcinomas and infections) whereby high local concentration can be achieved while minimizing side effects that occur because of release of drugs in the upper GIT or unnecessary systemic absorption.
2) The colon is rich in lymphoid tissue, uptake of antigens into the mast cells of the colonic mucosa produces rapid local production of antibodies and this helps in efficient vaccine delivery.
3) The colon is attracting interest as a site where poorly absorbed drug molecule may have an improved bioavailability.
4) This region of the colon is recognized as having a somewhat less hostile environment with less diversity and intensity of activity than the stomach and small intestine.
5) Additionally, the colon has a longer retention time and appears highly responsive to agents that enhance the absorption of poorly absorbed drugs.
6) Apart from retarding or targeting dosage forms, a reliable colonic drug delivery could also be an important starting position for the colonic absorption of per orally applied, undigested, unchanged and fully active peptide drugs.
7) As the large intestine is relatively free of peptidases such special delivery systems will have a fair chance to get their drug sufficiently absorbed after per oral application.
8) The simplest method for targeting of drugs to the colon to obtain slower release rates or longer release periods by the application of thicker layers of conventional enteric coatings or extremely slow releasing matrices.
1.3 VARIOUS pharmaceutical approaches to colon targeted drug delivery systems.
*Coating drug with pH-sensitive polymers.
*Encapsulation of drug with in biodegradable polysaccharide polymer.
*Covalent linkage of a drug with a carrier.
- Azo conjugates.
- Cyclodextrin conjugates.
- Glycoside conjugates.
- Glucuronate conjugates.
- Dextran conjugates.
- Polypeptide conjugates.
*Polymeric prodrug.
*Embedding drug in matrices.
*Embedding drug in biodegradable matrices and hydro gels.
*Embedding drug in pH-sensitive matrices.
*Formulation of Timed Released systems.
*Bioadhesive systems.
*Osmotic Controlled Drug Delivery systems.
*Coating with micro particles.
*Redox sensitive systems.
1.3.1 Coating with pH-sensitive polymers
The intact molecule can be delivered to the colon without absorbing at the upper part of the intestine by coating of the drug molecule with the suitable polymers, which degrade only in the colon. The pH-dependent systems exploit the generally accepted view that pH of the human GIT increases progressively from the stomach (pH 1-2 which increases to 4 during digestion), small intestine (pH 6-7) at the site of digestion and it increases to 7-8 in the distal ileum.
The coating of pH-sensitive polymers to the tablets, capsules or pellets provide delayed release and protect the active drug from gastric fluid. The polymers used for colon targeting, however, should be able to withstand the lower pH values of the stomach and of the proximal part of the small intestine and also be able to disintegrate at the neutral of slightly alkaline pH of the terminal ileum and preferably at the ileocecal junction. These processes distribute the drug throughout the large intestine and improve the potential of colon targeted delivery systems.
Commonly used pH sensitive with their threshold pH
|
Polymer |
Threshold pH |
|
Eudragit® L100 |
6.0 |
|
Eudragit® S100 |
7.0 |
|
Eudragit® L-30D |
5.6 |
|
Eudragit® FS 30D |
6.8 |
|
Eudragit® L100-55 |
5.5 |
|
Poly vinyl acetate phthalate |
5.0 |
|
Hydroxypropyl methyl cellulose phthalate |
4.5-4.8 |
|
Hydroxypropyl methyl cellulose phthalate SO |
5.2 |
|
Hydroxypropyl methyl cellulose phthalate SS |
5.4 |
|
Cellulose acetate phthalate |
5.0 |
Eudragit S 100
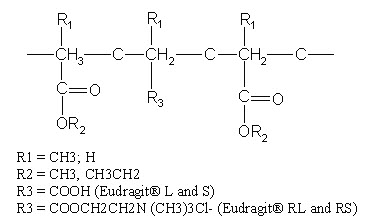
Most commonly used pH-dependent coating polymers are methacrylic acid copolymers, commonly known as Eudragit® S (Registered trademark of Rohm Pharmaceuticals, Darmstadt, Germany), more specifically Eudragit® L and Eudragit® S.
Eudragit® L100 and S 100 are copolymers of methacrylic acid and methyl methacrylate. The ratio of carboxyl to ester groups is approximately 1:1 in Eudragit® L100 and 1:2 in Eudragit® S 100. The polymers form salts and dissolve above pH 5.5 and disperse in water to form latex and thus avoid the use of organic solvents in the coating process.
The water solubility of the Eudragit® S depends on the ratio of free carboxyl groups to the esterifies groups. Polymers with ionizable phthalic acid groups dissolve much faster and at a lower pH than those with acrylic or methacrylic acid groups. The presence of plasticizer and the nature of the salt in the dissolution medium also influence the dissolution rate of Eudragit®. In addition, the permeability of the film formed may depend on the type of solvent used to dissolve Eudragit®
Colon targeted drug delivery systems based on methacrylic resins has described for insulin, prednisolone, quinolones, salsalazine cyclosporine, beclomethasone dipropionate and naproxane.
The polymer has a threshold pH is 7 and above, indicating that polymer can withstand the acidic pH of stomach and cannot dissolve in the entire stomach pH 1-2 and proximal part of small intestine where the pH is near to 6 and thus successfully delivers the drug in the colon by disintegrating at the favorable pH (neutral or slightly alkaline pH of the terminal ileum and preferably at the ileocecal junction). Polymer is soluble in polar organic solvents such as alcohol, acetone, chloroform etc. The polymer is non toxic to body. Its LD50 is 7.9g/kg body wt.
