About Authors: Kushwaha Anjali*
Department of Pharmaceutics, Institute of pharmacy,
Bundelkhand university, Jhansi (U.P.), India
Reference ID: PHARMATUTOR-ART-1085
Abstract
Solid dispersions is an efficient means of improving the dissolution rate and hence the bioavailability of a range of poorly soluble drugs. This article reviews the various types of solid dispersion, preparation techniques for solid dispersion and compiles some of the recent technologies. Some of the practical aspects to be considered for the preparation of solid dispersions, such as selection of carrier and methods of physicochemical characterization, along with nature of drugs in solid dispersions are also discussed. Finally, limited commercialization of solid dispersions and recent revival has been considered.
Introduction
Therapeutic effectiveness of a drug depends upon the bioavailability and ultimately upon the solubility of drug molecules. Solubility behavior of a drug is one of the key determinants of its oral bioavailability. In recent years, the number of poorly soluble drug candidates has increased tremendously. The formulation of poorly soluble drugs for oral delivery presents a challenge to the formulation scientists. The rate and extent of dissolution of the active ingredient from any dosage form often determines the rate of extent of absorption of the drug. When an active agent given orally, it must first dissolve in gastric and/or intestinal fluids before it can then permeate the membranes of the GI tract to reach systemic circulation. Therefore, a drug with poor aqueous solubility will typically exhibit dissolution rate limited absorption, and a drug with poor membrane permeability will typically exhibit permeation rate limited absorption. Hence, two areas focus on improving the oral bioavailability of active agents include: (i) enhancing solubility and dissolution rate of poorly water-soluble drugs and (ii) enhancing permeability of poorly permeable drugs. This article focuses on the former, in particular, the use of solid dispersion technologies to improve the dissolution characteristics of poorly water-soluble drugs and in turn their oral bioavailability. In case of poorly water soluble drugs, dissolution may be the rate-limiting step in the process of drug absorption. Drug with poor water solubility have been shown to be unpredictably and slowly absorbed compared with drugs of higher solubility. Therefore, a better oral, parenteral, or topical formulation can be developed by increasing the water solubility of the drugs. There are various techniques available to improve the solubility of poorly soluble drugs, such Micronization, Nanosuspension, Modification of the crystal habits, Eutectic mixtures, Solid dispersions, Micro emulsions, Self micro emulsifying drug delivery systems, cyclodextrin inclusion and lipid based delivery systems etc. This review focuses on the solid dispersion technique of solubilization for the attainment of effective absorption and improved bioavailability. Solid dispersion is one of the most promising approaches for solubility enhancement. In the biopharmaceutical classification system (BCS) drugs with low aqueous solubility and high membrane permeability are categorized as Class II drugs Therefore, solid dispersion technologies are particularly promising for improving the oral absorption and bioavailability of BCS Class II drugs. An estimated 40% of these drugs are poorly water soluble. Although most of the drugs have encouraging experimental data obtained in vitro, the in vivo results have been disappointing. The development of solid dispersions as a practically viable method to enhance bioavailability of poorly water-soluble drugs overcame the limitations of previous approaches such as salt formation, solubilization by co- solvents, and particle size reduction. In case of solid dispersion drug disperse in the matrix generally a hydrophilic matrix and a hydrophobic drug, thereby forming a solid dispersion. When the solid dispersion is exposed to aqueous media, the carrier dissolves and the drug releases as fine colloidal particles. The resulting enhanced surface area produces higher dissolution rate and bioavailability of poorly water-soluble drugs. (J Anil Shinde, 2007; Ghaste Rahul et al., 2009)
SOLID DISPERSIONS
“The term SD defined as the dispersion of one or more active ingredients (hydrophobic) in an inert carrier or matrix (hydrophilic) at solid state prepared by the melting (fusion), solvent, or melting-solvent method”. Solid dispersion refers to a group of solid products consisting of at least two different components, generally a hydrophilic matrix and a hydrophobic drug. The matrix can be either crystalline or amorphous. The drug can be dispersed molecularly, in amorphous particles (clusters) or in crystalline particles. Therefore, based on their molecular arrangement, six different types of solid dispersions can be distinguished. Moreover, not the preparation method but the molecular arrangement governs the properties of solid dispersions.
