About Authors: Seema Meena, Anil Kumar and Shikha Chauhan
a) Amity Institute of Pharmacy, Amity University, Noida,-201301, India.
b) Neuropharmacology division, University Institute of Pharmaceutical Sciences, UGC Centre for Advance Studies, Panjab University, chandigarh-160014, India.
Abstract:
Neuropathic pain represents a real clinical challenge because of its severity, chronic nature, and inadequate drug therapy. Recently, nitric oxide pathway has been proposed in the pathogenesis neuropathic pain like conditions. Curcumin is a well known for its antioxidant and medicinal values. The objective of the present study was to explore possible nitric oxide mechanism in the protective effect of curcumin against sciatic nerve ligation induced behavioral and biochemical alterations in rats.Sciatic nerve ligation was performed in Wistar rats. Various behavioral parameters (thermal hyperalgesia, cold allodynia) followed by assessment of biochemical parameters (lipid peroxidation, reduced glutathione, catalase, and nitrite) both on sciatic nerves and brain. Sciatic nerve ligation significantly caused thermal hyeralgesia, cold allodynia and oxidative damage as compared to sham (Sciatic nerve without ligation) and naïve animals. Chronic administration of curcumin (20 mg/kg, po) significantly reversed behavioral alteration and attenuated oxidative damage in both sciatic nerve and brain as compared to control. Further, L-NAME (5 mg/kg) pretreatment with curcumin (10 mg/kg, po) potentiated protective effect of curcumin as compared to their effect per se. However, L-arginine (100 mg/kg) pretreatment with curcumin (10 mg/kg, po) significantly reversed the protective effects of curcumin. Result of present study suggests that nitric oxide mechanism could be involved in the protective effect of curcumin against sciatic nerve ligation induced behavior and biochemical alterations in rats.
[adsense:336x280:8701650588]
INTRODUCTION:
Pain is a fundamental alarm system and is crucial for survival1. In contrast, neuropathic pain is a severe pathological condition of the nervous system that offers no adaptive advantage2. Neuropathic pain results from damage or abnormal function of the central or peripheral nervous system3. Neuropathic pain is caused by lesion or inflammation of the nervous system and is relatively common with an incidence estimated at 0.6% to 1.5% in the Indian population4. It is often severely debilitating and largely resistant to treatment mainly because the underlying mechanisms are still poorly understood5. Patients with neuropathic pain frequently report sensory abnormalities including burning sensations, exaggerated responses to noxious stimuli (hyperalgesia), pain sensations resulting from innocuous stimuli (allodynia) and spontaneous pain episodes (dysesthesia)6. Neuropathic pain is probably multifactorial pathophysiological process) 7. It is often refractory to the conventional treatment with analgesics such as opiates and nonsteroidal anti-inflammatory drugs8. Use of opioids in neuropathic pain is controversial because of their limited efficacy as compared to other pain states9.
Recently, significant scientific progress has been made to understand or explain the pathogenesis and cellular cascades underlying neuropathic pain, several treatments are being tried with inadequate therapeutic effect10. Oxidative damage, neuroinflammation and nitric oxide cascade involvement has been reported in its pathogenesis11. Curcumin, one of active components of Curcuma longa, has been used since time immemorial. Curcumin is known for its diverse biological actions such as free radical scavenging action12, anticarcinogenic, antimicrobial13, hepatoprotective14, hypoglycemic14, thrombosuppressive15, antiarthritic16, anti-inflammatory properties17 including the production of interleukin- 8 (IL-8), interleukin-1β (IL-1β) and TNF-α by lipopolysaccharide stimulated monocytes and alveolar macrophages18. Curcumin is a very potent free radical scavenger than vitamin E19.Studies have shown that curcumin is a powerful scavenger of the superoxide anion, the hydroxyl radical, and nitrogen dioxide20 and protects DNA against singlet-oxygen-induced strand breaks21. Curcumin have also been found to inhibit nitric oxide production as measured by the amount of nitrite released from the culture medium22. Together with the ability of curcumin to inhibit NO and TNF-α release which cause nitrosative stress in neuropathic pain and promote inflammatory signals respectively, the present study was designed to evaluate the effect of curcumin in neuropathic pain and an attempt was made to look for the participation of nitric oxide in curcumin's protective effect.