1.3.2 encapsulation of drug with in biodegradable polysaccharide polymers
The bioenvironment inside the human GIT is characterized by the presence of complex micro flora especially the colon that is rich in microorganisms that are involved in the process of reduction of dietary component or other materials. Drugs that are coated with the polymers, which are showing degradability due to the influence of colonic microorganisms, can be exploited in designing drugs for colon targeting. Actually, upon passage of the dosage form through the GIT, it remains intact in the stomach and small intestine where very little microbially degradable activity is present that is quiet insufficient for cleavage of polymer coating.
The Polysaccharides, the polymer of monosaccharides retains their integrity because they are resistant to the digestive action of gastrointestinal enzymes. The polysaccharides are assumed to remain intact in the physiological environment of stomach and small intestine but once they reach in the colon, they are acted upon by the bacterial polysaccharidases and results in the degradation.
This family of natural polymers has an appeal to the area of drug delivery as it is comprised of polymers with a large number of derivatizable groups, a wide range of molecular weights, varying chemical compositions, and for the most part, a low toxicity and biodegradability, yet a high stability. The most favourable property of these materials is that they are already approved as pharmaceutical excipients.
The most important fact in the development of polysaccharide derivatives for colon targeted drug delivery is the selection of a suitable biodegradable polysaccharide. As these polysaccharides are usually soluble in water, they must be made water insoluble by cross linking or hydrophobic derivatisation. Very important is an optimal proportional of the hydrophobic and hydrophilic parts respectively and the number of free hydroxy groups in the polymeric molecule.
A large number of polysaccharides such as amylose, guar gum, pectin, chitosan, inulin, cyclodextrins, chondroitin sulphate, dextrans and locust bean gum have been investigated for their use in colon targeted drug delivery systems.
The various bacteria present in colon and various enzymes secretes are as-
* Colonic bacteria-
- Bacteroides
- Bifidobacterium
- Eubacterium
- Peptococcus
- Lactobacillus
* Enzymes secreted-
- β-Glucuronidase
- β-Xylosidase
- β-Galactosidase
- α-Arabinosidase
- Nitroreductase
- Azoreductase
- Deaminase
- Urea Hydroxylase
commonly used bioegradable polysacharides and colonic bacteria that degrade them-
|
Polysaccharides |
Bacterial specics that degrade them |
|
Amylose |
Bacteroides ,Bifidobacterium |
|
Arabinogalactan |
Bifidobacterium |
|
Chitosan |
Bacteroides |
|
Chondroitin sulphate |
Bacteroides |
|
Cyclodextrin |
Bacteroides |
|
Dextran |
Bacteroides |
|
Guar-gum |
Bacteroides ,Ruminococcus |
|
Pectin |
Bifidobacterium , Bacteroides , Eubacterium |
|
Xylon |
Bifidobacterium , Bacteroides |
chitosan
Chitosan is a high molecular weight polycationic polysaccharide derived from naturally occurring chitin by alkaline deacetylation.
Chemically, it is a poly (N-glucosamine).
Chitosan has favourable biological properties such as nontoxicity, biocompatibility and biodegradability. Similar to other polysaccharides it also undergoes degradation by the action of colonic micro flora and hence poses its candidature for colon targeted drug delivery.
1.4 Associated Disease-
Amebiasis
It is an intestinal illness that's typically transmitted when someone eats or drinks something that's contaminated with a microscopic parasite called Entamoeba histolytica (E. histolytica). The parasite is an amoeba, a single-celled organism. That's how the illness got its name — amebiasis. In many cases, the parasite lives in a person's large intestine without causing any symptoms. But sometimes, it invades the lining of the large intestine, causing bloody diarrhea, stomach pains, cramping, nausea, loss of appetite, or fever. In rare cases, it can spread into other organs such as the liver, lungs, and brain. Amebiasis typically occurs in areas where living conditions are crowded and where there is a lack of adequate sanitation. The illness is very prevalent in parts of the developing world, including Africa, Latin America, India, and Southeast Asia. It is rare in the United States, occurring mostly in immigrants, recent travelers to high-risk countries, and people with HIV/AIDS.
1.4.1 Occurrence Of Intestinal Disease
Disease occurs when amoeba comes in contact with the cells, lining the intestine. It then secretes the substances to digest bacteria; the substances include enzymes that destroy cell membranes and proteins. Thus penetration and digestion of human tissue occur, resulting first in flask-shaped ulcers in the intestine. Entamoeba histolytica also ingests the destroyed cells by phagocytosis. Then a granulomateous mass (known as an amoeboma) may form in the wall of the ascending colon or rectum.
1.4.2 Occurrence Of Extraintestional disease
Cysts and trophozoites are passed in feces. Ingestion of mature cysts causes excystation in the small intestine. Trophozoites are released, which migrate to the large intestine where they multiply by binary fission. The trophozoites invade the intestinal mucosa enter the bloodstream reaches extra intestinal sites such as the liver, brain, and lungs causes extra intestinal disease.
1.4.3 Signs and Symptoms
Most kids who get amebiasis have minimal or no symptoms. When children do become ill, they experience abdominal pain that begins gradually, along with frequent loose or watery bowel movements, cramps, nausea, and a loss of appetite. In some cases they develop a fever and, possibly, bloody stools. For some people, symptoms of amebiasis can begin within days to weeks of swallowing food or water contaminated by amoebas. For other people, symptoms of amebiasis either take months to appear or never appear at all.