TYPES OF SOLID DISPERSION
Based on their molecular arrangement, six different types of solid dispersions can be distinguished as shown in table are ………
1. Simple eutectic mixture
2. Amorphous precipitations in crystalline matrix
3. Solid solutions
i. According to their miscibility
a. Continuous solid solutions
b. Discontinuous solid solutions
ii. According to the way in which the solvate molecules are distributed in the solvendum
a. Substitutional solid solutions
b. Interstitial solid solutions
4. Glass suspension
5. Glass suspension
6. Glass solution
SIMPLE EUTECTIC MIXTURES
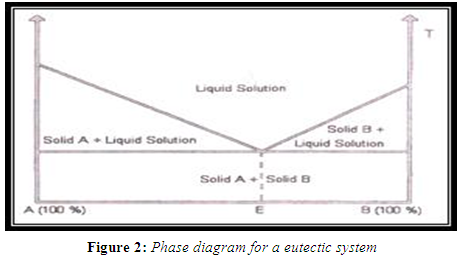
When a mixture of A and B with composition E is cooled, A and B crystallize out simultaneously, whereas when other compositions are cooled, one of the components starts to crystallize out before the other. Solid eutectic mixtures are usually prepared by rapid cooling of a co-melt of the two compounds in order to obtain a physical mixture of very fine crystals of the two components. When a mixture with composition E, consisting of a slightly soluble drug and an inert, highly water soluble carrier, is dissolved in an aqueous medium, the carrier will dissolve rapidly, releasing very fine crystals of the drug. The large surface area of the resulting suspension should result in an enhanced dissolution rate and thereby improved bioavailability. (Sekiguchi K et al.,1961; Goldberg A et al.1966)
SOLID SOLUTIONS
According to their miscibility two types of solid solution are-----
CONTINUOUS SOLID SOLUTIONS
In a continuous solid solution, the components are miscible in all proportions. Theoretically, this means that the bonding strength between the two components is stronger than the bonding strength between the molecules of each of the individual components. Solid solutions of this type have not been reported in the pharmaceutical literature to date.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
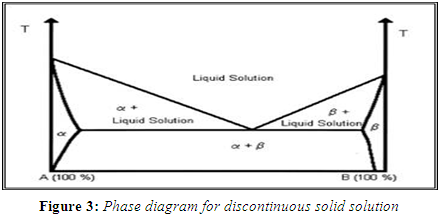
DISCONTINUOUS SOLID SOLUTIONS
In the case of discontinuous solid solutions, the solubility of each of the components in the other component is limited. A typical phase diagram, show the regions of true solid solutions. In these regions, one of the solid components is completely dissolved in the other solid component. Below a certain temperature, the mutual solubilities of the two components start to decrease. According to Goldberg that the term `solid solution' should only be applied when the mutual solubility of the two components exceeds 5%. (Goldberg H et al., 1965)
According to the way in which the solvate molecules are distributed in the solvendum the two type of solid solution are-----
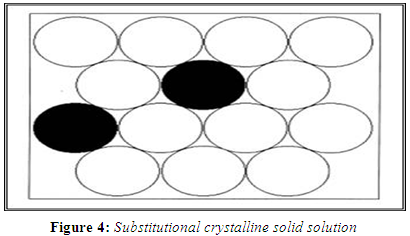
SUBSTITUTIONAL CRYSTALLINE SOLUTIONS
A substitutional crystalline solid dispersion is a type of solid solutions which have a crystalline structure, in which the solute molecules substitute for solvent molecules in the crystal lattice. Substitution is only possible when the size of the solute molecules differs by less than 15% or so from that of the solvent molecules. (Hume-Rotherly W et al., 1954)
INTERSTITIAL CRYSTALLINE SOLID SOLUTIONS
In interstitial solid solutions, the dissolved molecules occupy the interstitial spaces between the solvent molecules in the crystal lattice. As in the case of substitutional crystalline solid solutions, the relative molecular size is a crucial criterion for classifying the solid solution type. In the case of interstitial crystalline solid solutions, the solute molecules should have a molecular diameter that is no greater than 0.59 of the solvent molecule's molecular diameter. Furthermore, the volume of the solute molecules should be less than 20% of the solvent. (Reed-Hill R E, 1964)
AMORPHOUS SOLID SOLUTIONS
In an amorphous solid solution, the solute molecules are dispersed molecularly but irregularly within the amorphous solvent. Using griseofulvin in citric acid, Chiou and Riegelman were the first to report the formation of an amorphous solid solution to improve a drug's dissolution properties. Other carriers urea and sugars such as sucrose, dextrose and galactose, organic polymers such as polyvinylpyrrolidone (PVP), polyethylene glycol and various cellulose derivatives have been utilized for this purpose.
GLASS SOLUTIONS AND GLASS SUSPENSIONS
Chiou and Riegelman first introduced the concept of formation of a glass solution as another potential modification of dosage forms in increasing drug dissolution and absorption. A glass solution is a homogenous, glassy system in which a solute dissolves in a glassy solvent. The term glass can be used to describe either a pure chemical or a mixture of chemicals in a glassy or vitreous state. The glassy or vitreous state is usually obtained by an abrupt quenching of the melt. It is characterized by transparency & brittleness below the glass transition temperature Tg. (Reed-Hill RE,1964)
Table 1: Types of Solid Dispersion
|
Solid dispersion type |
Matrix* |
Drug** |
Remarks No.