Oxidative stress and reactive oxygen species (ROS) have been implicated in neuropathic pain23. Oxidative stress is imposed on the cells as a result of one of the three factors (i)an increase in oxidants generation (ii) decrease in antioxidant defense and (iii) a failure to repair oxidative damage24. Cell damage is induced by ROS which are either free radicals, reactive anions containing oxygen atoms or molecules containing oxygen atoms that can either produce free radicals or chemically activated by them. Examples are hydroxyl radicals, superoxide, hydrogen peroxide and peroxinitrite25. The main source of ROS in vivo is aerobic respiration, although ROS is also produced by peroxisomal-b oxidation of fatty acids, stimulation of phagocytosis by pathogens or lipopolysaccharides, arginine metabolism and tissue specific enzymes26. Unchecked ROS attacks many key molecules including enzymes, membranes, lipids and DNA. Under normal conditions, ROS is cleared from cells by the action of superoxide dismutase (SOD), catalase or glutathione, as well as antioxidant vitamin C and E. In spite of much work on oxidative stress and ROS, studies involving ROS in chronic pain are limited. In chronic constriction injury (CCI) model of rat neuropathic pain, heat hyperalgesia was reduced by systemically injected antioxidants26. Recently, in spinal nerve ligation (SNL) model of neuropathic pain, systemic injection of ROS scavenger phenyl-N-tert-butylnitrone (PBN) relieved mechanical allodynia27. Although, these studies suggest the involvement of free radicals/oxidative stress in neuropathic pain, little attention has been paid to critical role of antioxidant enzymes in neuropathic pain.
Studies indicate the potential role for nitric oxide (NO) in maintaining chronic painful states in animals after lesions of peripheral nervous system and CNS28. NO plays an important role in the maintenance of the behavioral signs of neuropathic pain and is involved in common steps in the maintenance of the different modalities of pain such as mechanical allodynia and cold allodynia29, 30, 31, 32. It has been reported that intrathecal administration of NOS inhibitors reverses thermal hyperalgesic responses in rats after sciatic nerve ligation33. There are also some reports that intracerebroventricular injection of L-arginine produces a biphasic effect on nociception, and NOS inhibitors have antinociceptive effects34, 35. Relatively, little has been known about the role of NO in the mediation of nociception at supraspinal level36. Sensory stimulation induces the release of L-arginine in the thalamus, which facilitate sensory transmission37. Therefore, it appears that physiological role for NO at supraspinal a site is likely to be pronociceptive, although the site(s) and mechanisms for such action remain to be clarified37. Together with the ability of curcumin to inhibit NO and TNF-α release which cause nitrosative stress in diabetes, promote pain and inflammatory signals respectively38. Therefore, present study was designed to evaluate the effect of curcumin in sciatic nerve ligation induced behavioral and biochemical alterations and an attempt was made to look for the participation of NO in curcumin's antinociceptive effect.
Materials and Methods:
Animals
Wistar rats (Central Animal House of the facility of the Panjab University, Chandigarh) weighing 180–200 g were used at the start of the surgery. Animals were acclimatized to laboratory conditions prior to experimentation. Following surgery, the animals were kept under standard conditions of light and dark cycle with food and water ad libitum in groups of single in plastic cages with soft bedding. All the experiments were carried out between 09:00 and 15:00 h. The protocol was approved by the Institutional Animal Ethics Committee and carried out in accordance with the Indian National Science Academy Guidelines for the use and care of animals.
Induction of peripheral neuropathy by sciatic nerve ligation
Peripheral neuropathy was induced by sciatic nerve ligation (SNL) in rats. In brief, rats were deeply anesthetized with thiopental sodium (40mg/kg i.p). The hair of rat’s lower back and thigh were shaved. The skin of the lateral surface of the right thigh was incised and a cut was made directly through the biceps. Expose the sciatic nerve and two ligatures (silk 4-0), were placed around the nerve. After performing the ligation, muscular and skin layer was immediately sutured with thread, and topical antibiotic was applied. Nociceptive threshold was assessed at weekly intervals on 7, 14, and 21st (39, 40).
