About Author: VIPUL P. PATEL1*, TUSHAR R. DESAI2, CHETAN R. MATHOLIYA, RAVI B. CHHAYANI
1. Assistant professor, Department of pharmaceutics,
R. K. College of Pharmacy, Kasturbadham,Rajkot.
2. Principal, Department of pharmacology,
R. K. College of Pharmacy, Kasturbadham,Rajkot.
3. Research Scholar, R. K. College of Pharmacy, Kasturbadham, Rajkot.
Reference ID: PHARMATUTOR-ART-1060
Abstract
Pulsatile Drug Delivery Systems are gaining a lot of interest as they deliver the drug at the right place at the right time and in the right amount, thus providing spatial and temporal delivery and increasing patient compliance. These systems are designed according to the circadian rhythm of the body. The principle rationale for the use of pulsatile release of the drugs is where a constant drug release is not desired. A pulse has to be designed in such a way that a complete and rapid drug release is achieved after the lag time. Various systems like capsular systems, osmotic systems, single- and multiple-unit systems based on the use of soluble or erodible polymer coating and use of rupturable membranes have been dealt with in the article. It summarizes the latest technological developments, formulation parameters, and release profiles of these systems. These systems are beneficial for the drugs having chronopharmacological behavior where night time dosing is required, such as anti-arhythmic and anti-asthmatic. Current review article discussed the reasons for development of pulsatile drug delivery system, types of the disease in which pulsatile release is required, classification, advantages, limitation, and future aspects of pulsatile drug delivery system.
Introduction
Over the last 30 years the pharmaceutical market has been demonstrated increasing preferably for controlled and targeted drug delivery system. Such systems have been focused on constant, variable; sustain drug release and/or targeting the therapeutic agent to a specific site/tissue/ organ. However, recently there are certain conditions for which such release pattern is not suitable. Such conditions that lead to the requirements of a time programmed therapeutic system, which capable of releasing drug after predetermined time delay and maintain constant drug levels throught the day. To introduce the concept of chronotherapeutics, it is important to define the following concepts.
Chronobiology: [4]
Chronobiology is the science concerned with the biological mechanism of the diseases according to a time structure.“Chrono” pertains to time and “biology” pertains to the study, or science, of life.
Chronopharmacology: [4]
Chronopharmacology is the science concerned with the variations in the pharmacological actions of various drugs over a period of time of the day.
Chronopharmacokinetics: [4,5]
Chronopharmacokinetics involves study of temporal changes in drug absorption, distribution, metabolism and excretion. Pharmacokinetic parameters, which are conventionally considered to be constant in time, are influenced by different physiological functions displaying circadian rhythm. Circadian changes in gastric acid secretion, gastrointestinal motility, gastrointestinal blood flow, drug protein binding, liver enzyme activity, renal blood flow and urinary pH can play role in time dependent variation of drug plasma concentrations.
Chronotherapy: [4]
Co-ordination of biological rhythms and medical treatment is called chronotherapy.
Chronotherapeutics: [4,6]
Chronotherapeutics is the discipline concerned with the delivery of drugs according to inherent activities of a disease over a certain period of time. it is becoming increasingly moreevident that the specific time that patients take their medication may be even more significant than was recognized in the past.
2. BIOLOGICAL RHYTHMS: [7]
1. Ultradian Rhythms:
Oscillations of shorter duration are termed Ultradian Rhythms (more than one cycle per 24 h). E.g.90 minutes sleep cycle.
2. Infradian Rhythms:
Oscillations that are longer than 24 hours are termed as Infradian Rhythms (less than one cycle per 24hours). E.g. Monthly Menstruation.
3. Circadian rhythms:
Circadian rhythms are self-sustaining, endogenous oscillations
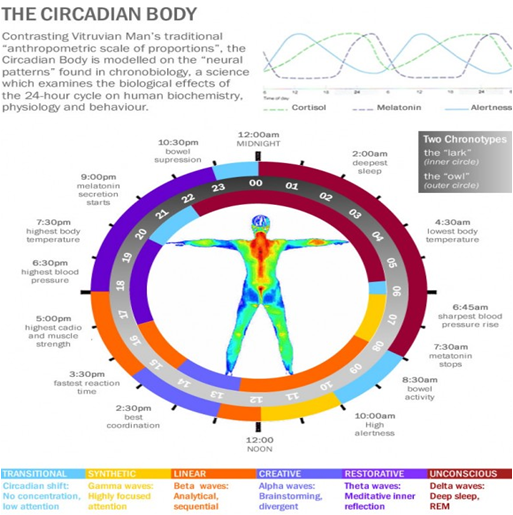
Figure 1: Diseases displaying circadian rhythm [8]
that occur with a periodicity of about 24 Hours. Interestingly, the term circadian is derived from the Latin circa which means “about” and dies which can be defined as “a day”. Normally, circadian rhythms are synchronized according to internal biologic clocks related to the sleep-wake cycle. [7]
DISEASES AND CHRONOTHERAPEUTICS:
The potential benefits of chronotherapeutics have been demonstrated in the management of a number of diseases. In particular there is a great deal of interest in how chronotherapy can particularly benefit patients suffering from allergic rhinitis, rheumatoid arthritis and related disorders, asthma, cancer, cardiovascular diseases, and peptic ulcer disease. [6]
Table: 1 Disease Influenced by Chronotherapy [6]
|
Diseases |
Influenced by Chronotherapy |
|
Cardiovascular |
Hypertension,angina , myocardial infarction |
|
Inflammatory |
Rheumatoid arthritis, related disorders |
|
Neoplastic |
Various forms of cancer |
|
Gastrointestinal |
Peptic ulcer disease |
|
Respiratory |
Allergic rhinitis, asthma |
Cardiovascular Diseases: [6]
Cardiovascular diseases such as hypertension and angina, or chest pain, also follow a definite circadian rhythm.