|
phases |
Reference |
|
|
1 |
Eutectics |
C |
C |
The first type of solid dispersion prepared |
2 |
(Chiou and Riegelman, 1971) |
|
2 |
Amorphous precipitations in crystalline matrix |
C |
A |
Rarely encountered |
2 |
(Breitenbach AH, 2002); (Mullins and Macek, 1960) |
|
3 |
Solid solutions |
|
|
|
|
|
|
A |
Continuous solid solutions |
C |
M |
Miscible at all composition, never prepared |
1 |
(Goldberg et al., 1965] |
|
B |
Discontinuous solid solutions |
C |
M |
Partially miscible, 2 phases even though drug is molecularly dispersed. |
2 |
Sekiguchi K and Obi N (1961)
|
|
C |
Substitutional solid solutions
|
C |
M |
Molecular diameter of drug (solute) differs less than 5% from the matrix (solvent) diameter. In that case the drug and matrix are substitutional. Can be continuous or discontinuous. When discontinuous: 2 phases even though drug is molecularly dispersed. |
1 or 2 |
(Rastogi and Verma,1956); (Wilcox et al., 1964) |
|
d |
Interstitial solid solutions |
C |
M |
Drug (solute) molecular diameter less than 59% of matrix (solvent) diameter. Usually limited miscibility, discontinuous. |
2 |
(Chiou and Riegelman, 1971); (Chiou and Riegelman, 1969) |
|
4 |
Glass suspension |
A |
C |
Particle size of dispersed phase dependent on cooling/evaporation rate. Obtained after crystallization of drug in amorphous matrix |
2 |
(Chiou and Riegelman, 1971); (Sarkari M et al., 2002) |
|
5 |
Glass suspension |
A |
A |
Particle size of dispersed phase dependent on cooling/evaporation rate many solid dispersions are of this type
|
2 |
(Chiou and Riegelman, 1971); (Sarkari M et al., 2002) |
|
6 |
Glass solution |
A |
M |
Requires miscibility OR solid solubility, complex formation or upon fast cooling OR evaporation during preparation, many (recent) examples especially with PVP |
1 |
Simonelli AP et al., 1969 |
*A: matrix in the amorphous state, C: matrix in the crystalline state
**: A: drug dispersed as amorphous clusters in the matrix, C: drug dispersed as crystalline particles in the matrix, M: drug molecularly dispersed throughout the matrix
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
METHODS OF PREPRATION
MELTING METHOD (FUSION METHOD)
The melting or fusion method, first proposed by Sekiguchi and Obi involves the preparation of physical mixture of a drug and a water-soluble carrier and heating it directly until it melted. The melted mixture is then solidified rapidly in an ice-bath under vigorous stirring. The final solid mass is crushed, pulverized and sieved. However many substances, either drugs or carriers, may decompose or evaporates during the fusion process which employs high temperature. Some of the means to overcome these problems could be heating the physical mixture in a sealed container or melting it under vacuum or in presence of inert gas like nitrogen to prevent oxidative degradation of drug or carrier. (Goldberg A et al. 1966)
MELT EXTRUSION METHOD
Melt extrusion method is same as the melt method except that intense mixing of drug/carrier mix is typically processed with a twin-screw extruder. The drug/carrier mix is simultaneously melted, homogenized and then extruded and shaped as tablets, granules, pellets, sheets, sticks or powder. The intermediates can then be further processed into conventional tablets. An important advantage of the hot melt extrusion method is that the drug/carrier mix is only subjected to an elevated temperature for about 1 min, which enables drugs that are somewhat thermo labile to be processed. (Narang A.et al., 2002; Breitenbach J., 2002)
SOLVENT EVAPORATION METHOD
In this method, the first step is formation of solution containing physical mixture of the drug and carrier dissolved in a common solvent and second step involve the removal of solvent resulting the formation of solid dispersion. First to dissolve both the drug and the carrier in a common solvent and then evaporate the solvent under vacuum to produce a solid solution. This enabled them to produce a solid solution of the highly lipophilic drug in the highly water soluble carrier polyvinylpyrrolidone. An important prerequisite for the manufacture of a solid dispersion using the solvent method is that both the drug and the carrier are sufficiently soluble in the solvent. The solvent can be removed by various methods like by spray-drying or by freeze-drying. Temperatures used for solvent evaporation generally lie in the range 23-65 °C. (Serajuddin A, 1999)
MELTING SOLVENT METHOD (MELT EVAPORATION)
It involves preparation of solid dispersions by dissolving the drug in a suitable liquid solvent and then incorporating the solution directly into the melt of polyethylene glycol, which is then evaporated until a clear, solvent free film is left. The film is further dried to constant weight. The 5 –10% (w/w) of liquid compounds can be incorporated into polymer without significant loss of its solid property. It is possible that the selected solvent or dissolved drug may not be miscible with the melt of the polymer. Also the liquid solvent used may affect the polymorphic form of the drug, which precipitates as the solid dispersion. This technique possesses unique advantages of both the fusion and solvent evaporation methods. From a practical standpoint, it is only limited to drugs with a low therapeutic dose e.g. below 50 mg. (Ghaste Rahul et al., 2009; Goldberg A et al. 1966)
PHYSICAL MIXTURE METHOD
The physical mixtures were prepared by weighing the calculated amount of drug and carriers and then mixing them in a glass mortar by triturating. The resultant physical mixtures were passed through 44-mesh sieve and stored in desiccators until used for further studies. (Jafar Mohammed et al.2010)
CO- GRINDING METHOD
The calculated amounts of drug and carriers where weighed and mixed together with one ml of water. The damp mass obtained was passed through a 44-mesh sieve; the resultant granules were dispersed in Petri dishes and dried at 60°C under vacuum, until a constant weight was obtained. The granules obtained were stored in desiccators until used for further studies.
