{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Kh. Hussan Reza 1*, Pranabesh Chakraborty2
1 Department of Pharmacy, Bengal School of Technology, Sugandha, Hooghly, West Bengal
2 Director, Department of Pharmacy, Bengal School of Technology, Sugandha, Hooghly, West Bengal
*hassan23pharma@gmail.com
ABSTRACT
Oral route of administration is most convenient route but often is disadvantageous for administering to geriatric and paediatric patients. Orally disintegrating film is found to be effective in delivering rapid drug action by dissolving the drug in buccal cavity. Since a decade oral thin films has got commercial importance in generic and non generic market projecting it as a multinational business asset. Current research in this field is found to be associated with industrialization and commercialization. Current article make an overview of not only technological changes in research and manufacturing but also gives an insight about growing market worldwide.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2428
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 8 Received On: 10/03/2016; Accepted On: 30/03/2016; Published On: 01/08/2016 How to cite this article: Reza KH, Chakraborty P; Recent industrial development in Oral Thin Film Technology: An Overview; PharmaTutor; 2016; 4(8); 17-22 |
INTRODUCTION
The oral route is most popular route for the administration of therapeutic agents because of the low cost of therapy and ease of administration lead to high levels of patient compliance. The most popular oral solid dosage forms are tablets and capsule[1]. Generally geriatric, paediatric and bedridden patient experience difficulties in swallowing the conventional oral dosage form. To overcome this problem a novel formulation was developed i.e. oral fast dissolving films or oral thin films[2]. Oral thin film was initially first developed in 1970 as a novel initiative of current dosage form[4]. Oral thin films were first launched in 2004 for systemic drug delivery and are now widely accepted[3].
Drug delivery via the oral mucosa is a promising route, when one wishes to achieve a rapid onset of action or improved bioavailability for drugs with high first pass metabolism. Thus, there is a growing interest in developing alternative dosage forms, i.e. orally fast disintegrating strip, which allow a rapidly dissolving drug to absorb directly into the systemic circulation through the oral mucosa. These kinds of dosage forms are also convenient for children, elderly patients with swallowing difficulties, and in the absence of portable liquids[1]. As mentioned in a study of 1576 patients 26 % faced difficulty in swallowing due to size, surface and taste[5]. Oral thin films are made up of water a soluble polymer which upon placing on tongue instantly dissolves or disintegrate the medication without the need of water. Thus suitable for patients: paediatrics and geriatrics, avoiding-nausea, vomiting and dysphasia[6].This system is found to be equally viable for unconscious or dementia patients as this system is capable to secrete saliva and get dissolved.
The concept of the oral dissolving film [6]
1. This delivery system consists of a thin film.
2. After placing it on the top of the tongue, the film dissolves within seconds, promoting first pass metabolism as compared to tablet and other immediate release oral solid dosage forms, and may increase the bioavailability of drug.
Special Features [2, 7]
1. Thin elegant film.
2. Available in various size and shapes.
3. Unobstructive.
4. Excellent mucoadhesion.
5. Fast disintegration and Rapid release.
6. They are made up of water soluble polymers that are inert to the drug substances incorporated, most commonly used polymers include- Hydroxy Propyl Methyl Celullose, Pullulan, Poly Ethylene Oxide,Poly Vinyl Alcohol, Maltodextrine, Starch, etc.
Criteria of Oral Dissolving Film [1, 7]
Fast dissolving film should
1. Have a pleasant mouth feel.
2. Not require water to swallow, but it should dissolve or disintegrate in the mouth in matter of seconds.
3. The drug should have pleasant taste.
4. Be compatible with taste masking.
5. Leave minimum or no residue in the mouth after oral administration.
6. Exhibit low sensitivity to environmental conditions such as temperature and humidity.
7. The drug to be incorporated should have low dose.
8. Have the ability to permeate oral mucosal tissue.
Advantages of Oral Dissolving Film [1, 2, 8, 9, 10]
1. Improved patient compliance by enhancing of administration to pediatric, geriatric, bedridden patients and psychiatric patients who refuse to swallow tablets.