1.4.4 Contagiousness
Amebiasis is contagious. Wherever living conditions are unsanitary and hygiene is poor, the chances are higher that the infection will pass from person to person. Someone carrying amoebas in his or her intestines can pass the infection to others through the stool. When infected stool contaminates food or water supplies, amebiasis can spread quickly to many people at once. This is especially true in developing countries where drinking water may be contaminated. Amebiasis can also be spread between people through inadequate hand washing, by using the same objects, and by sexual contact.
1.4.5 Prevention
There is no vaccine to prevent amebiasis. Because amoebas may contaminate food and water, you can help prevent the illness by being cautious about what you eat and drink, especially in developing countries, where a good rule regarding food is to cook it, boil it, peel it, or forget it.
1.4.6 treatment
|
Specific Form of Amebiasis |
Drug (s) of Choice |
Alternative Drug(s) |
|
Asymptomatic infection (intestinal) |
Diloxanide furoate |
Iodoquinol OR Paromomycin |
|
Mild-moderate infection; (non dysenteric colitis) |
Metronidazole Plus Diloxanide furoate Idoquinol OR Paromomycin |
Diloxanide furoate OR Iodoquinol Plus Tetracycline followed by Chloroquine OR Paromomycin followed by Chloroquine |
|
Severe intestinal infection (dysentery) |
Metronidazole Plus Diloxanide furoate OR Idoquinol |
Tetracycline Plus Diloxanide furoate OR Iodoquinol Followed By Chloroquine |
|
Hepatic Abscess |
Metronidazole Plus Diloxanide furoate OR Idoquinol Followed By Chloroquine |
Dehydroemetine OR Emetine Followed By Chloroquine Plus Diloxanide furoate OR Iodoquinol |
1.5NOVEL Colloidal Carrier systems-
* Lipid Carriers-
- Liposomes.
- Solid Lipid Nanoparticles.
- Fat Emulsions.
- Emulsomes.
* Bioadhesive system.
* Particulate Carriers-
- Nanoparticle.
- Microparticle.
1.5.1microparticle- (microspheres)
These are the spherical particles composed of various natural and synthetic materials with diameters in the micrometer range. These are freely flowing particles having size less than 200 micrometer.
Solid biodegradable microspheres incorporating a drug dispersed or dissolved throughout particle matrix have the potential for control release of drug. These carriers are also used for prolonged release as well as for targeting.
The incorporation of drug can be achieved by entrapment during production. Drug release is controlled by dissolution and diffusion of drug from the microsphere matrix and polymer degradation.
1.5.2 Various methods for preparation of microspheres
- Single emulsification techniques.
- Multiple emulsification techniques.
- Polymerization techniques.
* Normal polymerization
* Interfacial polymerization
- Phase separation coacervation.
- Spray drying and spray congealing.
- Solvent extraction
- Ionic gelation
- Cross linking
- Precipitation
1.5.3 advantages of microspheres
1. Controlled delivery of drugs.
2. High stability profile.
3. Relatively simple to prepare.
4. Allow convenience of individualized dose regimen.
5. Precise control over release of drug.
6. Drug Targeting
1.5.4 advantages in colon targeting
1. To deliver proteins and peptides.
2. To deliver the entire drug safely to colon.
3. A novel delivery system.
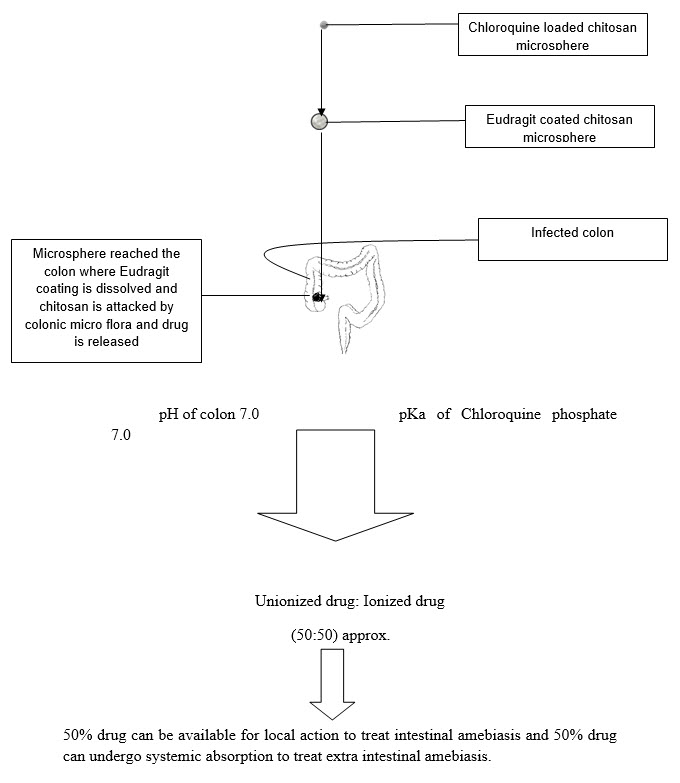
1.6 proposed system and its advantage
1.7 literature survey
Ravi et al., 2008 reviewed the potential for colon targeting to treat the colon diseases.
Chourasia and Jain 2003, described various approaches for colon targeting delivery, various polymers used for colon delivery system.