Table 2: List of Carriers Used In Solid Dispersion (Galia E et al., 1996)
|
S.No. |
NATURE |
CARRIER |
|
1 |
Acids |
Citric acid, tartaric acid, succinic acid, phosphoric acid |
|
2 |
Sugars
|
Dextrose, Mannitol, Sorbitol, Sucrose, Maltose, Galactose, Xylitol , Lactose, Soluble starch, D- glucose (Chitosan), Galactose, Xylitol, Galactomannan, British gum, Amylodextrin |
|
3 |
Polymeric Materials
|
Polyvinylpyrrolidone, PEG-4000, PEG-6000,PVP, CMC, Hydroxypropyl cellulose, Guar gum, Xanthan gum, Sodium alginate, Methyl cellulose, HPMC, Dextrin, ß- CD, HPß-CD, Eudragit® L100 sodium salts |
|
4 |
Surfactants
|
Polyoxyethylene stearate, Poloxamer, Deoxycholic acid, Tweens and Spans, Docusate sodium, Myrj-52, Pluronic-F68,SLS, Gelucire 44/14, Vitamine E TPGS NF |
|
5 |
Hydrotropes |
Sodium acetate, Sodium- o- hydroxy benzoate, Sodium- p- hydroxy benzoate, Sodium citrate,Resorcinol,Ascarbic acid |
|
6 |
Dendrimers |
polyamidoamine (PAMAM), Starburst |
|
7 |
Others |
Pentaerythritol,Urea, Urethane, Hydroxyalkyl xanthenes, Microcrystalline cellulose, Dicalcium phosphate, Silica gel, Sodium chloride, Skimmed milk |
Table 3: List of Solvents Used In Solid Dispersion
|
S.No. |
SOLVENT |
MELTING POINT (°C) |
BOILING POINT (°C) |
|
1 |
Water |
0 |
100 |
|
2 |
Methanol |
-93.9 |
65 |
|
3 |
Ethanol |
-117 |
78.5 |
|
4 |
Acetic acid |
17 |
118 |
|
5 |
1-propanol |
-85 |
97.4 |
|
6 |
2-propanol |
-127 |
82.4 |
|
7 |
Chloroform |
-63 |
62 |
|
8 |
DMSO |
19 |
189 |
Table 4: List of Poorly Soluble Drugs with Carriers
|
S. No. |
DRUG |
CARRIER |
|
1 |
Griseofulvin |
Polyethylene glycol (PEG) |
|
2 |
Acyclovir |
PEG, Urea, Mannitol, PVPK-30 |
|
3 |
Flufenamic acid |
PVP |
|
4 |
Aceclofenac |
PEG, Urea, Mannitol, Lactose |
|
5 |
Diazepam |
Sodium salicylates |
|
6 |
Glipizide |
Urea, Polaxamer-188, PVP |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
METHODS FOR THE CHARACTERIZATION OF SOLID DISPERSIONS
PARTICLE SIZE
Scanning electron microscopy (SEM) polarization microscopy method is used to study the microscopic surface morphology of drug and carriers and sometimes the polymorphism of drug. The fine dispersion of drug particles in the carrier matrix may be visualized.
DISSOLUTION TESTING
Drugs having intrinsic dissolution rate < 0.1 mg/cm2//min usually exhibit dissolution rate limited absorption. Comparison of dissolution profile of drug, physical mixtures of drug and carrier and solid dispersion may help to indicate the mechanism of improved release of drug in the formulation (solubilization / wetting / particle size reduction).
INFRARED SPECTROSCOPY
Infrared spectroscopy (IR) helpful in determining the solid state of the drug (molecular dispersion, amorphous, crystalline or a combination) in the carrier regardless of the state of the carrier. Crystallinities of under 5-10% cannot generally be detected. It also used to study the interaction occur between drug and polymer by matching the peaks of spectra. The absence of any significant change in the IR spectral pattern of drug & polymer physical mixture indicated the absence of any interaction between the drug and the polymer.
DIFFERENTIAL SCANNING CALORIMETRY
A frequently used technique to detect the amount of crystalline material is Differential Scanning Calorimetry (DSC) (Kerc and Srcic, 1995). It help to study the changes in the physical state of solid dispersion may occur during heating, and the presence of polymer may influence the melting behavior of drug (e.g. melting point depression). Results need to be confirmed by another technique. Crystallinities under 2% cannot generally be detected.
X-Ray DIFFRACTION
Powder X-ray diffraction can be used to qualitatively detect material with long range order. Sharper diffraction peaks indicate more crystalline material. Recently developed X-ray equipment is semiquantitative.
ADVANTAGES OF SOLID DISPRESION
Generally, solid dispersion is mainly used
o To reduced particle size
o To improve weetability
o To improve porosity of drug
o To decrease the crystalline structure of drug in to amorphous form
o To improve dissolvability in water of a poorly water-soluble drug in a pharmaceutical
o To mask the taste of the drug substance
o To prepare rapid disintegration oral tablets.
REDUCED PARTICLE SIZE
Solid dispersions represent the last state on particle size reduction, and after carrier dissolution the drug is molecularly dispersed in the dissolution medium. Solid dispersions apply this principle to drug release by creating a mixture of a poorly water soluble drug and highly soluble carriers (Leuner and Dressman, 2000). A high surface area is formed, resulting in an increased dissolution rate and, consequently, improved bioavailability (Leuner and Dressman, 2000 and Kang et al. 2004).
IMPROVED WETTABILITY
The enhancement of drug solubility is related to the drug wettability improvement verified in solid dispersions (Karavas et al., 2006). It was observed that even carriers without any surface activity, such as urea (Sekiguchi and Obi, 1964) improved drug wettability. Carriers with surface activity, such as cholic acid and bile salts. When used, can significantly increase the wettability property of drug. Moreover, carriers can influence the drug dissolution profile by direct dissolution or co-solvent effects (Pouton, 2006 and Kang et al., 2004).