2. No need of water to swallow or chew.
3. No risk of chocking.
4. Good mouth feel.
5. No special training is required for the administration of dosage form.
6. Bypassing the first pass effect leads to reduction in the dose which can lead to reduction in side effects associated with the molecule.
7. Precision in the administered dose.
8. Larger surface area promotes rapid disintegration and dissolution in the oral cavity.
9. Oral films are flexible and thus less fragile as compared to orally dissolving films. Hence, there is ease of transportation and during consumer handling and storage.
10. Easy transportation.
Limitataions of Oral Dissolving Film [1, 2, 9]
1. Drugs which are unstable at buccal pH cannot be administered.
2. These dosage forms are moisture sensitive.
3. They require special packaging for the products stability and safety.
4. High dose cannot be incorporated into the oral film.
[adsense:468x15:2204050025]
PHARMACEUTCIAL ASPECT
The pharmaceutical aspect generally includes the excipients used and the manufacturing process followed in the development of oral thin film, A brief general overview of the process is given below.
a. Exicipent:The excipients used are generally refered from official monographs and other used within the limits permissible in general the inactive ingredient list or IIG issued by FDA serves to us as a limit justified to be incorporated in formulations. The commonly used excipients are as follows:
i. Polymers [11,12,13]
To obtain the desired matrix of film, polymers can be used alone or in combination. In general water-soluble polymers are used as film formers as they undergo rapid disintegration, good mouth feel and mechanical properties to the films. The strength of the film depends on the type of polymer and the amount in the formulation. By increasing the molecular weight of polymer film bases, disintegration rate of the polymer decreases. Polymers frequently used as film formers are water soluble grades of cellulose ethers, polyvinyl alcohol, polysaccharides, polyvinyl pyrrolidone K-90, polyethylene glycols, pullulan, gelatin, carboxmethyl cellulose cekol 30, hydroxy propyl methyl cellulose E-3 and K-3, methyl cellulose A-3, A-6 and A-15, pectin, sodium alginate, hydroxypropylcellulose of grades E-5 ,E15 and LV grades, maltodextrins and eudragit RD10. Recent as per some patents event the use of starch as modified starch, pea starch is also reported. Polymers generally forms about 40% to 50% of the film matrix.
ii. Plasticizers [11,14,15]
Plasticizer enhances mechanical properties or durability of matrix such as tensile strength and elongation to the film by reducing the glass transition temperature of the polymer. It also reduces brittleness of the strip by fixing itself in between the polymer chains acting as a hinge to the support, as a result improves its flexibility. Choice of plasticizer depends upon type of solvent used and its compatibility with the polymer. Some of the commonly employed plasticizers are phthalate derivatives like dimethyl, diethyl and Dibutyl phthalate, glycerol mono oleate and low molecular weight polyethylene glycols, castor oil, citrate derivatives like tributyl,triethyl, acetyl citrate, triacetin and glycerol. The phthalates since it shows carcinogenegity are not used currently for formulation. Incorrect use of plasticizer may cause blooming, film cracking, splitting and peeling of the films. Plasticizers generally forms around 0-20 % of the film composition.
iii. Surfactant [12,14]
Surfactants are used multi dimentionally as solubilising agent, emulsifying agent, dispersing agent. Nonionic surfactants are in general preferred. The commonly used surfactants include Tweens, Sodium Laurly Sulphate, Cremophor, Polaxomer.
iv. Taste masking agents [16]
As most of the drugs are bitter, it’s a challenge to develop an oral thin film with a good acceptable taste. The taste masking techniques used involves complexation and use of artificial and natural sweeteners. The complexation of drug generally involves the use of pollacryline potassium, cyclodextrine, tannic acid, chitosan. Various artificial sweeteners used are aspartame, ascesulfame, xylitol, neotame on the other hand natural sweetners used are stevia, ammonium glycyrrizinate.
