 About Authors:
About Authors:
Nagasaraswathi.M*, Rafi khan.P, Aleemuddin.MA, Gopi Chand.K, Nagaprashanthi.Ch
Faculty of Pharmacy, PRIST University,
Thanjavur-614901, Tamilnadu (INDIA)
abdulaleemuddin@gmail.com
ABSTRACT
The importance for medicinal herbs is increasing day by day due to its high availability and lower side effects. The present study investigated the total phenolic and flavonoid content of various extractions of Indigofera tinctoria linn. The total phenolic content was determined by folin-ciocatechu reagent using Gallic acid as standard and total flavnoid content by aluminium chloride assay using Quercetin as a standard. The present study showed the ratio of phenolic and flavonoid content in different extracts of Indigofera tinctoria linn. Aqueous extract showed the more flavonoid and phenolic content in comparision with other extracts.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1591
INTRODUCTION :
Medicinal herbs are known to produce certain bioactive substances and secondary metabolites such as flavonoids, phenolic compounds to maintain healthy system of humans and animals. ( Amandeep Kaur et al., 2011, Jeevan K. Prasain et al.,2004 ). Phenolic compounds have one or more hydroxyl groups attached directly to an aromatic ring. Polyphenols are the compounds that have more than one phenolic hydroxyl group attached to one or more benzene rings(Wilfred vermerries, Ralph Nicholson,2006). Flavonoids are a group of polyphenolic compounds, which are widely distributed throughout the plant Kingdom. Flavonoids are largely planar molecules and their structural variation comes in part from the pattern of substitution: hydroxylation, methoxylation, prenylation, or glycosylation. (Sujatha V et al.,2005). Flavonoids are responsible for many activities like Hepatoprotective activity, CNS activity, cardio tonic activity, lipid lowering activity, antioxidant activity, anti neoplastic activity and anti microbial activity.( Raj Narayana K et al., 2001). Flavonoids are having the greatest antioxidant activity and the order of antioxidant power of flavonoids are catechins > dihydrochalcones > proanthocyanidins > flavonones > flavonols > flavones > anthocyanidins > flavonols. (david Hoffman,2003).
Reactive oxygen species (ROS) such as singlet oxygen (O-), superoxide anion (O2-) and hydroxyl (.OH) radical and hydrogen peroxide (H2O2) are often released by biological reactions or from exogenous factor. These ROS play major role in the degenerative diseases such as cancer, hypertension, atherogenesis, Alzheimers disease, and Parkinsons disease. flavonoids and other polyphenolic constituents have been reported to be act as potent antioxidant. ( Nagulendran KR et al., 2007,Maria Kratchanova et al.,2010). Due to chemical diversity and shape flavonoids interact with targets in different locations to influence the changes in biological activity(Charles S. Buer et al.,2010).
The plant Indigofera tinctoria Linn (fabaceae) is popularly known as neeli in tamil and common name is indigo (Amrith Singh, 2006). Indigofera tinctoria was traditionally using to treat various kinds of diseases such like constipation, liver diseases, heart palpitation, and gout (Amrith Singh, 2006) stimulant, deobstruent, purgative, antiseptic, astringent, antibacterial, antioxidant, cytotoxic effect, hepato protective activity (Renuka devi K.P et al., 2011), Antidyslipidemic activity (Anju Puri et al., 2007), Anti-hyperglycemic activity (Amarnath V Bangar et al., 2011), Anti-proliferative activity (Thiruvamiyoor Ravichandran Kameswaran et al., 2008), Anti-inflammatory activity (Pramod K Tyagi et al., 2010), Antihelmenthic activity (Gunasekaran Balamurugan et al., 2009). The juice of I.tinctoria leaves and indigo powder mixed with honey is used for enlargement of liver and spleen. Juice is also used in asthma, whooping cough, lung diseases and kidney disorders as in dropsy (Nadkarni et.,al 2002).The current study is to investigate the phenolic and flavonoidal content of different extracts of Indigofera tinctoria Linn.
MATERIALS AND METHODS
Plant material
A fresh aerial part of Indigofera tinctoria Linnwas collected from Thanjavur (India) and it was identified and authenticated by Dr.G.V.S.Murthy, Scientist ‘F’ & Head of Botanical Survey Of India, Coimbatore. Specimen number BSI/SRC/5/23/2011-12/Tech-1051
Instruments
UV/Vis double beam spectrophotometer (Techcomp UV-2310 spectrophotometer) and 1 cm quartz cells were used for all absorbance measurements.