INCREASE POROSITY
Particles in solid dispersions have been found to have a higher degree of porosity (Vasconcelos and Costa, 2007). The increase in porosity also depends on the carrier properties, for example, solid dispersions containing linear polymers produce larger and more porous particles than those containing reticular polymers and, therefore, result in a higher dissolution rate (Ghaderi et al., 1999). The increased porosity of solid dispersion particles also hastens the drug release profile.
DRUGS IN AMORPHOUS STATE
Poorly water soluble crystalline drugs, when in the amorphous state tend to have higher solubility (Pokharkar et al., 2006). The enhancement of drug release can usually be achieved using the drug in its amorphous state, because no energy is required to break up the crystal lattice during the dissolution process (Taylor and Zografi, 1997). In solid dispersions, drugs are presented as supersaturated solutions after system dissolution, if drugs precipitate it is as a metastable polymorphic form with higher solubility than the most stable crystal form (Leuner and Dressman, 2000, Karavas et al., 2006). For drugs with low crystal energy (low melting temperature or heat of fusion), the amorphous composition is primarily dictated by the difference in melting temperature between drug and carrier. For drugs with high crystal energy, higher amorphous compositions can be obtained by choosing carriers, which exhibit specific interactions with them (Vippagunta et al., 2002).
ALTERNATIVE STRATEGIES
LYOPHILISATION TECHNIQUE
Freeze-drying involves transfer of heat and mass to and from the product under preparation. This technique was proposed as an alternative technique to solvent evaporation. Lyophilisation has been thought of a molecular mixing technique where the drug and carrier are co-dissolved in a common solvent, frozen and sublimed to obtain a lyophilized molecular dispersion. (Perissutti B et al., 2002)
SPRAYING ON SUGAR BEADS USING FLUIDIZED BED COATING
The approach involves fluidized bed coating system, where-in a drug-carrier solution is sprayed onto the granular surface of excipients or sugar spheres to produce either granules ready for tableting or drug-coated pellets for encapsulation in one step. This method has been applied for both controlled-and immediate-release solid dispersions (Beten et al., 1995; Ho Ho et al., 1996)
For e. g., Itraconazole coated on sugar sphere, is made by layering onto sugar beads a solution of drug and hydroxypropylmethylcellulose (HPMC) in an organic solvent of dichloromethane and ethanol.
DIRECT CAPSULE FILLING
The filling of semi solid materials into hard gelatin capsules as melts, which solidify at room temperature, was first done in1978. Direct filling of hard gelatin capsules with the liquid melt of solid dispersions avoids grinding-induced changes in the crystallinity of the drug. A surfactant must be mixed with the carrier to avoid formation of a drug-rich surface layer (e.g., poly-sorbate80 with PEG, phosphatidyl choline with PEG). The temperature of the molten solution should not exceed ~70-C because it might compromise the hard-gelatin capsule shell.
THE USE OF SURFACTANT
The utility of the surfactant systems in solubilization is well known. Adsorption of surfactant on solid surface can modify their hydrophobisity, surface charge, and other key properties that govern interfacial processes such as flocculation/dispersion, floatation, wetting, solubilization, detergency, enhanced oil recovery and corrosion inhibition. Surfactants have also been reported tocause solvation /plasticization, manifesting in reduction of melting the active pharmaceutical ingredients, glass transition temperature and the combined glass transition temperature of solid dispersions. Because of these unique properties, surfactants have attracted the attention of investigators for preparation of solid dispersions. Two of the important surface-active carriers used are Gelucire 44/14and Vitamin ER-alpha- tocopherylpolyethyleneglycol 1000 succinate (TPGS). In which Gelucire44/14 has commonly been used in solid dispersion for the bioavailability enhancement of drugs. A commonly used surfactant, Polysorbate 80, when mixed with solid PEG, has also been reported to be an alternative surface-active carrier.( Ghebremeskel A N et al.,2007; Zhang R et al.,2006)
ELECTROSTATIC SPINNING METHOD
This technology used in the polymer industry combines solid solution/dispersion technology with nanotechnology (Reneker, 1993; Reneker and Chun, 1996).This technology is now applied in the pharmaceutical field (Ignatious and Baldoni, 2001). Electrospinning is a process in which solid fibers are produced from a polymeric fluid stream solution or melt delivered through a millimeter-scale nozzle. In this process, a liquid stream of a drug/polymer solution is subjected to a potential between 5 and 30 kV. When electrical forces overcome the surface tension of the drug/polymer solution at the air interface, fibers of submicron diameters are formed. As the solvent evaporates, the formed fibers can be collected on a screen to give a nonwoven fabric, or they can be collected on a spinning mandril. The fiber diameters depend on surface tension, dielectric constant, feeding rate, and electric field strength (Deitzel et al., 2001). Water-soluble polymers would be useful in the formulation of immediate release dosage forms, and water-insoluble (both biodegradable and nonbiodegradable) polymers are useful in controllable dissolution properties. Fabrics generated by water-soluble carriers could be used in oral dosage formulations by direct incorporation of the materials into a capsule. Itraconazole/HPMC nanofibers have been prepared using this technique (Verreck et al., 2003).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
SUPER CRITICAL FLUID (SCF) TECHNOLOGY