Hypertension: [6]
Heart rate and blood pressure are increased in the early morning hours (morning or A.M. surge). The blood pressure declines form mid afternoon and is minimum at midnight. In most hypertensive patients, there is a rather marked rise in blood pressure upon awakening that is called the morning or “a.m.” Systolic blood pressure rises approximately 3mm Hg/hour for the first 4-6 hours post-awakening, while the rate of rise of diastolic blood pressure is approximately 2mm Hg/hour.
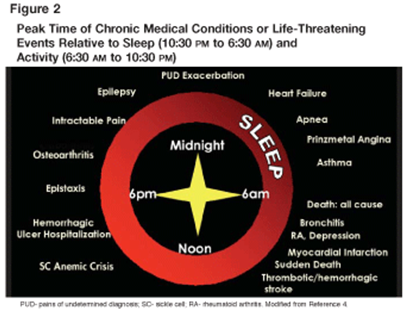
Fig.2: circadian variations in Blood Pressure [9]
Myocardial Infarction:
Onset of myocardial infarction has been shown to be more frequent in the morning with 34% events occurring between 6 A.M. and noon. Acute cardiac arrest and transient myocardial ischemia shows an increased frequency in morning. The causes for these findings have been suggested to be release of catecholamine, cortisol increase in the platelet aggregation and vascular tone. [4]
ACE inhibitors are more effective when administered during night. Atenolol, Nifedipine and amolodipine are more effective when administered at night.[4]
Arthritis:
Patients suffering from osteoarthritis are reported to have less pain in morning hours than night, while patients suffering from rheumatoid arthritis feel more pain in the morning hours. 7. In this case taking medication at night is an obvious solution.
NSAIDs such as ibuprofen need to be administered 4 to 6 hours before achieving their maximum benefit, as a result peak will occur at patients waking and the effect will be decline as patient start to wake up. [4,6]
Neoplastics:
Antineoplastic drugs cause cytotoxic effects on healthy and diseased tissues. As would be expected, the biological rhythms of both healthy and tumor cells may influence the susceptibility of normal and malignant cells to these agents. It has been demonstrated that “susceptibility rhythms” to drugs may differ between healthy tissue and cancerous tissue. Therefore, the “correct” timing of drug treatment may reduce host toxicity, increase the maximum drug tolerance, and ultimately result in better tumor management. [7]
Peptic ulcer disease:
Because of maximal acid secretion, peptic ulcer disease pain, and perforation of gastric and duodenal ulcers are more common at night, administration of drugs at bedtime is more effective. Nocturnal administration not only reduces acid secretion more effectively but also promotes ulcer healing and reduces ulcer recurrence. Bedtime H2-receptor blockade is one such regimen.
Bronchial asthma:
Asthma is a chronic inflammatory disease of the airways, characterized by hyper responsiveness to a variety of stimuli. The role of circadian rhythms in the pathogenesis and treatment of asthma indicates that airway resistance increases progressively at night in asthmatic patients. Circadian changes are seen in normal lung function, which reaches a low point in the early morning hours. [10]
The worsening of asthma at night, commonly referred to as nocturnal asthma (NA). A drug delivery system administered at bedtime but releasing drug during morning hours would be ideal in this case. [11]
Table 1. Diseases required pulsatile delivery [12]
|
Chronological behavior |
Drugs used |
Diseases |
|
Acid secretion is high in the Aternoon and at night |
H2 blockers |
Peptic ulcer |
|
Precipitation of attacks during night or at early morning |
β2 agonist, Antihistamines |
Asthma |
|
BP is at its lowest during the sleep cycle and rises steeply during the early morning |
Nitroglycerin, calcium channel blocker, ACE inhibitors |
Cardiovascular diseases |
|
Pain in the morning and more pain at night |
NSAIDs, Glucocorticoids |
Arthritis |
|
Increase in the blood sugar level after meal |
Sulfonylurea, Insulin |
Diabetes mellitus |
|
Cholesterol synthesis is generally higher during night than day time |
HMG CoA reductase inhibitors |
Hypercholesterolemi |
Table 2. Marketed technologies of pulsatile drug delivery [13-17]
|
Technology |
Mechanism |
Proprietary name and dosage form |
API |
Disease |
References |
|
OROS* |
Osmotic mechanism |
Covera-H5*; XL tablet |
Verapamil HCL |
Hypertension |
14 |
|
Three dimentional printing* |
Externally regulated system |
Their Form* |
Diclofenac sodium |
Inflammation |
15 |
|
DIFFUCAPS* |
Multiparticulate system |
Innopran*; XL tablets |
Verapamil HCL, propranoll HCL |
Hypertension |
16 |
|
PulsincapTM |
Rupturable system |
PulsincapTM |
Dofetilide |
Hypertension |
17 |
3. CLASSIFICATION OF PULSATILE DRUG DELIVERY SYSTEMS:
A. Various approaches of pulsatile drug:
Pulsatile drug delivery system can be broadly classified into three classes;
I. Time controlled pulsatile drug delivery
II. Stimuli induced pulsatile drug delivery
III. Externally regulated pulsatile drug delivery
IV. Time controlled pulsatile drug delivery
A. Single unit pulsatile systems
1. Capsule based systems
E.g. Pulisincap system
2. Capsular system based on Osmosis
a. ‘PORT’ System
b. System based on expandable orifice
c. Delivery by series of stops.