Reagents and solutions
The extracting solvents used were all of analytical grade, including ethanol, ethyl acetate, acetone, chloroform, petroleum ether and other reagents and standards: Gallic acid , sodium nitrite, aluminium chloride, sodium hydroxide, sodium carbonate, folin-ciocalteu reagent, Quercetin and Gallic acid, were purchased from Merck mumbai.
Preparation of plant extracts :
Aqueous extraction( Decoction method)
150 g of coarse powder of Indigofera tinctoria Linnleaves and mixture of stem and root powder were boiled with 600ml of distilled water (Sukhdev Swami Handa et al.,2008). Then it was cooled to room temperature and filterate was filtered.
Solvent Extraction:
100gm of powder was subjected to successive soxhlet extraction by various solvent such as petroleum ether, chloroform, acetone, ethylacetate and ethanol. The percentage yield was showed in table I .
Extractive values calculated by following formulae:
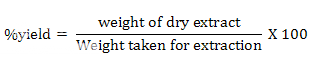
Preparation of Standard Solutions:
Gallic acid and Quercetin 10mg were accurately weighed into a 10 mL volumetric flask, dissolved in 10mL methanol and the solution was made up to 10 mL with the same solvent [1mg/mL].
Determination of total phenolics
To analyze the total phenolic content (TPC), the method of Folin Ciocalteu assay was carried out (Marinova.D et al.,2005). In this method Gallic acid was used as standard and Total phenolic content was expressed as mg gallic acid equivalent (GAE)/100 g fresh weight.. For this purpose, the calibration curve of Gallic acid was drawn (Figure I). 1ml of standard or extract solution (20, 40, 60, 80,100 mg/l) was taken into 25ml volumetric flask, containing 9ml of HPLC grade distill water.1ml of Folin-Ciocalteu’s reagent was added to the mixture and shaken. After 5min, 10ml of 7%Na2Co3 solution was added to the mixture. The solution was diluted upto 25ml with HPLC grade distill water. Incubate the solution at room temperature for 90 min . A reagent blank using HPLC Grade distill water was prepared. The absorbance was noted at 750nm using UV-Visible spectrophotometer.
Determination of total flavonoids
Colorimetric aluminum chloride method was used for flavonoid determination (Marinova.D et al.,2005). in this method Quercetin was used as standard and flavonoid contents were measured as quercetin equivalent. For this purpose, the calibration curve of quercetin was drawn(Figure II). 1ml of standard or extract solution (20, 40, 60, 80,100 mg/l) was taken into 10ml volumetric flask, containing 4ml of HPLC grade distill water. 0.3ml of 5%NaNo2 added to the flask. After 5min, 0.3ml 10%AlCl3 was added to the mixture. At the 6th min add 2ml of 1M NaOH was added and volume made up to 10ml with HPLC grade distills water. The absorbance was noted at 750nm using UV-Visible spectrophotometer. A reagent blank using HPLC Grade distill water was prepared. The absorbance was noted at 415nm using UV-Visible spectrophotometer.
Results and Discussion :
Phytochemical analysis
The results of preliminary phytochemical studies are as follows for different extracts.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Preliminary phytochemical analysis of different extracts
|
S.No |
CHEMICAL TEST |
Aque |
Ethanol |
Chloro |
Ace |
Ethyl acetate |
Pet ether |
Hydro Alcoholic |
|
1 |
Alkoloids |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
2 |
Glycosides |
+ |
+ |
-- |
+ |
- |
- |
+ |
|
3 |
Flavonoids |
+ |
+ |
+ |
+ |
- |
- |
+ |
|
4 |
Saponins |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
5 |
Steroids |
+ |
- |
- |
- |
+ |
- |
- |
|
6 |
Carbohy |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
7 |
Protiens |
+ |
+ |
- |
- |
- |
- |
+ |
|
8 |
Tannins |
+ |
- |
+ |
+ |
+ |
+ |
+ |
|
9 |
Phenolic Group |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ = present, - =Absent
Preliminary phytochemical analysis revealed that the presence of flavonoids and Phenolics abundantly. Besides this hydroalcoholic extracts shows presence of alkaloids, glycosoide, proteins, saponons, carbohydrates, Tannins, Proteins.