This technology has been introduced in the late 1980s and early 1990s, From the very beginning of supercritical fluid particle generation research, the formation of biocompatible polymer and drug-loaded biopolymer micro-particles for pharmaceutical applications has been studied intensively by a number of researcher groups CFs either as solvent: rapid expansion from supercritical solution (RESS) or antisolvent: gas antisolvent (GAS), supercritical antisolvent (SAS), solution enhanced dispersion by supercritical fluids (SEDS) and/or dispersing fluid: GAS, SEDS, particles from gas-saturated solution (PGSS). Conventional methods, i.e. Spray drying, solvent evaporation and hot melt method often result in low yield, high residual solvent content or thermal degradation of the active substance. In the supercritical fluid carbon dioxide is used used as either a solvent for drug and matrix or as an anti-solvent (Kompella and Koushik, 2001; Palakodaty and York, 1999). When supercritical CO2 is used as solvent, matrix and drug are dissolved and sprayed through a nozzle, into an expansion vessel with lower pressure and particles are immediately formed. The adiabatic expansion of the mixture results in rapid cooling. This technique does not require the use of organic solvents and since CO2 is considered environmentally friendly, this technique is referred to as ‘solvent free’. The technique is known as Rapid Expansion of Supercritical Solution (RESS). However, the application of this technique is very limited, because the solubility in CO2 of most pharmaceutical compounds is very low (<0.01wt-%) (Subramaniam et al., 1997) and decreases with increasing polarity. Different acronyms were used by various authors to denote micronization processes: aerosol solvent extraction system (ASES), precipitation with a compressed fluid antisolvent (PCA), gas anti-solvent (GAS), solution enhanced dispersion by supercritical fluids (SEDS) and supercritical anti-solvent (SAS). The SAS process involves the spraying of the solution composed of the solute and of the organic solvent into a continuous supercritical phase flowing concurrently use of supercritical carbon dioxide is advantageous as it is much easier to remove from the polymeric materials when the process is complete, even though a small amount of carbon dioxide remains trapped inside the polymer; it poses no danger to the patient. In addition the ability of carbon dioxide to plasticize and swell polymers can also be exploited and the process can be carried out near room temperature Moreover, supercritical fluids are used to lower the temperature of melt dispersion process by reducing the melting temperature of dispersed active agent. The reason for this depression is the solubility of the lighter component (dense gas) in the forming phase (heavier component).
CONCLUSION
The solubility of the drug is the factor that controls the formulation of the drug as well as therapeutic efficacy of the drug, hence the most critical factor in the formulation development. There are various available techniques, alone or in combination can be used to enhance the solubility of the drug. Although in all techniques mentioned solid dispersion systems have been realized as extremely useful tool in improving the dissolution properties of poorly water-soluble drugs. In recent years, a great deal of knowledge has been accumulated about solid dispersion technology, but their commercial application is limited. Various methods have been tried recently to overcome the limitation and make the preparation practically feasible. The problems involved in incorporating into formulation of dosage forms have been gradually resolved with the advent of alternative strategies. These include methods like spraying on sugar beads and direct capsule filling. Although there are some hurdles like scale up and manufacturing cost to overcome, there lies a great promise that solid dispersion technology will hasten the drug release profile of poorly water soluble drugs.
REFERENCES
1. J Anil Shinde. Solubilization of Poorly Soluble Drugs: A Review. pharmainfo.net Vol. 5, Issue 6, 2007.
2. Ghaste Rahul, C D Dhanyakumar, R Rohit Shah, S. Dhananja Ghodke. Solid Dispersions: An Overview. Latest review.Pharmainfo.net 2009; vol. 7 Issue 5.
3. Sekiguchi K, Obi N, “Studies on absorption of eutectic mixtures. I. A comparison of the behavior of eutectic mixtures of sulphathiazole and that of ordinary sulphathiazole in man”, Chem. Pharm. Bull., 1961; 9; 866-872.
4. Sekiguchi K, Obi N, “Studies on absorption of eutectic mixtures. I. A comparison of the behavior of eutectic mixtures of sulphathiazole and that of ordinary sulphathiazole in man”, Chem. Pharm. Bull., 1961; 9; 866-872.
5. Sekiguchi K and Obi N (1964). Studies on Absorption of Eutectic Mixture II. Absorption of Fused Conglomerates of Chloramphenicol and Urea in Rabbits. Chem. Pharm. Bull. (Tokyo), 12: 134-144.
6. Goldberg AH, Gibaldi M, Kanig JL. “Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures. II. Experimental evaluation of eutectic mixture: urea-acetaminophen system”. J Pharm Sci. 1966; 55:482-487.
7. Goldberg AH, Gibaldi M, Kanig JL. “Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures. III. Experimental evaluation of griseofulvin-succinic acid solution”. J Pharm Sci. 1966; 55:487-492.
8. Goldberg H, Gibaldi M, Kanig L, “Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures-I- theoretical considerations and discussion of the literature”, J. Pharm. Sci.,1965; 54;1145-1148.
9. Hume-Rotherly W, Raynor G V, “The Structure of Metals and Alloys”, Institute of Metals, London , 1954.
10. Reed-Hill R E, “Physical Metallurgy Principles”, Van-Nostrand, Princetown, NJ , 1964.
11. Chiou W L, Riegelman S, “Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin”, J. Pharm. Sci.,1969; 1505-1510.
12. Breitenbach J. “Melt extrusion: from process to drug delivery technology.”, Eur J Pharm Biopharm,. 2002;54;107-117.
13. Narang A., Shrivastava A. ”Melt extrusion solid dispersion technique”, Drug Dev. Ind. Pharm., 2002;26(8);111-115
14. Serajuddin A., “solid dispersion technique”, J.Pharm.Sci. 1999; 88(10); 891-900.
15. Jafar Mohammed, Mhg Dehghan, Shareef Adi. Enhancement of dissolution and anti-inflammatory effect of meloxicam. International Journal of Applied Pharmaceutics, Vol. 2 Issue 1, 2010.