Paharia et al., 2007 described the Eudragit coated pectin microspheres for colon targeting.
Kreidl et al., 1999 reviewed the colon disease amebiasis.
Goldsmith 1988, studied the treatment for amebiasis and various complications of amebiasis
Park et al., 2002 prepared the TPP cross linked chitosan micro particles.
Rahman et al., 2006 described the characterization of Eudragit coated pectin micro particles.
Bajpai et al., described the design of Chloroquine phosphate nanoparticles.
Walker et al ., 1971 reviewed the molecular parameters influencing rate of reduction of azo compounds by intestinal micro flora.
Spitael et al., 1979 studied the Solubility and dissolution rate of enteric polymers.
Ashford et al. 1993 carried out an in vitro investigation into the suitability of pH-dependent polymers for colon targeting.
Shu et al., 2001 prepared the novel pH-sensitive citrate cross-linked chitosan film for drug controlled release.
Lim et al., 1997 showed Chitosan microspheres prepared by emulsification and ionotropic gelation.
Ravi Kumar, M.N.V., 2001. reviewed chitin and chitosan applications.
Malick et al., 1998 described the Controlled drug delivery by biodegradable poly (ester) devices.
Prasad et al., 1998 reviewed the In vitro evaluation of guar gum as a carrier for colon-specific drug delivery.
1.8 Research envisage
The colon targeted drug delivery system has potential to reduce the side effects and improve the pharmacological response of drugs at local site. The treatment of large intestine disorders, where high concentration of drug is required, can be improved by colon specific delivery system. Various approaches have been reported for the colon targeting. Among them encapsulation of drug within biodegradable polysaccharide and then coating the core microsphere with pH sensitive polymer improve the efficacy of delivery system.
The biodegradable polysaccharide can be degraded by colonic bacteria but can also be degraded by acidic pH of stomach which can be prevented by coating the core microspheres by pH sensitive polymer. Hence this combined system is advantageous in delivering the entire drug safely to colon. The disease amebiasis caused by E. histolytica occurs in large intestine with or without any symptoms and becomes severe if not treated. The treatment includes the combination of drugs. One serves as luminal amebicidal and other as systemic.
Thus research work envisages include development and characterization of novel carrier system, microsphere that target the colon and deliver the drug, Chloroquine phosphate, in controlled rate to treat amebiasis. Thus by this novel approach we can limit to only one drug for treatment, irrespective of conventional, combination drug regimens.
1.9 Plan of work-
*Literature Survey-
*Pre formulation Studies –
- Drug Identification and Characterization -
- I.R. Spectroscopy.
- U.V. Spectroscopy.
- Solubility.
- Partition Coefficient.
* Formulation and Optimization Of Microsphere-
- Ionic Gelation Method.
- Optimization-
- Chitosan Concentration.
- Stirring Time.
- TPP. Concentration.
- Chitosan: TPP. Ratio
- Coating By Eudragit S-100-
- Coat: Core Ratio.
- StirringTime.
* Characterization-
- Surface morphology.
- Particle size and particle size distribution.
- Entrapment efficiency.
- In-vitro Release studies.
* Compilation of data and Submission.
2 DRUG PROFILE AND CHARACTERIZATION
2.1 Drug selected-
Chloroquine phosphate
2.2 Drug Profile-
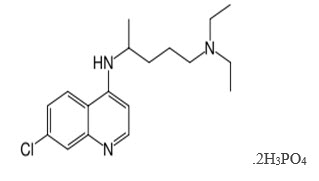
N'-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diaminediphosphate
Group : - Amino-4-quinoline (quinoline derivative)
Chemical formula: - C18H26ClN3, 2H3Po4
Molecular mass : - 515.9 g.
Solubility: - Freely soluble in water 50mg/ml
Partition coefficient: - 4.39
pKa: - 7.0, 9.2
Melting Point: - 194°C (381.2°F) - 213 C.
Color: - White to yellowish.
2.3 drug pharmacology
Mechanism of action
Chloroquine has been found to be active against the asexual erythrocytic forms of all species of malaria parasites; P. vivax, P. ovale, P. malaria and susceptible strains of P. falciparum. It is a rapid acting blood schizontocide with some gametocytocidal activity against P. ovale, P. vivax, P. malariae and immature gametocytes of P. falciparum.
The mechanism of plasmodicidal action of Chloroquine is not completely certain. Its effect is believed to result, at least in part, from its interaction with DNA. It acts mainly on the large ring form and mature trophozoite stages of the parasite.
Chloroquine also is taken up into the acidic food vacuoles of the parasite in the erythrocyte. It increases the pH of the acid vesicles, interfering with vesicle functions and possibly inhibiting phospholipids metabolism.
In suppressive treatment, Chloroquine inhibits the erythrocytic stage of development of plasmodia. In acute attacks of malaria, Chloroquine interrupts erythrocytic schizogony of the parasite. Its ability to concentrate in parasitized erythrocytes may account for its selective toxicity against the erythrocytic stages of plasmodial infection.
pharmacokinetics
Chloroquine is rapidly and almost completely absorbed from the gastrointestinal tract following oral administration and peak plasma concentrations of the drug are generally attained within 1-2 hours.
Chloroquine is widely distributed into body tissues such as the eyes, heart, kidneys, liver and lungs, where retention is prolonged. Concentrations are two to five times higher in erythrocytes than in plasma. Very low concentrations are found in intestinal wall. Chloroquine crosses the placenta and is distributed into breast milk.