d. Pulsatile delivery by solubility modulation
3. Pulsatile system with Erodible or soluble barrier coatings.
a. The chronotropic system
b. ‘TIME CLOCK’ System
c. Compressed tablets
d. Multilayered Tablets
4. Pulsatile system with rupturable coating
B. Multiparticulate / Multiple unit systems:
1. Pulsatic system with rupturable coating
E.g. Time –controlled Explosion system (TCES)
2. Osmotic based rupturable coating system
E.g. Permeability controlled system
3. Pulsatilc delivery by change in membrane permeability
E.g.Sigmoidal release system. [18]
A. Single unit pulsatile systems
1. Capsule based systems:
Single-unit systems are mostly developed in capsule form. The lag time is controlled by a plug, which gets pushed away by swelling or erosion, and the drug is released as a “Pulse” from the insoluble capsule body. [19]
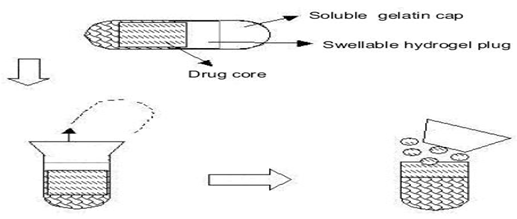
Fig 3: Design of Pulsincap system
The lag time can be controlled by manipulating the dimension and the position of the plug. [20,21]
Polymers used for designing of the hydrogel plug
1) Insoluble but permeable and swellable polymers
(e.g., polymethacrylates)
2) Erodible compressed polymers
(e.g., hydroxypropylmethyl cellulose, polyvinyl alcohol, Polyethylene oxide)
3) Congealed melted polymers
(e.g., saturated polyglycolated glycerides, glyceryl monooleate)
4) Enzymatically controlled erodible polymer (e.g., pectin). [22,23]
The preparation and invitro release of tetramethylpyrazine phosphate pulsincap capsule has been reported. It was prepared by sealing the drug tablet and fillers inside an impermeable capsule body with erodible plug. To meet the chronotherapeutic requirements, a suitable lag time can be achieved by adjusting the content of gel-forming polymer (HPMC) and the erodible plug weight. [24]
2. Capsular system based on Osmosis
a. ‘PORT’ System
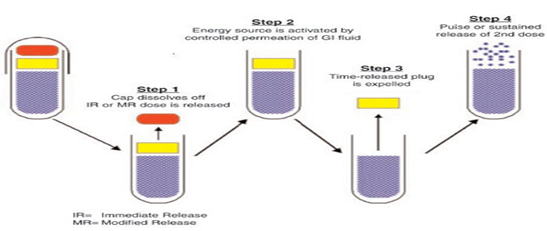
Fig 4: Drug release mechanism from PORT system
The Port system fig. (4) was developed by Therapeutic system research laboratory Ann Arbor, Michigan, USA, and consists of a capsule coated with a semipermeable membrane. Inside the capsule was an insoluble plug consisting of osmotically active agent and the drug formulation.25 When this capsule came in contact with the dissolution fluid, the semipermeable membrane allowed the entry of water, which caused the pressure to develop and the insoluble plug expelled after a lag time. Such a system was utilized to deliver methylphenidate used in the treatment of attention deficit hyperactivity disorder as the pulsatile port system. This system avoided second time dosing, which was beneficial for school children during daytime.
b. System based on expandable orifice:
To deliver the drug in liquid form, an osmotically driven capsular system was developed in which the liquid drug is absorbed into highly porous particles, which release the drug through an orifice of a semipermeable capsule supported by an expanding osmotic layer after the barrier layer is dissolved. 26
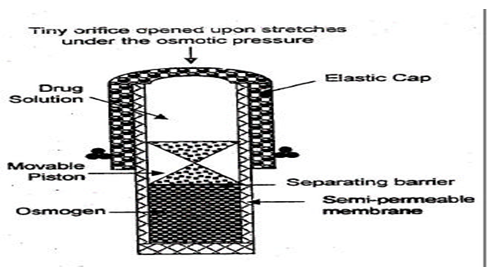
Fig. 5: System based on expandable orifice
The orifice is small enough so that when the elastic wall relaxes, the flow of the drug through the orifice essentially stops, but when the elastic wall is distended beyond threshold value, the orifice expands sufficiently to allow drug release at a required rate. E.g. Elastomers, such as styrene-butadiene copolymer have been suggested. [27,28]
c. Delivery by series of stops:
This system is described for implantable capsules. The capsule contains a drug and a water absorptive osmotic engine that are placed in compartments separated by a movable partition. The pulsatile delivery is achieved by a series of stops along the inner wall of the capsule. These stops obstruct the movement of the partition but are overcome in succession as the osmotic pressure rises above a threshold level. [29]
d. Pulsatile delivery by solubility modulation:
Such systems contain a solubility modulator for pulsed delivery of variety of drugs. The system was especially developed for delivery of salbutamol sulphate. [30-32] The compositions contains the drug (salbutamol sulphate) and a modulating agent (sodium chloride). The amount of NaCl was such that it was less than the amount needed to maintain saturation in a fluid that enters the osmotic device. The pulsed delivery is based on drug solubility. Salbutamol has solubility of 275mg/ml in water and 16 mg/ml in saturated solution of NaCl, while NaCl has solubility of 321 mg/ml in water, and its saturation solubility is 320 mg/ml.