Drugs and treatment schedule
Animals were divided into eleven groups (5 animals in each). First, second and third group were treated as naïve (vehicle treated), sham group (expose the sciatic nerve without ligation) and sciatic nerve ligation (SNL ligated animals) respectively. Curcumin (10 and 20 mg/kg, po), L-arginine (100 mg/kg, ip) and L-NAME (5 mg/kg, ip) were treated as groups 4–7, respectively. L-arginine (100 mg/kg, ip) or L-NAME (5 mg/kg) pretreatments with curcumin (10 and 20 mg/kg, po) were treated as groups 8–11, respectively. L-arginine and L-NAME were freshly prepared in distilled water and administered intraperitoneally 15 minutes before curcumin treatment. Curcumin was suspended freshly in 0.5% w/v (sodium carboxy-methyl-cellulose) before administration in a constant volume of 5 ml/kg body weight. Curcumin treatment was given, once daily for the duration of 3 weeks by oral route. However, L-arginine and NAME treatment were given 10 minutes before. Doses of Curcumin, L-arginine and L-NAME were selected based on reported literature and laboratory reports 41.
Behavioral Examinations:
Hot plate test
Thermal hyperalgesia was assessed by placing animals individual on a hot plate (Eddy’s Hot Plate) maintained at 55 0C on weekly interval on 7th, 14 and 21st day after SNL. The latency to first sign of paw licking or jumping response to avoid thermal pain was taken as an index of pain threshold. A cut off time of 15 sec was maintained42.
Cold allodynia
Cold allodynia was assessed by measuring paw (both ipsilateral and collateral) withdrawal latency (PWL), when dipped in water bath maintained at 40C 20C on weekly interval on 7th, 14 and 21st days after SNL. A cut off 15 sec was maintained during the experiment43.
Biochemical parameters:
Dissection and homogenization
On 21st day, animals were sacrificed by decapitation immediately after behavioral assessments. The complete sciatic nerve and brains were removed and 10% (w / v-1) tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4). Homogenate were centrifuged for 20 minutes at 15000 rpm and supernatant was used for estimation of lipid peroxidation and reduced glutathione levels. The post nuclear fractions for catalase assay are obtained by centrifugation of the homogenate at 1000 × g for 20 min, at 4°C and for other enzyme assays centrifuged at 12,000 × g for 60 min at 4°C.
Lipid peroxidation assay
The quantitative measurement of lipid peroxidation in the whole brain was measured according to the method of Wills44. The amount of malondialdehyde formed was measured by the reaction with thiobarbituric acid at 532 nm using Perkin Elmer lambda 20 spectrophotometer. The results were expressed as nanomole of malondialdehyde per milligram protein using the molar extinction coefficient of chromophore (1.56×10M¯¹ cm¯¹).
Estimation of reduced glutathione
Reduced glutathione in the brain was estimated according to the method of Ellman45. A 1.0 ml of homogenate was precipitated with 1.0 ml of 4% sulfosalicylic acid by keeping the mixture at 4°C for 1h and the samples were immediately centrifuged at 1200 ×g for 15 min at 4°C. The assay mixture contains 0.1ml of supernatant, 2.7 ml of phosphate buffer of pH 8.0 and 0.2 ml of 0.01M-dithiobisnitrobenzoic acid (DTNB). The yellow color developed was read immediately at 412nm using Perkin Elmer lambda 20 spectrophotometer. The results were expressed as nanomole GSH per milligram protein.
Nitrite estimation
Nitrite is the stable end product of nitric oxide (NO) in living system. Accumulation of nitrite was measured in cell free supernatants from brain homogenates by spectrophotometer assay based on Greiss reagent 15 (1% sulphanilamide/ 0.1% naphthylethylenediamine dihydrochloride/ 2.5% phosphoric acid) and incubated at room temperature for 10 min to yield a chromophore. Absorbance was read at 543 nm spectrophotometrically. The nitrite concentration was calculated from a standard curve using sodium nitrite as standard and expressed as micro molar nitrite per milliliter homogenate46.