v.Saliva stimulating agents[17]
Saliva stimulating agents are used to increase the rate of production of saliva that would help in the faster disintegration of the rapid dissolving strip formulations. Examples of salivary stimulants are citric acid, malic acid, lactic acid, ascorbic acid and tartaric acid. Among these the most preferred one is citric acid.
vi. Flavoring agents
Various types of flavoring agent are used in the formulation of oral thin films. Flavoring agent also accelerates the taste masking effect of sweeteners. In general use flvouring agents available as oil soluble or water soluble form. The oil soluble form is preferred more. Flouring agents are used along with menthol that provides a typical cooling sensation to the flavor. Apart from regular oil soluble flavors some volatile flavors are also used as eucalyptol, eugenol, thymol. Some exotic flavors such as vanilla, chocolate, coffee are also used depending upon the targeted market.
vii. Colouring agents
Generally incorporated colouring agents are FD&C colours, natural colours, pigments such as titanium dioxide. The colour selection is generally done based on the formulation and its colour acceptable in the market by customers.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
b. Manufacturing process:
To manufacture fast dissolving oral films, following methods are generally employed:
a. Semisolid casting or solvent casting.
b. Rolling.
c. Solid dispersion extrusion.
d. Hot melt extrusion.
a. Semisolid casting or solvent casting
This is most widely used method for preparing oral thin film. In a industrial scale up this method at first a solution of water soluble film forming polymer is prepared. Then the resulting slurry drug and other substances are added. There after it is layered on a layering machine diagram of this is given in Figure 1. After layering the prepared films are obtained as mother rolls. The mother rolls are slitted to form daughter rolls which run for strip packing. A flow chart for this process is given in Figure 2. In a small scale or lab scale it can be prepared by using table top layering device as shown in Figure 3.
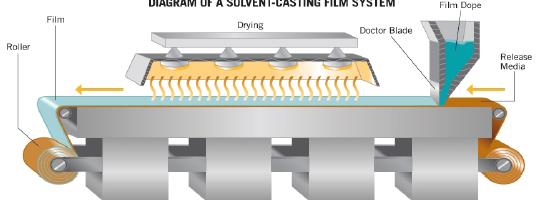
Figure 1 : Solvent casting film system/ Layering machine.[9]
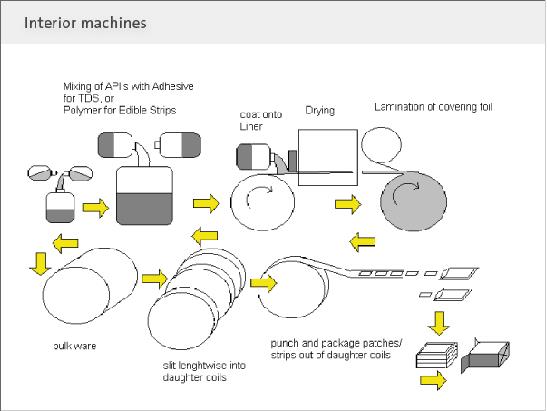
Figure 2 : The manufacturing Techniques for oral thin films.[23]

Figure 3: Table top laboratory scale layering or film forming device.[22]
b. Rolling
Solvents mainly used in this method are water and mixture of water and alcohol. By the means of high shear processor, active agent and other ingredients are dissolved in small portion of aqueous solvent. Water soluble hydrocolloids are dissolved in water to form homogenous viscous solution. Then the resultant solution or suspension containing drug is rolled on a carrier. Finally the obtained film is cut in to desired shapes and sizes as shown in figure 4 [19].
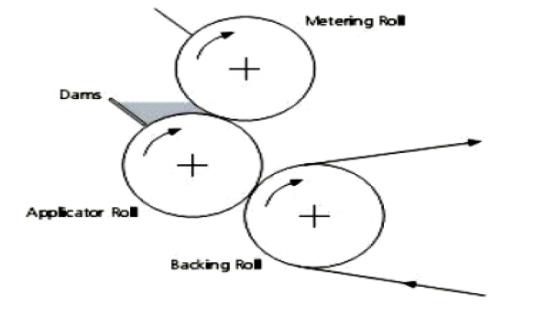
Figure 4: Rolling Method[9]
c. Solid dispersion extrusion
Firstly solid dispersion is prepared by extruding immiscible components with drug and then shaped in to films by the means of dies [19].
d.Hot melt extrusion
In hot melt extrusion method at first drug is mixed with carriers in solid form. Then the mixture is molten by the means of extruder having heaters. Lastly the melt is shaped in to films by the dies [20]. The diagram of the machine is given in Figure 5.