3. Pulsatile system with Erodble or soluble barrier coatings:
Most of the pulsatile drug delivery systems are reservoir devices coated with a barrier layer. This barrier erodes or dissolves after a specific lag period, and the drug is subsequently released rapidly from reservoir core. The lag time depends on the thickness of the coating layer.
a. The chronotropic system:
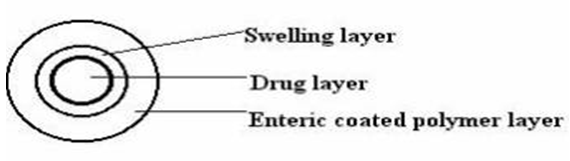
Fig. 6: The chronotropic system
The Chronotropic® system consists of a drug-containing core coated by hydrophilic swellable hydroxypropylmethyl cellulose (HPMC), which is responsible for a lag phase in the onset of release. [33-35] In addition, through the application of an outer gastric-resistant enteric film, the variability in gastric emptying time can be overcome, and a colon-specific release can be obtained, relying on the relative reproducibility of small intestinal transit time. [36] The lag time is controlled by the thickness and the viscosity grades of HPMC. [37] Both in-vitro and in-vivo lag times correlate well with the applied amount of the hydrophilic retardin polymer. The system is suitable for both tablets and capsules. [38]
b. ‘TIME CLOCK’ System:

Fig.7: ‘TIME CLOCK’ System
The lag time could be controlled by varying the thickness of the film. After the lag time, i.e., the time required for rehydration, the core immediately releases the drug. This system has shown reproducible results in vitro and in vivo. The effect of low calorie and high calorie meal on the lag time was studied using gamma scintigraphy. The mean lag time of drug release was 345 and 333 min respectively.
c. Compressed Tablets:
Compression coating can involve direct compression of both the core and the coat, obviating needs for separate coating process and use of coating solutions. The outer tablet of the compression-coated tablet provides the initial dose, rapidly disintegrating in the stomach and the inner layer is formulated with components that are insoluble in gastric media but are released in the intestinal environment. [39] Materials such as hydrophilic cellulose derivates can be used. Compression is easy on laboratory scale. The major drawbacks of the technique are that relatively large amounts of coating materials are needed and it is difficult to position the cores correctly.[39]
Press-coated pulsatile drug delivery systems:
1. Press-coated pulsatile drug delivery systems can be used to protect hygroscopic, light-sensitive, oxygenlabile or acid-labile drugs.
2. Press-coated pulsatile drug delivery systems are relatively simple and cheap.
3. These systems can involve direct compression of both the core and the coat.
4. Materials Such as hydrophobic, hydrophilic can be used in press-coated pulsatile drug delivery system.
5. Press-coated pulsatile drug delivery systems involve compression which is easy on laboratory scale.
6. Press-coated pulsatile formulations relese drug after “lag-time”.
7. Press-coated pulsatile drug delivery formulations can be used to separate incompatible drugs from each other or to achieve sustained release.
d. Multilayered Tablets:
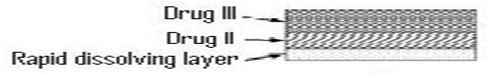
Fig.8: Multilayered Tablet
A release pattern with two pulses was obtained from a threelayered tablet containing two drug containing layers separated by a drug-free gellable polymeric barrier layer. [40-42]
4. Pulsatile system with rupturable coating:
These systems depend on the disintegration of the coating for the release of drug. The pressure necessary for the rupture of the coating can be achieved by the effervescent excipients, swelling agents, or osmotic pressure. An effervescent mixture of citric acid and sodium bicarbonate was incorporated in a tablet core coated with ethyl cellulose. The carbon dioxide developed after penetration of water into the core resulted in a pulsatile release of drug after rupture of the coating. [43] The release may depend on the mechanical properties of the coating layer. [43]
a) Pulsatile system based on rupturable coating:
E.g. Time –controlled Explosion system (TCES):
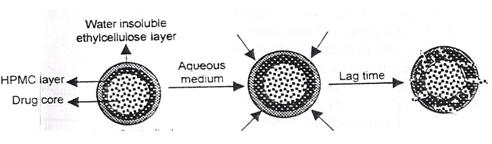
Fig.9: Time –controlled Explosion system (TCES)
This is a multiparticulate system in which drug is coated on non-pareil sugar seeds followed by a swellable layer and an insoluble top layer. [44-46] The swelling agents used include Superdisintegrants like sodium carboxymethyl cellulose, sodium starch glycollate, L hydroxypropyl cellulose. Polymers like polyvinyl acetate, polyacrylic acid, polyethylene glycol, etc.