Protein estimation
The protein content was measured according to the method of Lowry using bovine serum albumin as standard47.
Catalase estimation
Catalase activity was assayed by the method of Luck48, wherein the breakdown of hydrogen peroxide (H2O2) is measured at 240 nm. Briefly, the assay mixture consisted of 3 ml of H2O2 phosphate buffer and 0.05 ml of supernatant of tissue homogenate (10%), and the change in absorbance was recorded at 240 nm. The results were expressed as micromole H2O2 decomposed per milligram of protein/min.
Statistical Analysis
All the values are expressed as mean ± SEM. The data were analyzed by using one way analysis of variance followed by Tukey’s test. P< 0.05 was considered statistically significant.
Results:
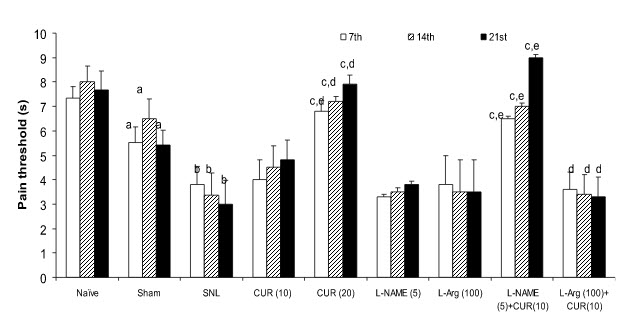
Figure 1. L-NAME and L-arginine modulation on the protective effects of curcuminagainst thermal hyperalgesia Values are expressed as mean ± SEM, n=5 rats per group, aP<0.05 as compared to naive group,bP<0.05 as compared to sham and naive,cP<0.05 as compared to Control (SNL), dP<0.05 as compared to Curcumin (10), eP<0.05 as compare to L-NAME (5).
Effects of Curcumin and its modulation by L-NAME and L-arginine on thermal hyperalgesia in SNL rats- Sciatic nerve ligation (SNL) significantly caused hyperalgesiaas reflected by shorten pain threshold (Jump or licking time latency) on weekly intervals as compared to naïve and sham group. Curcumin (20 mg/kg, po) treatment significantly reversed SNL induced hyperalgesia on weekly intervals as compared to control (SNL) (Figure 1). However, lower dose of curcumin (10mg/kg, po) did not show any significant effect as compare to control group (SNL). Further, L-NAME (5mg/kg) pretreatment with curcumin (10 mg/kg, po) significantly potentiated the protective effect of curcumin on 2nd and 3rd week which was significant as compared to their effect per se (Figure 1). Similarly, L-arginine (100 mg/kg) pretreatment with curcumin (10mg/kg, po) significantly caused hyperalgesic response on 2nd and 3rd week as compared to control (Figure 1). L-arginine (100 mg/kg, per se) treatment did not produce any significant effect on hyperalgesia even after 3weeks.

Figure 2. Effects of Curcumin and its interaction with L-NAME and L-arginine on cold allodynia Values are expressed as mean ± SEM, n=5 rats per group, aP<0.05 as compared to naive group,bP<0.05 as compared to sham and naive,cP<0.05 as compared to Control (SNL), dP<0.05 as compared to Curcumin (10), eP<0.05 as compare to L-NAME (5).
Effects of curcuminand its modulation by L-NAME and L-arginine on cold allodynia in SNL rats- Sciatic nerve ligation (SNL) significantly caused cold allodynia as reflected by decreased paw withdrawal latency on weekly intervals as compared to naïve and sham group. Curcumin (20mg/kg, po) treatment significantly delayed withdrawal latency on 3rd week as compared to control (SNL) (Figure 2). But lower dose of curcumin (10mg/kg, po) did not show any significance difference as compare to control (SNL). L-NAME (5mg/kg) pretreatment with curcumin (10 mg/kg, po) caused potentiation in antiallodynic effect of curcumin on 2nd and 3rd week which was significant as compared their effect per se. Further, L-arginine (100mg/kg,per se) treatmentdid not produce any significant antiallodynic like behavior as compared to control (SNL) (P < 0.05). However, L-arginine (100 mg/kg, ip) pretreatment with curcumin (10 mg/kg, po) significantly reversed its antiallodynic effect on 2nd and 3rd weeks as compared to their effect per se (Figure 2).