In the present study, total phenolic content present in Indigofera tinctoria Linn extract was estimated using modified Folin- ciocalteau method and total flavonoid content by aluminium chloride assay method. The results of total phenolic and total flavonoid content and ratio of total flavonoids/phenolic content of Indigofera tinctoria Linn were represented in table II. The data clearly indicating that Indigofera tinctoria Linn, medicinal herb is rich of phenols and flavonoids. Aqueous extract contains more phenolic and flavonoid content in comparision with other solvent extracts.
Conclusion:
Phenolics are the widely spread as secondary metabolites in plant kingdom(Aleemuddin MA et al, 2012). These are potential natural antioxidant and act by efficient radical scavengers and metal chelator(Tapas et al., 2008). Aqueous extract of Indigofera tinctoria Linnleaves and ethanolic extract of stem and root of Indigofera tinctoria Linnis a potential source of phenols and flavonoids. Further deeper studies have to be carried out to isolate new natural molecules.
Tables and figures:
Table 1 percentage of yield of Indigofera tinctoria Linnextract
|
Solvent |
Yield of extract(%) |
|
Aqueous |
23.3 |
|
Ethanol |
14.2 |
|
Ethyl acetate |
3.3 |
|
Chloroform |
3.8 |
|
Acetone |
5 |
|
Petroleum ether |
0.6 |
Table II Total phenolic and flavonoid content of leaves extract of Indigofera tinctoria
|
solvent |
Total phenolics mg GAE/100g fresh mass |
Total flavonoids mg QC/100g fresh mass |
Flavonoids/Phenolics |
|
Aqueous |
73.48 |
55.9 |
1.31449 |
|
Ethanol |
67.43 |
50.11 |
1.34564 |
|
Chloroform |
8.09 |
2.2 |
3.677273 |
|
Acetone |
5.93 |
3.85 |
1.54026 |
|
Ethyl acetate |
25.29 |
18.99 |
1.331754 |
|
Petroleum ether |
55.26 |
43.01 |
1.284817 |
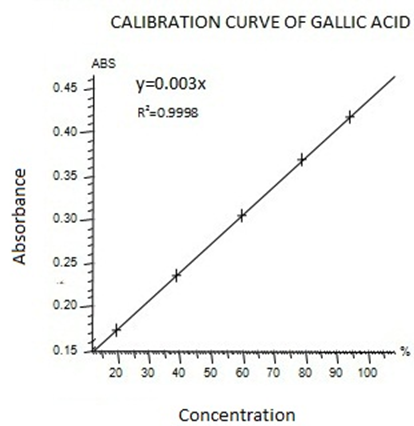
Figure I standard calibration curve of Gallic acid
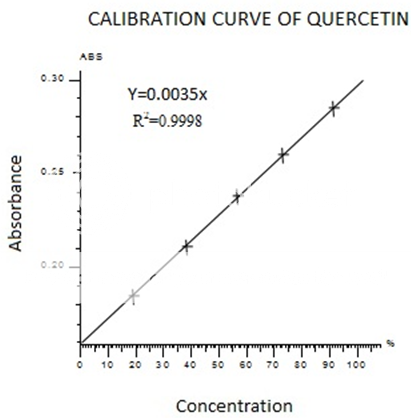
FigureII standard calibration curve of Quercetin
REFERENCES:
- Amrithpal Singh. Medicinal Plants of the World, Special indin edition, Oxford & IBH” Publishing Co.Pvt Ltd, , New delhi( 2006):168.
- Renukadevi KP and Suhani Sultana S. “Determination of Anti bacterial, Antioxidant and Cytotoxicity effect of Indigofera tinctoria on Lung cancer cell line NCI-h69”. International journal of pharmacology.;7(3):356-362, (2011)
- Anju puri,Tanvir khaliq,Rajendran SM,Geetika Bhatia, Ramesh Chandra and Tadigoppula Narender. Antidyslipidemic activity of indigofera tinctoria. Journal of Herbal Pharmacotherapy.7(1): 59-64 (2007).
- Amarnath V Bangar and MG Saralaya. Anti Hyperglycemic activity of ethanol extract and chloroform extract of Indigofera tinctoria leaves in streptozotocin induced diabetic mice. Research Journal of Pharmaceutical , Biological and Chemical Sciences. 2(1) 445-455 (2011).