b) Osmotic based rupturable coating system:
This system is based on a combination of osmotic and swelling effects. The core containing the drug, a low bulk density solid and/or liquid lipid material (eg, mineral oil) and a disintegrant was prepared. This core was then coated with cellulose acetate. Upon immersion in aqueous medium, water penetrates the core displacing lipid material. After the depletion of lipid material, internal pressure increases until a critical stress is reached, which results in rupture of coating. [47]
c) Pulsatile delivery by change in membrane permeability:
The permeability and water uptake of acrylic polymers with quaternary ammonium groups can be influenced by the presence of different counter-ions in the medium. 48 Several delivery systems based on this ion exchange have been developed. Eudragit RS 30D is reported to be a polymer of choice for this purpose. It typically contains positively polarized quaternary ammonium group in the polymer side chain, which is always accompanied by negative hydrochloride counter-ions. The ammonium group being hydrophilic facilitates the interaction of polymer with water, thereby changing its permeability and allowing water to permeate the active core in a controlled manner. This property is essential to achieve a precisely defined lag time. [49]
B. Stimuli-induced pulsatile release
1. Temperature-induced pulsatile release
Thermoresponsive hydrogels have been investigated as possible drug delivery carriers for stimuli responsive drug delivery systems. [50-52] PIPAAm cross-linked gels have shown thermoresponsive, discontinuous swelling / deswelling phases: swelling, for example, at temperatures below 328C, whileshrinking above this temperature. Thermoresponsive polymeric micelle systems As Kataoka et al. [53] comprehensively reviewed, the properties and biological interests of polymeric micelles make them a most noteworthy candidate as drug carrier for the treatment of cancer. The polymeric micelle is composed of amphiphilic block copolymers exhibiting a hydrophobic core with a hydrophilic corona. The application of a temperature gradient induced an on–off drug release regulation from PIPAAm PBMA micelles between 4 and 378C.
2. Chemical stimuli-induced pulsatile release
a) Glucose-responsive insulin release devices
A decrease in or the absence of insulin secretion from pancreatic islets is the cause of diabetes mellitus. Diabetes mellitus patients suffer long term from a gradual decline in the efficiency of various organs, such as the occasional loss of eyesight. Several systems have already been developed which are able to respond to glucose concentration changes. Glucose oxidase (GOD) catalyzes glucose oxidation. Utilizing this reaction, Ishihara et al. [54] prepared two types of gel membrane systems to regulate insulin permeability. They prepared and nicotinamide-immobilized gel membranes, separately.
Inflammation-induced pulsatile release
When human beings receive physical or chemical stress, such as injury, broken bones, etc., inflammation reactions take place at the injured sites. At the inflammatory sites, inflammation-responsive phagocytic cells, such as macrophages and polymorphonuclear cells, play a role in the healing process of the injury. During inflammation, hydroxyl radicals (OH) are produced from these inflammation-responsive cells. Yui and co-workers 55-56. used hyaluronic acid (HA), a linear mucopolysaccharide composed of repeating disaccharide subunits of N-acetyl-D-glucosamine and D-guluronic acid. In the body, HA is mainly degraded either by a specific enzyme, hyaluronidase, or hydroxyl radicals. Degradation of HA via the hyaluronidase is very low in a normal state of health. Degradation via hydroxyl radicals however, is usually dominant and rapid when HA is injected at inflammatory sites. Thus,Yui and co-workers [55-56] prepared cross-linked HA with ethyleneglycol diglycidylether or polyglycerol polyglycidylether. These HA gels degraded only when the hydroxyl radicals were generated through the Fenton reaction between Fe+2 ions and hydrogen peroxide in vitro.
Thus, a surface erosion type of degradation was achieved. When microspheres were incorporated in the HA hydrogels as a model drug, these microspheres were released only when hydroxyl radicals induced HA gel degradation. The microsphere release was regulated by the surface erosion type of degradation.
b) Drug release from intelligent gels responding to antibody concentration.
There are numerous kinds of bioactive compounds which exist in the body. Recently, novel gels were developed which responded to the change in conbecentration of bioactive compounds to alter their swelling/deswelling characteristics. Miyata and co-workers [57-58] focused on the introduction of stimuli-responsive cross-linking structures into hy-drogels. Special attention was given to antigen antibody complex formation as the cross-linking units in the gel, because specific antigen recognition of an antibody can provide the basis for a new device fabrication.
c) Electric stimuli-responsive pulsatile release
The combination of developments in several technologies, such as microelectronics and micromachin ing, as well as the potential need for chronotherapy, have currently assisted the development of electronically assisted drug delivery technologies. These technologies include iontophoresis, infusion pumps, and sonophoresis [59]. Several approaches have also been presented in the literature describing the preparation of electric stimuli-responsive drug delivery systems using hydrogels.