Table 1. Effects of Curcuminand its interaction with L-NAME and L-arginine on oxidative damage on sciatic nerves
|
Treatment |
LPO(nM of MDA/mgpr) (% of Control) |
Nitrite (μg/ml) (% o Control) |
GSH (µmoles of GSH/mg protein) (% of Control) |
Catalase (µ moles of H2O2 /min/mgpr) (% of Control) |
|
Naïve |
1.1±0.025 (52.63) |
20±0.29 (50.0) |
22±0.22 (200.0) |
4.023±0.4 (257.8) |
|
Sham |
1.23±0.024a (58.85) |
30±0.2a (75.0) |
20±.19a (181.1) |
3.12±0.71a (200.0) |
|
SNL |
2.09±0.024b (100) |
40±0.2b (100) |
11±0.27b (100.0) |
1.56±0.42b (100±34) |
|
CUR(10) |
1.8±0.051c (86.12) |
36.0±0.11c (90.0) |
12.1±0.33c (110.0) |
1.74±0.11c (111.53) |
|
CUR(20) |
1.18±0.012d (56.4) |
25.1±0.3d (62.75) |
17.1±0.11d (155) |
2.45±0.33d (157) |
|
L-NAME (5) |
1.94±0.01 (92.82) |
38±0.36 (95.0) |
13±0.21 (118.18) |
1.60±0.06 (102.56) |
|
L-arg (100) |
2.21±0.002 (105.74) |
43±0.42 (107.5) |
10.0±0.12 (90.9) |
1.44±0.045 (92.30) |
|
L-NAME (5) +CUR(10) |
1.32±0.04 d,e (63.15) |
24±0.56d,e (60) |
20±0.34d,e (181.8) |
2.65±0.086d,e (169.87) |
|
L-Arg (100) +CUR(10) |
1.88±0.034d (89.95) |
40.5±0.11d (101.25) |
9.42±0.01d (85.6) |
1.39±0.64d (89.10) |
Values are expressed as mean ± SEM, n=5 rats per group, aP<0.05 as compared to naive group,bP<0.05 as compared to sham and naive,cP<0.05 as compared to Control (SNL), dP<0.05 as compared to Curcumin (10), eP<0.05 as compare to L-NAME (5).
Effects of curcumin and its modulation by L-NAME and L-arginine on oxidative damage in SNL rats- Sciatic nerve ligation significantly caused oxidative damage as indicated by increased lipid peroxidation, nitrite concentration, depletion of reduced glutathione level and catalase activity in both sciatic nerve as well as whole brain respectively as compared to naïve or sham treated animals. Treatment with curcumin (10 and 20 mg/kg, po) significantly caused attenuation of lipid peroxidation, nitrite concentration and restoration of depleted reduced glutathione and catalase activity as compared to the control (SNL) both in brain and sciatic nerves respectively (P<0.05) (Table 1 and Table 2).