- Thiruvanmiyoor Ravichandran,Kameswaran and Ravichandran Ramani ,The Anti proliferative acitivity of Biological Sciences;1-7, (2008).
- Pramod KTyagi K. Preliminary phyto chemical Screening and Evaluttion of Anti - inflammatory activity of Ethanolic extract of leaves of Indigofera tinctoria.;3(1):47-50 (2010)
- Gunasekaran Balamurugan and Shinnaraj Selvarajan. Preliminary Phytochemical Screening and Anthelmintic Activity of Indigofera tinctoria Linn. International Journal of Drug development and Research. 1(1):157-160, (2009)
- Nadkarni K M, Indian meteria medica, 3rd revised enlarged edition, Ramdas Bhatkat for popular prakashanpvt. LtdMumbai.( 2002);680-681
- Amandeep kaur, Rana A.C, Vineeta tiwari , ramica Sharma and sunil kumar, Review on ethanomedicina and pharmacological properties of Ficus religiosa, J.App.Pharm.Sci. 2011, 1 ( 8 ), 06-11.
- Charles S. Buer, Nijat Imin and Michael A. Djordjevic, Flavonoids: New Roles for Old Molecules, Journal of Integrative Plant Biology (2010), 52 (1): 98–111.
- David Hoffmann, Medical herbalism: the science and practice of herbal medicine, inner traditions and bear &co(2003),103.
- Jeevan K. Prasain,y Chao-Cheng Wang,y and Stephen Barne, Mass spectrometric methods for the determination of flavonoids in biological samples, Free Radical Biology & Medicine (2004), 37( 9),1324–1350,
- Krishnarao Mangeshrao Nadkarni,. Nadkarni A. K. Indian materia medica, 3, Vol 1, Bombay, Popular Book Depot, (1955);371.
- Maria Kratchanova, Petko Denev, Milan Ciz, Antonin Lojek and Atanas Mihailov, Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems,acta biochimica polonica,(2010),57(2) 229–234.
- Marinova D, F. Ribarova, M. Atanassova, Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables, Journal of the University of Chemical Technology and Metallurgy, (2005),40( 3), 255-260.
- Nagulendran KR. S. Velavan, R. Mahesh and V. Hazeena begum, In Vitro Antioxidant Activity and Total Polyphenolic Content of Cyperus rotundus Rhizomes, E-Journal of Chemistry(2007), 4, (3),440-449.
- Patel N, Raval S, Goriya H, Jhala M,Joshi B, Evaluation Of Ant Diabetic Activity Of Indigofera tinctoria LinnIn Alloxan –Induced Diabetes In Rat, J Herb Pharmacother (2007);7(1):13-23.
- Raj Narayana K., M. Sripal Reddy, M.R. Chaluvadi, D.R. Krishna, Bioflavonoids classification,pharmacological , biochemical effects and therapeutic potential, Indian Journal of Pharmacology (2001); 33: 2-16.
- Sujata V. Bhat, Bhimsen A, Nagasampagi, Meenakshi sivakumar,Chemistry of natural products,Birkhauser(2005),585-587.
- Sukhdev Swami Handa, Suman Preet Sing Khanuja, Gennaro Longo, Dev Dutt Rakesh, Extraction technologies for medicinal and aromatic plants, Italy, (2008), 23.
- Tapas AR , DM Sakarkar1, and RB Kakde, Flavonoids as Nutraceuticals: A Review, Tropical Journal of Pharmaceutical Research, (2008); 7 (3): 1089-1099.
- White Law Anisile. Materia Indica, Vol II, Rees, London, Longman, Orme, Brown, and Green, (1826);435-436.
- Wilfred vermerries, Ralph Nicholson ,Phenolic Compound Biochemistry,springer(2006), 2-3.
- Amrith Singh ,2006 Medicinal plants of the world, Mohan Primlani for Oxford&IBH Publishing Co.Pvt.Ltd ,New Delhi, Special Indian Edition ,168.
- Aleemuddin MA et al, (2012). Total Phenolic and Flavonoid Content of Different Extract of Coldenia procumbens Linn. Journal Of Pharmacy Research ( New Journal BioMedRx), 5(4)., 2012.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