Kishi et al. [60] developed an electric stimuli induced drug release system using the electrically stimulated swelling /deswelling characteristics of polyelectrolyte hydrogels. They utilized a chemomechanical system, which contained a drug model within the polyelectrolyte gel structure. These gels exhibited reversible swelling / shrinking behavior in response to on–off switching of an electric stimulus. Thus, drug molecules within the polyelectrolyte gels might be squeezed out from the electric stimuli-induced gel contraction along with the solvent flow. To realize this mechanism, poly(sodium acrylate) microparticulate gels containing pilocarpine as a model drug were prepared. [61]
4. RECENT ADVANCES IN THE PULSATILE DRUG DELIVERY
Nowadays pulsatile drug delivery systems are gaining importance in various disease conditions specifically in diabetes where dose is required at different time intervals. Among these systems, multi-particulate systems (e.g. pellets) offer various advantages over single unit which include no risk of dose dumping, flexibility of blending units with different release patterns, as well as short and reproducible gastric residence time [62]. Multiparticulate systems consists pellets of different release profile which can be of any type like time dependent, pH dependent, micro flora activated system as discussed in the previous sections. Site and time specific oral drug delivery have recently been of great interest in pharmaceutical field to achieve improved therapeutic efficacy. Gastroretentive drug delivery system is an approach to prolong gastric residence time, thereby targeting sitespecific drug release in upper gastrointestinal (GI) tract. Floating drug delivery system (FDDS) and bioadhesive drug delivery are widely used techniques for gastro retention. Low density porous multiparticulate systems have been used by researchers for formulation of FDDS. Sharma and Pawar developed multiparticulate floating pulsatile drug delivery system using porous calcium silicate and sodium alginate for time and site specific drug release of meloxicam [63]. Various pulsatile technologies have been developed on the basis of methodologies as discussed previously. These includes OROS® technology, CODAS® technology, CEFORM® technology, DIFFUCAPS® technology, Three-dimensional printing®, timerx® etc.
5. CURRENT SITUATION AND FUTURE SCOPE
Now a day's pulsatile drug delivery is gaining popularity. The prime advantage in this drug delivery is that drug is released when necessity comes. As a result chance of development of drug resistance which is seen in conventional and sustained release formulations can be reduced. Furthermore, some anticancer drugs are very toxic. These drugs give hazardous problems in conventional and sustained release therapies. Now many FDA approved chronotherapeutic drugs are available in the market. This therapy is mainly applicable where sustained action is not required and drugs are toxic. Key point of development of this formulation is to find out circadian rhythm i.e. suitable indicator which will trigger the release of drug from the device. Another point is absence of suitable rhythmic biomaterial which should be biodegradable, biocompatible and reversibly responsive to specific biomarkers in rhythmic manner. Regulatory is another big question. In preapproval phase it is sometimes difficult to show chronotherapeutic advantage in clinical settings. In postapproval phase causal recreational drug abuse along with on a much larger scale, by the criminal diversion of these modified formulations for profit have arisen problems. The FDA has now heavily relied on the development and implementation of risk management programs as a strategy to allow an approval of a drug to go forward while exercising some restrictions. Many researches are going on the pulsatile drug delivery to discover circadian rhythm with suitable device in the world. In future this delivery will be a leading way to deliver therapeutic agents due to its some unique characters like low chance of dose dumping, patient compliance and the above factors. [64]
6. ADVANTAGES OF PULSATILE DRUG DELIVERY SYSTEM: [65,66]
1. Extended daytime or nighttime activity
2. Reduced side effects
3. Reduced dosage frequency
4. Reduction in dose size
5. Improved patient compliance
6. Lower daily cost to patient due to fewer dosage units are required by the patient in therapy.
7. Drug adapts to suit circadian rhythms of body functions or diseases.
8. Drug targeting to specific site like colon.
9. Protection of mucosa from irritating drugs.
10. Drug loss is prevented by extensive first pass metabolism .
11. Patient comfort and compliance: Oral drug delivery is the most common and convenient for patients, and a reduction in dosing frequency enhances compliance.
7. LIMITATIONS OF PULSATILE DRUG DELIVERY SYSTEM: [66]
Pulsatile drug deleivery system have certain limitation, so in many cases these drug delivery system is fails,
• Multiple manufacturing steps in case of Multiparticulate pulsatile drug delivery system.
• Low drug load
• Incomplete release
• In-vivo variability in single unit pulsatile drug delivery system.
References:
1. Janugade BU, Patil SS, Patil SV, Lade PD: Pulsatile drug delivery system for chronopharmacological disorders: an overview. Journal of Pharmacy Research , 2009; 2 (1):133-143.
2. Ramesh D. Parmar, Rajesh K. Parikh, G. Vidyasagar, Dhaval V. Patel, Chirag J. Patel and Biraju D. Patel: Pulsatile Drug Delivery Systems: An Overview International Journal of Pharmaceutical Sciences and Nanotechnology 2009; 2(3): 605-607.
3. Nitin Saigal, Sanjula Baboota, Alka Ahuja and Javed Ali: Site Specific Chronotherapeutic Drug Delivery Systems: A Patent Review. Recent Patents on Drug Delivery & Formulation 2009; 3: 64-70.
4. Jha N, Bapat S: Chronobiology and chronotherapeutics. Kathmandu University Med. Jour. 2004; 2(8): 384-388.
5. Bruguolle B, Lemmer B: Recent advances in chronopharmacokinetics: methodological problems, Life Sci. 1993; 52 (23): 1809-1824.
6. https://www.uspharmacist.com/oldformat.asp?url=newlook/files/ Feat/ACF2F15.cfm&pub_id, accesed on 15/9/10.
7. Botti B, Youan C: Chronopharmaceutics: gimmick or clinically relevant approach to drug delivery, Jorn. Control. Rel. 2004; 98(3): 337-353.
8. https://thred.org/wp-content/uploads/2010/06/circadian-body-e1283009674721.jpg assessed on 1-2-2011
9. https://www.pharmacytimes.com/files/ArticleFiles/PTMAR05p115_f2.gif assessed on 1-2-2011
10. Gothoskar AV, Joshi AM, Joshi NH: Pulsatile Drug Delivery Systems: A Review. Drug Del. Tech. 2004; 4: 5-8.
11. Skloot, G.: Nocturnal Asthma: Mechanisms and Management. The Mount Sinai J. of Med. 2002; 69: 140-147.
12. Survase S, Kumar N: Pulsatile Drug Delivery: Current Scenario. Current Research & Information on Pharmaceutical Sciences. 2007; 8(2): 27-33.