Table 2. Effects of Curcuminand its interaction with L-NAME and L-arginine on oxidative damage in SNL brain
|
Treatment |
LPO(nM of MDA/mgpr) (% of Control) |
Nitrite (% of Control) |
GSH (µg/mg of protein) (% of Control) |
Catalase (µ moles of H2O2 /min/mgpr) (% of Control) |
|
Naïve |
3.12±0.63 (53.6) |
20±0.25 (50) |
21.6±0.15 (466) |
5.81±0.5 (352.12) |
|
Sham |
3.11±0.4a (53.4) |
20±0.28a (50) |
22±0.19a (488) |
4.82±0.58a (292) |
|
SNL |
5.82±0.58b (100) |
40±0.22b (100) |
4.5±0.16b (100) |
1.65±0.56b (100) |
|
CUR(10) |
5.1±0.14c (87.6) |
37±0.31c (92.5) |
6.2±0.05c (137.7) |
1.6±0.41c (96.9) |
|
CUR(20) |
3.89±0.02d (66.8) |
29±0.11d (72.5) |
9.1±0.03d (202.2) |
3.21±0.22d (194) |
|
L-arg (100) |
6.01±0.08 (103.26) |
45±0.34 (112.5) |
3.9±0.18 (86.66) |
1.41±0.03 (85.45) |
|
L-NAME (5) |
5.41±0.65 (92.95) |
33±.11 (82.5) |
5.3±0.35 (117.7) |
1.91±0.08 (115.75) |
|
L-Arg (100) +CUR(10) |
5.6 ± 0.55d (96.21) |
41 ± 0.11d (102.5) |
3.1 ± 0.66d (68.8) |
1.78 ± 0.28d (107.8) |
|
L-NAME (5) +CUR(10) |
3.42±0.04ef (58.76) |
25±0.21ef (62.5) |
11.8±0.8ef (262.2) |
4.1±0.38ef (248.4) |
Values are expressed as mean ± SEM n=5 rats per group: aP<0.05vs naive group,bP<0.05 as compared to control group,c P<0.05 as compared to Curcumin (10),dP<0.05 as compared to l-arginine (100),ep≤0.05 as compare to Curcumin (20),fp≤0.05vsl-NAME (5)
L-NAME (5mg/kg) pretreatment with lower dose of curcumin (10 mg/kg, po) caused potentiation in the antioxidant effect of curcumin both in brain and sciatic nerves which was significant as compared their effect per se ( Table 1 and Table 2). However, L-NAME (5mg/kg, per se) treatment did not influence significantly these oxidative stress parameters as compared to SNL group in both brain and sciatic nerves. Further, L-arginine (100 mg/kg/ip) pretreatment with curcumin (10 mg/kg, po) significantly reversed the protective effect of curcumin in both brain and sciatic nerve (Table 1 and Table 2). L-arginine (100 mg/kg, per se) did not produce any significant effect on oxidative stress parameters as compared to SNL group in both brain and sciatic nerves.
Discussion:
Neuropathic pain is a complexdebilitating and chronic disease that usually is accompanied by tissue injury49. Peripheral nerve injury produces a persistent neuropathic pain state characterized by spontaneous pain, allodynia and hyperalgesia50. With neuropathic pain, the nerve fibers themselves may be damaged, dysfunctional or injured. These damaged nerve fibers send incorrect signals to other pain centers51. In present study, unilateral sciatic nerve ligation induced significant behavioral and biochemical alterations resulting ipsilateral cold allodynia, thermal hyperalgesia and oxidative damage both in sciatic nerves as well as brain.
In the present study, partial sciatic nerve ligation (SNL) caused thermal hyperalgesia as well as cold allodynia in response to non-noxious cold stimulus which was more significant in 2nd and 3rd week of SNL. Studies have also reported that partial ligation of sciatic nerve induced hyperalgesia and cold allodynia like behavior in animals52.Reports further suggest the mechanism of hyperalgesia action could be due to sensitization of primary afferent nerves53. In the present study, partial sciatic nerve ligation significantly raised lipid peroxidation, nitric concentration and depletion of reduced glutathione and catalase activity suggesting the involvement of oxidative damage during neuropathic pain.
In the present study, chronic treatment with curcumin significantly reversed thermal hyperalgesia, cold allodynia in sciatic nerve ligated animals indicating its therapeutic potential against neuropathic pain. The results of our study coincided with the earlier works which proved the analgesic activity of curcumin in neuropathic pain54. These findings suggest that curcumin plays an important role in pain regulation at central and peripheral level. Studies also reported the therapeutic potentials of curcumin against hyperalgesia and related states55.
Curcumin exhibit differential antioxidant activity in several in vitro and in vivo models56. The significance of Curcuma longa Linn (Turmeric) in health and nutrition has changed considerably since the discovery of the antioxidant property of naturally occurring phenolic compounds, curcuminoids. Earlier studies have shown that curcumin inhibits reactive oxygen species (ROS) production57 as well as calcium entry58 It can also affect different other cellular processes such as activation of apoptosis, inhibition of platelet aggregation, inhibition of inflammatory cytokine production, inhibition of cyclooxygenase and lipoxygenase isoenzymes. It may also affect the activity of different key enzymes such as PKC, protein tyrosine kinases and calcium dependent endonuclease59. In present study, curcumin treatment significantly attenuated oxidative damage in both sciatic nerves as well as in brain. Curcumintreatment significantly attenuated rise in lipid peroxidation, nitrite level and restored the depleted reduced glutatathione and catalase activity, suggesting its antioxidant like action against SNL. Although, pathogenesis of neuropathic pain is not clear so far. Recent evidences suggested that antioxidant agents could be used to reverse peripheral nerve injuries60.