Approximately 55% of the drug in the plasma is bound to plasma proteins. It is metabolized in the liver to active de-ethylated metabolites. Principal metabolite is desethylchloroquine. The plasma half life of Chloroquine in healthy individuals is generally reported to be 72-120 hours. Chloroquine is eliminated by renal route. 42 to 47% of Chloroquine is excreted unchanged in the urine; 7 to 12% desethylchloroquine is excreted in urine. Chloroquine is excreted very slowly and may persist in urine for months or years after medication is discontinued. Urine acidification increases renal excretion by 20 to 90%.
indications
Treatment of acute attacks of malaria due to P. vivax, P. malariae, P. ovale, and susceptible strains of P. falciparum. Chemoprophylaxis in non-immune individuals at risk. Chloroquine does not prevent relapses in patients with vivax or malariae malaria because it is not effective against exoerythrocytic forms of the parasite. The drug is also indicated for the treatment of extra intestinal amebiasis.
Adverse effects
Ocular reactions:Irreversible retinal damage; visual disturbances; nyctalopia; scotomatous vision with field defects of paracentral, pericentral ring types, and typically temporal scotomas.
Neuromuscular reactions:Convulsive seizures.
Auditory reactions:Nerve type deafness; tinnitus, reduced hearing in patients with preexisting auditory damage.
Gastrointestinal system: Anorexia, nausea, vomiting, diarrhea, abdominal cramps.
CNS reactions:Mild and transient headache, psychic stimulation, fatigue, nervousness, anxiety, apathy, irritability, agitation, aggressiveness, confusion, personality changes, depression.
Cardiovascular reactions:Rarely, hypotension, electrocardiographic change, cardiomyopathy.
Others:Allergic and anaphylactic reactions, peripheral neuritis, neuromyopathy, changes in liver function including hepatitis and abnormal liver function tests occur rarely.
dosage
Children and adults, for whom the use of Chloroquine is indicated, should receive a full treatment dose of 25 mg of Chloroquine base per kg given over 3 days. The pharmacokinetically superior regimen consists of 10 mg of base per kg followed by 5 mg/kg 6-8 h later and 5 mg/kg on each of the following 2 days.
A more practical regimen used in many areas consists of 10 mg/kg on the first and second days and 5 mg/kg on the third. Both these regimens provide a total dose of 25 mg/kg (e.g. 1 500 mg of base for a 60-kg adult).
There is no evidence to suggest that increasing the dosage will increase the clinical cure rate in such situations and repeated administration of such high doses may produce adverse reactions. GI upsets may be avoided by administering the dose after a meal.
Recommended
5 mg of base per kg weekly in a single dose, or 10 mg of base per kg weekly, divided into 6 daily doses.
Extra intestinal Amebiasis
Adults, 1 g (600 mg base) daily for two days, followed by 500 mg (300 mg base) daily for at least two to three weeks. Treatment is usually combined with an effective intestinal amebicide.
toxicity
Toxic doses of Chloroquine can be fatal. As little as 1 g may be fatal in children. Toxic symptoms can occur within minutes. These consist of headache, drowsiness, visual disturbances, nausea and vomiting, cardiovascular collapse, shock and convulsions followed by sudden and early respiratory and cardiac arrest. The electrocardiogram may reveal atrial standstill, nodal rhythm, prolonged intraventricular conduction time, and progressive bradycardia leading to ventricular fibrillation and/or arrest.
2.4 Drug Identification and Characterization–
* I.R. Spectroscopy.
* U.V. Spectroscopy.
* Solubility.
* Partition Coefficient.
2.4.1I.R. Spectroscopy of drug -
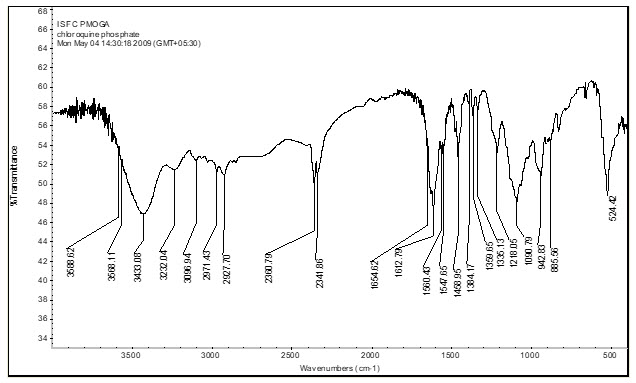
Spectrum- Chloroquine Phosphate
Library Record Match-
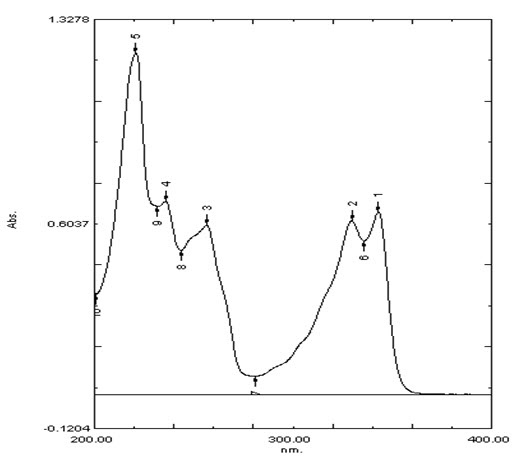
2.4.2U.V. Spectroscopy. Of drug-
Table showing absorbance maximum for chloroquine phosphate at 220nm, 235nm, 256nm, 329nm, 342nm as Stated in I.P.
|
S.No. |
Wavelength λmax |
Absorbance |
|
1 |
343 |
0.645 |
|
2 |
229.6 |
0.614 |
|
3 |
256.4 |
0.599 |
|
4 |
235.8 |
0.683 |
|
5 |
220.8 |
1.207 |
2.4.3 Solubility
The drug is soluble in cold water 50 mg/ml.