13. Lemmer: Circadian rhythms and drug delivery. Jou. Control. Rel. 1991; 16: 63-74.
14. Jao F, Wong P, Huynh H, et al. (1992):17
15. Percel P, Vishnupad K and Venkatesh G (2002):13
16. Katstra WE, Palazzolo RD, Rowe CW, et al. (2000) J. Control. Rel. 66:1-9
17. Stevens HNE, Wilson CG, Welling PG, et al. (2002) Int. J. pharm. 236:27-34
18. Janugade B.U1*, Patil S. S2., Patil S.V. 1, Lade P. D.1 Pulsatile drug delivery system for chronopharmacological disorders: an overview Review Article Journal of Pharmacy Research 2009;2;1:136
19. Neill MC, Rashid A, Stevens HN, GB Patent No. GB2230442, 1993.
20. Sarasija S, Hota A, Colon-specific drug delivery systems, Ind. J. Pharm. Sci., 62(1), 2002, 1-8.
21. Kinget R, Kalala W, Vervoort L, Mooter GV, Colonic drug targeting, J. Drug Targeting, 6(2), 1998, 129-149.
22. Krögel I, Bodmeier R, Pulsatile drug release from an insoluble capsule body controlled by an erodible plug, Pharm. Res., 15(3),1998, 474-481.
23. Krögel I, Bodmeier R, Evaluation of an enzyme-containing capsular shaped pulsatile drug delivery system, Pharm. Res.16 (9), 1999, 1424-1429.
24. Wu F, Zhang ZR, He WL, Zhang Y, Preparation and in vitro release of tetramethylpyrazine phosphate pulsincap capsule controlled by an erodible plug. Yao Xue Xue Bao., 37(9), 2002, 733-738.
25. Crison JR., Siersma PR, Amidon GL, A novel programmable oral release technology for delivering drugs: human feasibility testing using gamma scintigraphy, Proceed Intern Symp Control Rel. Bioact. Mater., 23, 1996, 51-52.
26. Pollock DC, Dong L, Wong P, A new system to deliver a delayed bolus of liquid drug formulation, Proceed Intern Symp, Control. Rel. Bioact. Mater., 28, 2001, 6033.
27. Linkwitz A, Magruder JA, Merrill S, Osmotically Driven Delivery Device with Expandable Orifice for Pulsatile Delivery Effect, US Patent No. 5,318,558, 1994.
28. Linkwitz A., Magruder JA, Merrill S, Osmotically Driven Delivery Device with Expandable Orifice for Pulsatile Delivery Effect, US Patent No. 5,221,278, 1993.
29. Balaban SM, Pike JB, Smith JP, Baile CA, Osmotically Driven Delivery Devices with Pulsatile Effect, US Patent No. 5209746, 1993.
30. Magruder PR, Barclay B, Wong PS, Theeuwes F, Composition Comprising Salbutamol, US Patent No. 4751071, 1988.
31. Magruder PR, Barclay B, Wong PS, Theeuwes F, Constant Release System with Pulsed Release, US Patent No. 4777049, 1988.
32. Magruder PR., Barclay B, Wong PS, Theeuwes F, Composition Comprising a Therapeutic Agent and a Modulating Agent. US Patent No. 4851229, 1989.
33. Gazzaniga A, Iamartino P, Maffione G, Sangalli ME, Oral delayed- release system for colonic specific delivery, Int. J. Pharm., 2(108), 1994, 77-83.
34. Gazzaniga A, Sangalli ME, Giordano F. Oral chronotopic drug delivery systems: achievement of time and/or site specifity, Eur. J. Biopharm., 1994; 40(4): 246-250.
35. Gazzaniga A, Busetti C, Moro L, Crimella T, Sangalli ME, Giordano F. Evaluation of low viscosity HPMC as retarding coating material in the preparation of a time-based oral colon specific delivery system. Proceed Intern Symp Control. Rel. Bioact. Mater., 1995; 22: 242-243.
36. Poli S, Busetti C, Moro L: Oral Pharmaceutical Composition for Specific Colon Delivery, EP Patent No. 0,572,942, 1993.
37. Sangalli ME, Maroni A, Zema L, Busetti C, Giordano F, Gazzaniga A: In vitro and in vivo evaluation of an oral system for time and/or site-specific drug delivery. J. Control. Rel., 2001; 73: 103-110.
38. Maroni A, Sangalli ME, Cerea M, Busetti C, Giordano F, Gazzaniga A: Low viscosity HPMC coating of soft and hard gelatin capsules for delayed and colonic release: preliminary investigations on process parameters and in vitro release performances. Proceed Int. Control. Rel. Bioact. Mater., 1999; 26: 887-888.
39. Patel G: Specialized chronotherapeutic drug delivery systems, Pharmainfo.net
40. Conte U, Colombo P, Manna A, Gazzaniga A: A new ibuprofen pulsed release oral dosage form. Drug Dev. Ind. Pharm., 1989; 15(14- 16): 2583-2596.