Figure 5: Film extrusion system [9]
MARKET FOR ORAL THIN FILMS [21]
The study of oral thin film market is essential with industrial point of view, judgment and market analysis of this formulation ensures its future growth. At present around ten prescription films are in market and twenty nine are currently in stages of various clinical and pre-clinical development of which six products to be lunched in 2016. The key players are MonoSol Rx, Labtec GmbH, Biodelivery system and Nal Pharma. 38 % of the formulation are based on Mono Sol’s PharmFilm technology or Labtec’s Rapid film technology. Some of the start up like FFT Medicals and Cynapsus Therapeutics have recently emerged fostering innovations in recent years. Out of marketed product, Suboxone (Buprenorphine and Naloxone) by MonoSol Rx is found to have a strong acceptance worldwide. This is followed by other drugs like Fentanyl formulation of MonoSol Rx (Breakyl) and Meda phrama ( Onsolis). Ondansetron formulation like Zuplenz of by MonoSol Rx is found to have a good acceptance throughout the globe. Apart from this the oral thin films have a good market of OTC and food products including, Simethicone, vitamins, Melatonine and flavored breath freshening strips. The most propular OTC includes Gas X by Novartis and Listerine breath freshener
Even Indian investors are also coming up to invest on this technology. Within a short period of time new companies have emerged that are exclusively concentrating on this technology. These includes Aavishkar Oral strips Pvt.Ltd, Hyderabad; NU Therapeutics, Hyderabad; and ZYM Laboratories, Nagpur are working on this technology. Also big pharmaceutical companies like Cipla, Mankind and Dr. Reddy’s laboratory are working on its development. Sildenafil, Tadalafil, Ondansetron are some of the major drugs running in Indian market at present.
CURRENT STATUS OF RESEARCH WORKS ON ORAL THIN FILMS
The study is based on thorough survey of patents from various patent regulatory bodies across the globe. By analyzing patents issued from USPTO (United States Patent and Trademark Office), WIPO (World Intellectual Property Rights Organization), EPO (European Patent Office) and IPO (Indian Patent Office), maximum patent filing was found to be in USPTO. Sildenafil Citrate, Dextromethorphan, Acetominophaen, Buprenorphine, Naloxone are some of the drugs which were used to file the patents. Most of the assignee of patents are generally pharmaceutical companies based across the globe, includes Johnson & Johnson, Novartis, Warner Lambert, Monosol Rx, Lts Lohmann, Colgate Palmolive. This shows the industrial demand of formulation and development for oral thin films. As the market of this technology is increasing which spontaneously increasing the patent filing by these companies. Another observation made shows that maximum application on this technology was filed within last decade of 2003 to 2015. India has proved to be a would be market of oral thin films as companies like Monosol Rx, Warner Lambert, Lts Lohmann and gone for filing patents in IPO. Thus we can conclude that there is an exponential increase in research in the field of oral thin film technology and will substantially influence the pharmaceutical market in future.
CONCLUSION
Thus we can define an oral thin film or orally dissolving film as advanced form of oral solid dosage form that improves the drug efficacy by dissolving it in the buccal cavity within defined duration of time with better patient compatibility. This novel technology is found to be booming up in International as well as Indian market as a billion dollar market with equal penetration in pharmaceutical, food and confectionary sectors. A promising drug delivery for coming decade and can prove to be a better alternative for conventional dosage forms in the near future.