16. Jafar Mohammed, Mhg Dehghan, Shareef Adi. Improving Dissolution of Meloxicam Using Solid Dispersions. Iranian Journal of Pharmaceutical Research 2006; 4: 231-238.
17. Galia E, Nicolaides E, Hoerter D, Loebenberg R, Reppas C and Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res 1998; 15: 698-705.
18. Wadke DA, Serajuddin A, Jacobson H,“Preformulation testing”. In: Lieberman HA, Lachman L, Schwartz JB, eds. Pharmaceutical Dosage Forms: Tablets. New York , NY : Marcel Dekker; 1989:1-73.
19. Kerc J and Srcic S (1995). Thermal analysis of glassy pharmaceuticals. Thermochim. Acta., 248: 81-95.
20. Leuner C and Dressman J (2000). Improving drug solubility for oral delivery using solid dispersions. Eur.J. Pharm. Biopharm. 50: 47-60.
21. Karavas E, Ktistis G, Xenakis A and Georgarakis E (2006). Effect of hydrogen bonding interactions on the release mechanism of felodipine from nanodispersions with polyvinylpyrrolidone. Eur. J. Pharm. Biopharm., 63: 103-114.
22. Pouton CW. “Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems”. Eur. J. Pharm. Sci. 2000; 11 Suppl. 2; 93-98.
23. Pouton CW (2006). Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci., 29: 278-287.
24. B.AppaRao., R.M. Shivalingam, V.Y. Kishore Reddy, Rao Somesekhara, K.Rajesh. Formulation and Evaluation of Aceclofenac Solid Dispersions for Dissolution Rate Enhancement. International Journal of Pharmaceutical Sciences and Drug Research 2010; 2(2): 146-150
25. Vasconcelos T and Costa P (2007). Development of a rapid dissolving ibuprofen solid dispersion. In: PSWC – Pharmaceutical Sciences World Conference, DD-W- 103.
26. Ghaderi R, Artursson P and Carifors J (1999). Preparation of biodegradable microparticles using solution enhanced dispersion by supercritical fluids (SEDS).Pharm. Res., 16: 676-681.
27. Pokharkar VB, Leenata P Mandpe, Mahesh N Padamwar, Anshuman A. Ambike, Kakasaheb R. Mahadik and Anant Paradkar (2006). evelopment, characterization and stabilization of amorphous form of a low Tg drug. Powder Technol., 167: 20-25.
28. Taylor LS and Zografi G (1997). Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res., 14: 1691-1698.
29. Perissutti B, Newton JM, Podezeck F, Rubessa F. “Preparation of extruded Carbamazepine and PEG 4000 as a potential rapid release dosage form”, Eur J Pharm Biopharm., 2002;53; 125-132.
30. Beten DB, Amighi K and Moes AJ (1995). Preparation of controlled- elease coevaporates of dipyridamole by loading neutral pellets in a fluidized-bed coating system. Pharm Res., 12: 1269-1272.
31. Ghebremeskel A N, Vemavarapu C and Lodaya M., “Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer surfactant combinations using solubility parameters and testing the processability”, Int. J. Pharm., 2007; 308; 119-129.
32. Reneker DH and Chun I (1996). Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology, 7: 216-223.
33. Chauhan B, Shimpi S, Paradkar A. Preparation and Characterization of Etoricoxib Solid Dispersions Using Lipid Carriers by Spray Drying Technique. AAPS Pharm. Sci. Tech. 2005; 6(3): E405-E412.
34. Ignatious F, Baldoni JM and inventors (2001). Smithkline Beecham Corp. Electrospun pharmaceutical compositions. World patent 0 154 667. August 2.
35. Verreck G, Chun I, Peeters J, Rosenblatt J and Brewster ME (2003). Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res., 20: 810-817.
36. Kompella UB and Koushik K (2001). Preparation of drug delivery systems using supercritical fluid technology. Crit. Rev. Ther. Drug Carrier Syst., 18(2): 173-199.
37. Subramaniam B, Rajewski RA and Snavely K (1997).Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci., 86(8): 885-890.
38. Choudhary D, Kumar S, Gupta GD. Enhancement of solubility and dissolution of glipizide by solid dispersion technique. Asian journal of pharmaceutics, Vol. 3 Issue 3, 2009; 245-251.
39. Deitzel JM, Kleinmeyer J, Harris D and Beck Tan NC (2001). The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polym., 42: 261-272.
40. David J. H, “Lipid-Based Systems for Oral Drug Delivery. Enhancing the Bioavailability of Poorly Water Soluble Drugs” APR, 2002; 5; 88-93.
41. Geneidi A. S., ElShamy A.H., Awad G. A. S., Hassan A. O. K. and A. O. K, Demerdash E. EL. Solid dispersion of Famotidine: factorially designed capsule formulation and in vivo evaluation of antiulcer activity. Saudi Pharmaceutical Journal, Vol. 12, No. 4, 2004.