2.4.4 partition coefficient
The drug has partition coefficient value is around 5.0
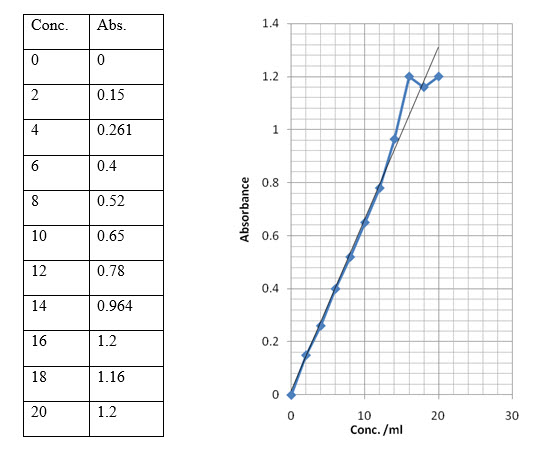
2.4.5 Std. plot of Chloroquine Phosphate in water at λmax 220nm
3.0 FORMULATION AND OPTIMIZATION OF CHITOSAN MICROSPHERE
3.1 FORMULATION Method - Ionic gelation method
The method involves the drop wise addition of tripolyphosphate solution in the chitosan solution with constant stirring. The prepared microspheres were collected by centrifugation at 12000 rpm.
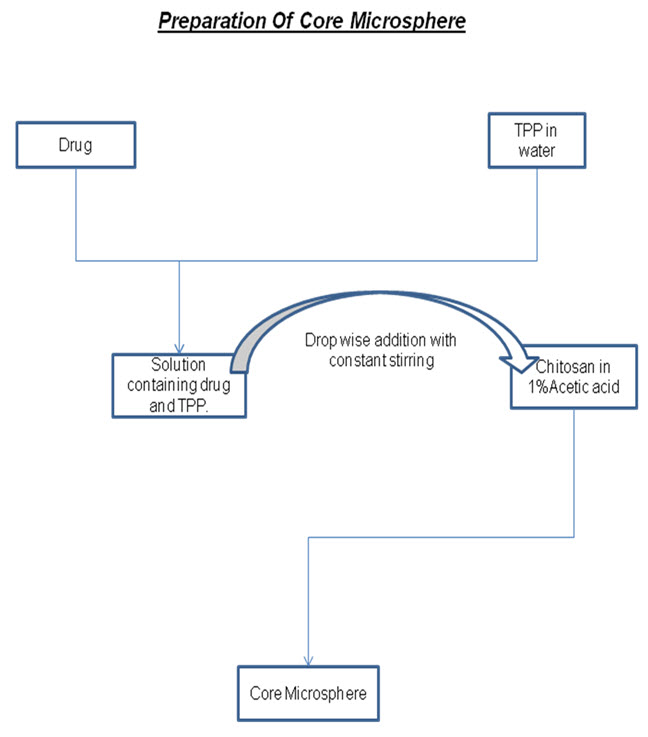
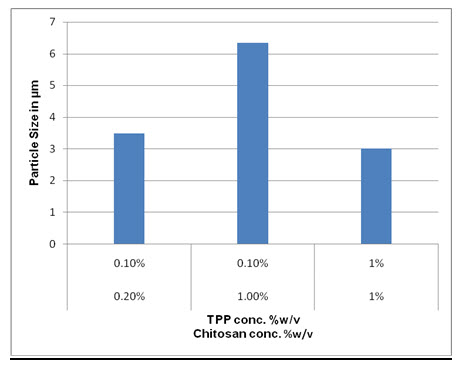
3.2 Formulation and Optimization Of Chitosan Microsphere without drug-
|
Acetic Acid conc. |
Chitosan conc. |
TPP. Conc. |
Partical Size |
|
1% |
0.1% |
0.1% |
----- |
|
1% |
0.2% |
0.1% |
2.5-5 μ |
|
1% |
1% |
0.1% |
5-8 μ |
|
1% |
1% |
1% |
1.5-2.5 μ |
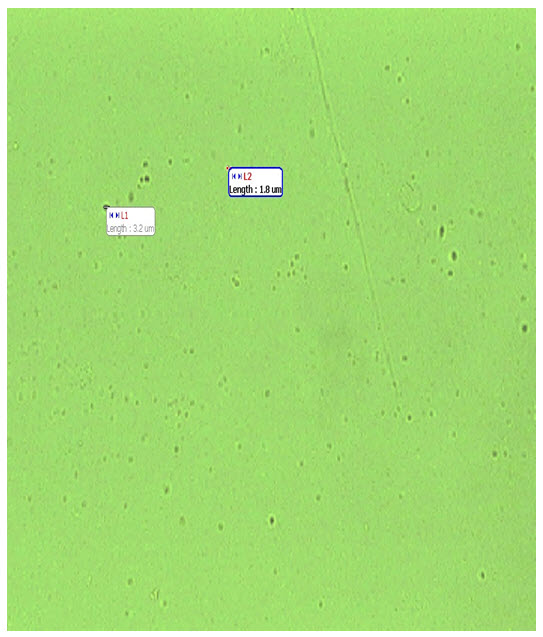
3.3 Drug loaded microsphere
|
Amount of drug |
Chitosan conc. |
TPP. Conc. |
Partical Size |
|
50 mg |
1% |
1% |
2.5-4 μ |
3.4 Entrapment efficiency-
For the determination of entrapment efficiency, the prepared microspheres were centrifuged at 12000rpm for 20 min. The supernatant was collected and analyzed on U.V. at 220 nm. The amount of drug present in the supernatant can be calculated using standard plot for Chloroquine.