41. Conte U, Manna A, Colombo P: Tablet for Pharmaceutical Use Able to Release Active Substances at Successive Times, US Patent No. 4,865,849, 1989.
42. Conte U, Giunchedi P, Maggi L, Sangalli ME, Gazzaniga A, Colombo P, Manna A, Ibuprofen delayed release dosage forms: a proposal for the preparation of an in vitro/in vivo pulsatile system, Eur. J. Pharm., 1992; 38(6): 209-212.
43. Krögel I, Bodmeier R: Floating or pulsatile drug delivery systems based on coated effervescent cores. Int. J. Pharm., 1999; 187: 175-184.
44. Ueda Y, Hata T, Yamaguchi H, Ueda S, Kotani M: Time Controlled Explosion System and Process for Preparation for the Same, US Patent No. 4,871,549, 1989.
45. Ueda Y, Hata T, Yamaguchi H, Kotani M, Ueda S: Development of a novel drug release system, time-controlled explosion system (TES), Part 1: concept and design. J. Drug Targeting. 1994; 2: 35-44.
46. Ueda S, Yamaguchi H, Kotani M, Kimura S, Tokunaga Y, Kagayama A, Hata T: Development of a novel drug release system, time-controlled explosion system (TES), Part II: design of multiparticulate TES and in vitro drug release properties, Chem. Pharm. Bull., 1994; 42(2): 359-363.
47. Amidon GL, Leesman GD: Pulsatile Drug Delivery System, US Patent No. 1993; 5,229,131.
48. Bodmeier R, Guo X, Sarabia RE, Skultety P: The influence of buffer species and strength on diltiazem HCl release from beads coated with aqueous cationic polymer dispersions, Eudragit RS, RL 30D, Pharm. Res.,1996; 13 (1): 52-56.
49. Beckert TE, Pogarell K, Hack I, Petereit HU: Pulsed drug release with film coatings of Eudragit & Mac226; RS 30D, Proceed Int’l Symp Control. Rel. Bioact. Mater., 1999; 26: 533- 534.
50. T. Okano, N. Yui, M. Yokoyama, R. Yoshida: Advances in Polymeric Systems for Drug Delivery, Gordon and Breach, Yverdon, Switzerland, 1994.
51. Y.H. Bae, T. Okano, S.W. Kim: ‘On–off’ thermocontrol of solute transport. I. Temperature dependence of swelling of N-isopropylacrylamide networks modified with hydrophobic components in water, Pharm. Res. 1991; 8 (4): 531–537.
52. Y.H. Bae, T. Okano, S.W. Kim: ‘On–off’ thermocontrol of solute transport. II. Solute release from thermosensitive hydrogels. Pharm. Res. 1991; 8 (5): 624–628.
53. K. Kataoka, A. Harada, Y. Nagasaki: Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001; 47: 113–131.
54. K. Ishihara, M. Kobayashi, I. Shinohara: Control of insulin permeation through a polymer membrane with responsive function for glucose, Makromol. Chem. Rapid Commun. 1983; 4: 327–331.
55. N. Yui, T. Okano, Y. Sakurai, Inflammation responsive degradation of crosslinked hyaluronic acid gels. J. Control. Release 1992; 22: 105–116.
56. N. Yui, J. Nihira, T. Okano, Y. Sakurai: Regulated release of drug microspheres from inflammation responsive degradable matrices of crosslinked hyaluronic acid. J. Control. Release 1993; 25: 133–143.
57. T. Miyata, N. Asami, T. Uragami: A reversibly antigen responsive hydrogel. Nature 1999; 399: 766–769.
58. T. Miyata, N. Asami, T. Uragami: Preparation of an antigen sensitive hydrogel using antigen–antibody bindings, Macro molecules 1999; 32: 2082–2084.
59. B. Berner, S.M. Dinh: Electronically assisted drug delivery: an overview, in: B. Berner, S.M. Dinh (Eds.), Electronically Controlled Drug Delivery, CRC Press, Boca Raton, FL, 1998, pp. 3–7.
60. R. Kishi, M. Hara, K. Sawahata, Y. Osada. Conversion of chemical into mechanical energy by synthetic polymer gels (chemomechanical system), in: D. DeRossi, K. Kajiwara, Y. Osada, A. Yamauchi (Eds.), Polymer Gels — Fundamentals and Biomedical Applications, Plenum Press, New York, 1991, pp. 205–220.
61. I.C. Kwon, Y.H. Bae, T. Okano, B. Berner, S.W. Kim: Stimuli sensitive polymers for drug delivery systems. Makro mol. Chem. Macromol. Symp. 1990; 33: 265–277.
62. Dashevsky A and Mohamad A: Int. J. pharm. 2006; 318: 124-131.
63. Sharma S and Pawar A.Int. J. pharm. 2006; 313
64. Asim Sattwa Mandal, Nikhil Biswas, Kazi Masud Karim, Arijit Guha, Sugata Chatterjee, Mamata Behera, Ketousetuo Kuotsu: Drug delivery system based on chronobiology. A review Journal of Controlled Release 2010;10
65. Sunil kamboj, G D Gupta, Jagmohan Oberoy: Matrix Tablets : An Important Tool for Oral Controlled-Release Dosage Forms, Pharmainfo net 2009; 7 (6), 1-9.
66. Rathod Shruti: Colon Targeted Pulsatile Drug Delivery : A Review. Pharmainfo net 2007; 5(2), 1-11.










.png)