42. Gupta Sachin, Srivastav Shruti, Vajpai Meenakshi. Comparative study of solubility enhancement of poorly soluble drug by solid Dispersion and Inclusion complex. Journal of Pharmacy Research 2010; 3(4): 692-696.
43. Hasan S., Ali J.,Baboota S., Ali M. Comparative analysis of Acyclovir-PEG solid dispersion for bioavailability enhancement. AAPSJ.org/abstracts, 2007;
44. Ho HO, Shu HL, Tsai T and Sheu MT (1996). The preparation and characterization of solid dispersions on pellets using a fluidized bed system. Int. J. Pharm., 139: 223-229.
45. Inamdar Nazma, Bhise Kiran, Memon Shakeel. Solubility enhancement and development of dispersible tablet of meloxicam. Asian journal of pharmaceutics vol.2, issue 2, 2008; 128-132.
46. Kang BK JS Lee, SK Chon, SY Jeong, SH Yuk, G (2004). Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J.Pharm., 274: 65-73.
47. Lakshmi Narasaiah.V, Kalyan Reddy. B, Kishore. K, Raj Kumar.M, Srinivasa Rao.P, Venkateswara Reddy.B. Enhanced Dissolution Rate of Atorvastatin Calcium using Solid Dispersion with PEG 6000 by Dropping Method. J. Pharm. Sci. & Res. Vol.2 (8), 2010; 484-491.
48. Lee Sibeum, Kyungwan Nam, Min Soo Kim, Seoung Wook Jun, Jeong-Sook Park, Jong Soo Woo and Sung-Joo Hwang. Preparation and Characterization of Solid Dispersions of Itraconazole by using Aerosol Solvent Extraction System for Improvement in Drug Solubility and Bioavailability Arch Pharm Res, Vol 28, No 7, 2005; 866-874.
49. Leonardi D, Barrera MG, Lamas MC, Salomón CJ. Development of Prednisone: Polyethylene Glycol 6000 Fast-Release Tablets From Solid Dispersions: Solid-State Characterization, Dissolution Behavior, and Formulation Parameters. AAPS Pharm. Sci. Tech. 2007; 8(4): Article 108.
50. Modi A, Tayade P. Enhancement of Dissolution Profile by Solid Dispersion Technique. AAPS Pharm. Sci. Tech. 2006; 7(3): Article 68.
51. Narasaiah Lakshmi V, Reddy. B, K.Kishore, Kumar Raj.M1, Rao. Srinivasa P, Reddy. Venkateswara B Enhanced Dissolution Rate of Atorvastatin Calcium using Solid Dispersion with PEG 6000 by Dropping Method. J. Pharm. Sci. & Res. Vol.2 (8), 2010; 484-491.
52. Patel R.P., Patel M.M. Physicochemical Characterization and Dissolution Study of Solid Dispersions of Lovastatin with Polyethylene Glycol 4000 and Polyvinylpyrrolidone K30. Journal of Informa health care, Volume 12, Issue 1 , 2007; 21 – 33.
53. Pathak deepa,Dahiya Sunita, Pathak Kamla. Solid dispersion of meloxicam: Factorially designed dosage form for geriatric population. Acta Pharm. 58 (2008); 99–110.
54. S.Amol Malpani. Promising Novel Approaches for Oral Delivery of Poorly Soluble Drugs. Pharmainfo.net, Vol. 7 Issue 4 2009.
55. Shah Jigar, Vasanti S., Anroop B. Hiral Vyas. Enhancement of dissolution rate of valdecoxib by solid dispersions technique with PVP K30 & PEG 4000: preparation and in vitro evaluation. J Incl Phenom Macrocycl Chem 2009; 63:69–75.
56. Sharma A.,Jain C.P. Techniques to enhance solubility of poorly soluble drugs: a review. Journal of Global Pharma Technology 2010; 2 (2): 18-28.
57. Zhang R, Somasundaran P, “Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Advances in colloid and interface science”, Int. J. Pharm, 2006;123; 213-229.
58. Chaudhari. P.D. Current trends in solid dispersions techniques Latest Reviews. , 2006; Vol. 4, Issue 3
59. K. ArunPrasad1, N. Narayanan, G. Rajalakshmi. Preparation and evaluation of solid dispersion of Terbinafine hydrochloride. International Journal of Pharmaceutical Sciences Review and Research 2010; Volume 3,130-134
60. Ganesh Chaulang, Piyush Patel, Sharwaree Hardikar, Mukul Kelkar, Ashok Bhosale, Sagar Bhise. Formulation and Evaluation of Solid Dispersions of Furosemide in Sodium Starch Glycolate. Tropical Journal of Pharmaceutical Research, 2009; 8 (1): 43-51
61.Dehghan M H G. Comparative Dissolution Study of Glipizide by Solid Dispersion Technique. Journal of Pharmaceutical Science and Technology. 2010, Vol. 2 (9), 293-297
62. Saritha Alladi, Shastr Nalini. Preparation, Physico Chemical Characterization of Solid Dispersions of Tenoxicam with Poloxamer. Journal of Pharmaceutical Science and Technology. 2010,Vol. 2 (9), 308-311
63. Kumar Narendra, Jain AK, Akhilesh, Singh Chhater, Agarwal Kshitij, RK Nema. Development, characterization and solubility study of solid dispersion of terbinafine hydrochloride. International Journal of Pharmaceutical Sciences and Nanotechnology. 2008, Vol. 1, 170-176
64. drugbank.com
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org