Further, nitric oxide is an unconventional intercellular messenger in the nervous system. NO reacts with reactive oxygen species and acts as an oxidant61. The oxidation is not specific and affects any cell molecule. In the present study, pretreatment with L-arginine, nitric oxide precursor produced hyperalgesia, allodynic like conditions and oxidative damage suggesting the involvement of nitric oxide pathway in sciatic nerve ligation induced behavioral. Studies reported the involvement of nitric oxide cascade in the pathogenesis of neuropathic pain62. However, an exact molecular and cellular cascade in the pathogenesis of neuropathic pain is still unclear. Besides, role of reactive oxygen species–reactive nitrogen species (ROS–RNS) and its interaction with NO in the regulation of stress responses are not clearly understood so far. Further, L-NAME pretreatment with curcumin caused potentiation of antihyperalgesic, antiallodynic and antioxidant like effect of curcumin, suggesting the possible involvement of NO pathway in its protective action. It seems nitric oxide mechanism is involved in the protective action of curcumin against sciatic nerve ligation induced neuropathic pain like states and oxidative damage.
In summary, nitric oxide mechanism could be involved in the protective action of curcumin against SNL induced neuropathic pain and oxidative damage. Study further suggests the potential of curcumin in effect management of neuropathic pain and related conditions.
References:
1. Attal N, Bshassria D: Can pain be more or less neuropathic? Pain 2004; 110: 5101.
2. Rajalu E, Waltisperger S, Doridot P, Poisbeau M, Freund-Mercier M: Sciatic nerve cuffing in mice: A model of sustained neuropathic pain. Eur J P 2001; 12(5): 591 - 599.
3. Gila moalem, davidj Tracey: Immune and inflammatory mechanism in neuropathic pain. Behav Brain Res Review 2006
4. Bridges D, Thompson SW, Rice AS: Mechanisms of neuropathic pain. Br. J Anaesth 2001; 87: 12–26.
5. Dyck PJ: Current concepts in neurology. The causes, classification, and treatment of peripheral neuropathy. N Engl J Med 1982; 307 (5): 283-6.
6. Zimmermann M: Pathobiloglogy of neuropathic pain. Eur J Pharmacol 2001; 429: 23-37.
7. Michael I, Bennett, Blair H, Nicola T, Amanda j: Can pain be more or less neuropathic? Comparison of symptom assement tools with rating of certainty by clinicians. Pain 2006; 122: 289-296.
8. McQuay HJ, Tramer M, Nye BA: A systematic review of antidepressants in neuropathic pain. Pain 1996; 68: 217–27.
9. Price DD, Bennett GJ: Psychophysical observations on patients with neuropathic pain relieved by a sympathetic block. Pain 1989; 36:273-88.
10. Muthuraman A, Jaggi AS, Singh N, Singh D: Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol 2008; 587(1-3):104-11.
11. Kim HK, Park SK, Zhou JL, Tagliatela G, chung K, Coggeshall RE, chung J M: Reactive oxygen species plays an important role in neuropathic pain. Pain 2004; 111:116-124.
12. Sreejayan N, Rao MNA, Priyadarsini KI, Devasagayam T PA: Inhibition of radiation-induced lipid peroxidation by Curcumin. Int J Pharm1997; 151(1) 12:127-130.
13. Bhaumi KS, Jyothi MD, A. Khar: Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett 2000; 483:78–82.
14. Johnston BD, DeMaster EG: Suppression of nitric oxide oxidation to nitrite by curcumin is due to the sequestration of the reaction intermediate nitrogen dioxide, not nitric oxide. Nitric Oxide 2003; 8:231–234.