Once the amount of drug in supernatant was found, the entrapped drug in microspheres can be calculated.
|
Drug Taken |
Abs of Supernatant |
Conc. Of drug in supernatant |
Amt of drug in 1 ml supernatant |
Amt of drug in 11 ml supernatant |
Amount of drug in microspheres |
% entrapment |
|
50mg |
0.450 |
7μg/ml |
0.7mg |
7.7mg |
42.3mg |
84.6% |
4.0 Coating Of Core Microsphere
4.1 Method of coating- O/O Emulsification
The method involves dispersing core microspheres in coating solution prepared by dissolution of Eudragit in ethanol and acetone (2:1). the organic phase is poured in light liquid paraffin containing 1%w/v span 80 the system is made under 1000 rpm at room temperature for 3 hours to allow evaporation of solvent. Coated microspheres were centrifuged, washed with n-hexane and freeze dried overnight.
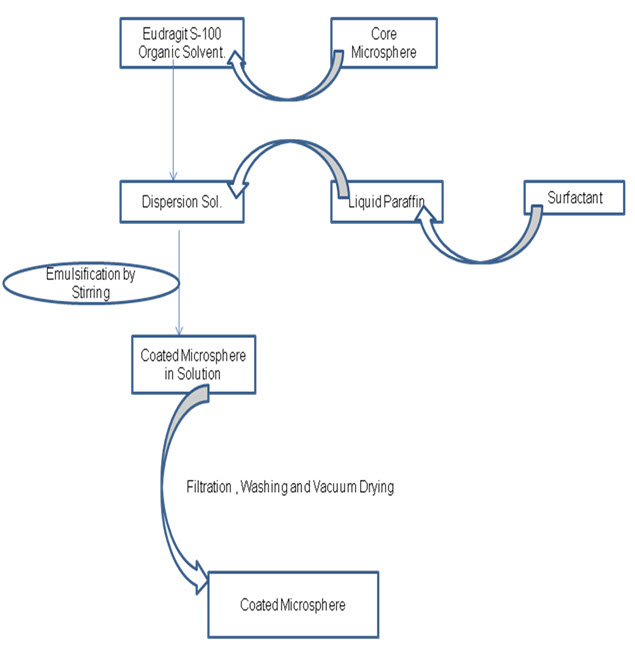
4.2 Coating of drug loaded core microspheres by
Eudragit S-100
|
Coat : Core Ratio |
Particle Size |
Population |
|
1:1 |
25-40 μ |
Collectable |
SUMMARY AND CONCLUSION
The diseases associated with colon are asymptomatic in the initial stages and become sever in later stages so the treatment become difficult for both patient and physician and it includes multiple drug therapy which has in addition to pharmacological advantages more side effects, so to overcome these side effects and to go for a single drug therapy various colon targeting drug delivery system has been proposed and developed for treating IBD, Chron’s disease, ulcerative colitis, amebiasis etc.
This study offers a system based on combined approach for the treatment of amebiasis that is Eudragit coated Chloroquine loaded chitosan micro particles.
The system employs chitosan as biodegradable polysaccharide that forms the drug containing core and Eudragit S100 as pH sensitive polymer that forms the coating over microsphere. The chitosan microspheres were prepared by ionic gelation method. Further microspheres were coated with Eudragit s-100 to protect them from acidic environment of stomach and to deliver chitosan microspheres to colon.
In the colon the chitosan is degraded by colonic bacteria and by various enzymes and releases drug. As the pH of colon varies from 6.4-7.0 (6.4 ascending colon, 6.6 transverse colon, 7.0 descending colon, Meldrum et al., 1972; Evans et al., 1988) and pKa of Chloroquine phosphate is 7.0 indicates that approx. 50% drug is available in the unionized form and aprox.50% is available in ionized form upon reaching to colon, so, 50%drug can be used for intestinal amebiasis and 50% can be absorbed from colon for extra intestinal amebiasis by systemic absorption.
With this system we can treat amebiasis by single drug i.e. Chloroquine rather than going for conventional multiple drug therapy.
Thus system can be used to treat both intestinal and extra intestinal amebiasis.
For the preparation of microspheres, various concentrations from 0.1-1%w/v of chitosan in 1%v/v acetic acid solution were prepared and various concentrations from 0.1-1%w/v of Tri Poly Phosphate were prepared and added to the above chitosan solutions. And final formulation was prepared by employing 1%w/v chitosan solution and 1% w/v TPP solution. Then the core microspheres were coated with Eudragit in such a way to give 1:1 coat core ratio.
The prepared microspheres were characterized for particle size, population and entrapment efficiency.
The particle size of the core microspheres was found to be 1.5-2.5 microns and drug loaded microspheres were of size 2.5-4 microns. The size of coated microspheres was found to be 40 microns.