REFERENCES
1. Patil S L, Mahaparale P L, Tiwari S S, Pavour K V, Sane P N; Fast Dissolving Oral Films: An Innovative Drug Delivery System; International Journal of Research and Reviews in Pharmacy and Applied Science; 2012; 2(3); 482-496.
2. Thakur N, Bansal M, Sharma N, Yadav G, Khare P; Overview A Novel Approach of Fast Dissolving Films and Their Patients; Advances in Biological Research; 2013; 7(2); 50-58.
3. Liew K.B, Tan Y.T, Pen K.K.; Characterization of orally disentrigating film containing donepozil for Alzheimer disease; AAPS Pharm Sci Tech; 2012; 13(1); 134-142.
4. Sloboda M, Barnhart S; Formulation Flexibility Broadens The Scope For Oral Thin Film Technology; Adhesives Research; 2011; 22-24.
5. Patel R, Prajapati S, Raval A; Fast dissolving films as a newer venture in fast dissolving film dosage form; Int J Drug Dev Res; 2010; 2(2); 232-236
6. Hideaki Okabe; Development of easily swallowed thin film formulation; International journal of pharmaceutics; 2008; 355(1-2); 62-66.
7. Bansal S, Bansal M, Garg G; Formulation And Evaluation of Fast Dissolving Film of an Antihypertensive Drug; International Journal of Pharmaceutical Chemical And Biological Sciences; 2013; 3(4); 1097-1108.
8. Aggarwal J, Singh G, Saini S, Rana A C; Fast Dissolving Films: A Novel Approach to Oral Drug Delivery; International Research Journal of Pharmacy; 2011; 2(12); 69-71.
9. Radhakisan U R, Chavan V, Tribhuvan N; Mouth Dissolving Film and Their Patent: An Overview; International Research Journal of Pharmacy. 2012; 3(9); 39-42.
10. Arya A, Chandra A, Sharma V, Pathak K; Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form; International journal of Chem Tech Research; 2010; 2(1); 576-583.
11. Chien. M J, Tirol. G, Chien. C and Schmitt. R.; Film forming polymers in oral films; Poster presented at the 2006 Annual Meeting and Exposition of the American Association of Pharmaceutical Scientist; Oct 29-Nov 2, AAPS. 2006, 1-5.
12. Cilurzo. F, Minghetti. P, Como. A, Montanari. L.,; Feasibility study of fastdissolving film containing Piroxicam; The AAPS Journal. - ISSN 1550-7416. - 7:S2 ;2005; W4148-W4148. AAPS Annual Meeting and Exposition, Nashville, 2005.
13. Chien. M J, Tirol. G, Charles. B, Corniello. C, Waston. G, Sanchez. I.; Castable edible pharmaceutical films; Dow Chemical Company; West Haven; USA; 2007; 1-7.
14. Sakellariou. P and Rowe. R.C.; Interactions in cellulose derivative films for oral drug delivery; Prog. Polym. Sci.; 1995; 20; 889 ? 942.
15. Banker GS; Film coating theory and practice; J. Pharm. Sci.; 1966; 55(1); 81? 89.
16. Sharma S, Lewis S; Taste masking technology: A Review; International journal of pharmacy and pharmaceutical sciences; 2010; 2(2); 6-13
17. Chapdelaine. A H, Zyck. D J and Dzija. M R.; Edible film formulations containing Maltodextrin; US Patent May 25, 2004; US Patent 6740332.
18. Mishra. R, Amin. A.; Quick API Delivery; Pharmaceutical Technology Europe; 1- 5.
19. Frey; Film Strips and Pharmaceuticals, Pharma Mfg & Packag Sourcer; 2006; 92– 93.
20. Coppens. K A, Hall. M J, Mitchell. S A and Read. M D; Hypromellose, Ethyl cellulose and Polyethylene oxideused in hot melt extrusion; Pharmaceutical Technology; 2005; 1-6.
21. www. researchandmarkets.com/research/ckvqw7/oral_thin_film.
22. vjinstruments.com/index.php/products/pharmaceutics/item/1-film-former
23. www. optimags.de/tds-odf.php
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