The % entrapment of drug in chitosan microspheres was found to be 84.6%.
Thus it could be concluded from the present study that the system “Eudragit coated Chloroquine loaded chitosan micro particles” can be capable of delivering drug to colon for the treatment of amebiasis (intestinal and extra intestinal) and thus advantageous over conventional multiple drug therapy.
Further research is focused on the drug release studies, animal studies, dose optimizing and finally focusing on the development of system which provides maximum benefits with minimum side effects to treat amebiasis.
References
* Chourasia M K, Jain S K, 2003. “Pharmaceutical approaches to colon targeted drug delivery systems”. J Pharm Pharmaceut Sci ,6(1):33-66
* Paharia A, Yadav AK, Rai G, Jain SK, Pancholi SS, Agrawal GP, . 2007. “Eudragit-coated Pectin Microspheres of 5-Fluorouracil for Colon Targeting”. AAPS PharmSciTech., 8 (1): 12.
* Ravi V, Pramod Kumar TM, S, 2008.”Novel colon targeted drug delivery system using natural polymers”. Indian J Pharm Sci. 70 (1): 111-3.
* Bajpai A K, Choubey J, 2006 “Design of gelatin nanoparticles as swelling controlled delivery system for Chloroquine phosphate” J Mater Sci. (17 ): 345–358.
* Shantha K, (June 2005). “Review of Polysaccharides for Encapsulation and Delivery”. Food Science and Nutrition Rev., 45 (4):251-258.
* Pramod K, Singh S, Mishra B, 2008. “Colon Targeted Delivery Systems of Metronidazole Based on Osmotic Technology: Development and Evaluation”. Chem. Pharm. Bull., 56 (9): 1234-1242.
* Rahman Z, Kohli K, Khar RK, Ali M, Charoo NA, Shamsher AA, 2006 “Characterization of 5-Fluorouracil Microspheres for Colonic Delivery”. AAPS PharmSciTech. 2 (7): 47.
* Sebastiaan J van Hal, Damien J Stark, Debbie Marriott and Jock L Harkness. 2007. “Amebiasis: current status in Australia” Med J Aust, 6 (187): 372-374.
* Kreidl P, Imnadze P, Baidoshvili L, Greco D (October 1999). “Investigation of an outbreak of amebiasis in Georgia". Euro Surveill. 10 (4): 103–104.
* Goldsmith R S, 1998 “Antiprotozoal Drugs in Basic and Clinical Pharmacology” Appleton-Lange,:838-861.
* Oladapo A, Adetunji, Michael A, Odeniyi, Oludele A, 2006. “Research Article” Trop J Pharm Res,(5):2
* J.A. Koa, H.J. Park b,*, S.J. Hwang c, J.B. Park d, J.S. Lee e, (2002) International Journal of Pharmaceutics,( 249): 165/174
* Spitael J., Kinget R., 1980 “Influence of solvent composition upon film-coating”. Pharm Acta Helv, (55): 157-160
* Ashford M., Fell J.T., Attwood D., and Woodhead P.J., 1993 “An in vitro investigation into the suitability of pH-dependent polymers for colon targeting”. Int J Pharm, (91): 241-243
* Spitael J., Kinget R., 1979 “Solubility and dissolution rate of enteric polymers”. Acta Pharm Technol, (25): 163-168
* Walker R., and Ryan A.J., 1971 “Some molecular parameters influencing rate of reduction of azo compounds by intestinal micro flora”. Xenobiotica, (1): 483-486
* Shu X.Z., Zhu K.J., Song W., 2001” Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release”. Int. J. Pharm. (212): 19_/28
* Ravi Kumar, M.N.V., 2001. “A review of chitin and chitosan Applications” . Reactive Funct. Polymers (46): 1_/27
* Jain R, Shah NH, Malick AW, Rhodes CT., 1998 “Controlled drug delivery by biodegradable poly (ester) devices : different preparative approaches”. Drug Dev Indian pharm, (24 ): 703-27
* Lim L.Y., Wan L.S.C., Thai P.Y., 1997. “Chitosan microspheres prepared by emulsification and ionotropic gelation”. Drug Dev. Ind. Pharm, (23): 981_/985.
* Prasad YV, Krishnaiah YS, Satyanarayana S., 1998 “In vitro evaluation of guar gum as a carrier for colon-specific drug delivery”. J Cont Rel, (51) :281-7
* Mi F.L., Shyu S.S., Lee S.T., Wong T.B., 1999” Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method”. J. Polym. Sci: Polym. Phys,( 37): 1551_/1564.
* Salles J M, Moraes L A, 2003 “Hepatic Amebiasis Rev.” Brazilian J Inf Dis., 2 (7) : 96-110.
* Kosaraju S.L., 2005 “Colon-targeted delivery systems ; Review of polysaccharides for encapsulation and delivery”. Crit Rev Food Sci Nut,( 45): 251-258
* Lamprecht A., Yamamoto H., Takeuchi H., Kawashima Y., 2003 “Microsphere design for the colonic delivery of 5-Fluorouracil” J Control Rel,( 90): 313- 322
* Shu X.Z., Zhu K.J., 2000.” A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled drug delivery”. Int. J. Pharm,( 201): 51_/58
* US Patent “6039975” Colon targeted delivery system.
* US Patent “6682758” Water-insoluble drug delivery system.
* US Patent “5525634” Colonic drug delivery system