15. Joe B, Rao UJ, Lokesh BR: Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol Cell Biochem 1997; 169:125–34.
16. Satoskar RR, Shah SJ, Shenoy SG: Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol 1986; 24:651–4.
17. Jiang MC, Yang-Yen HF, Yen JJ, Lin JK: Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr Cancer 1996; 26:111–20.
18. Jagetia GC, Aggarwal BB: Spicing up, of the immune system by curcumin. J Clin Immunol 2007; 27:19–35.
19. Aggarwal BB, Harikumar KB: Potential therapeutic effect of curcumin, the anti-inflammatory agent, agains neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 2008.
20. Ramsewak RS, DeWitt DL, Nair MG: Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I–III from Curcuma longa. Phytomedicine 2000; 7:303–8.
21. Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R: Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 1995; 94:79–83.
22. Sugiyama Y, Kawakishi S, Osawa T: Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem Pharmacol 1996; 52:519–25.
23. Kim HK, Park SK, Zhou JL, Tagliatela G, chung K, Coggeshall RE, chung JM: Reactive oxygen species plays an important role in neuropathic pain. Pain 2004; 111:116-124.
24. Yu BP: Cellular defenses against damage from reactive oxygen species. Physiol Rev 1994; 74:139–162.
25. Fridovich I: Oxygen toxicity: a radical explanation. J Exp Biol 1998; 201:1203–1209.
26. Terence J, Coderre, Dimitris N, Xanthos, Laura Francisc, Gary J, Bennett: Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-Type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain 2004; 112 94–105.
27. Kim HK, Park SK, Zhou JL, Tagliatela G, chung K, Coggeshall RE, chung J M Reactive oxygen species plays an important role in neuropathic pain. Pain 2004; 111:116-124.
28. Khalil Z, Liu T, Helme R: Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal in rats with chronic constriction injury. Pain 1999; 79:31–37.
29. Fan L, Zuo X, Chen ZW, Fang M, Dai LM, and Song BW: The role of hydroxyl radical in pain perception. Chin J of pain Med 2003; 9:23–27.
30. Kitto KF, Haley JE, Wilcox GL: Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett 1992; 148:1–5.
31. Hao JX, Xu XJ: Treatment of a chronic allodynia-like response in spinally injured rats: effects of systemically administered nitric oxide synthase inhibitors. Pain 1996; 66:313–319.
32. Nohl H: Involvement of free radicals in ageing: a consequence or cause of senescence. Br. Med. Bull 1993; 49:653–667.
33. Gordh T: The role of nitric oxide in neuropathic pain and neurodegeneration. Acta Anaesthesiol. Scand1998; 42:29–30.
34. Meller ST, Pechman PS, Gebhart G F, Maves T J: Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience 1992; 50:7–10.
35. Wiesenfeld-Hallin Z, Hao J X, Xu X J, Hokfelt T: Nitric oxide mediates ongoing discharges in dorsal root ganglion cell after peripheral nerve injury. J. Neurophysiol 1993; 70:2350–2353.
36. Levy D, Hoke A, Zochodne D W: Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci. Lett 1999; 260:207–209.
37. Levy D, Zochodne D W: Local nitric oxide synthase activity in a model of neuropathic pain. Eur. J. Neurosci 2001; 10:1846–1855.
38. Choi Y, Raja SN, Moore LC, Tobin JR: A neuropathic pain rat is associated with altered nitric oxide synthase activity in neural tissue. J Neurol Sci 1996; 138:14–20.
39. Bennett GJ, Xie YK: A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33:87-107.
40. Kwang Jin, Kim Young, Wook Yoon and Jin Mo Chung: Comparison of three rodent neuropathic pain models. Exp Brain Res 1997; 113:200-206.
41. Kumar A, Singh A: Possible nitric oxide modulation in protective effect of (Curcuma longa, Zingiberaceae) against sleep deprivation-induced behavioral alterations and oxidative damage in mice. Phytomedicine 2008; 15(8):577-86.
42. Osikowicz M, Makuch W, Przewlocka BMika J: Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic. Pain. 2008 Apr 26.[ Epub ahead of print]
43. Dyck PJ: Current concepts in neurology. The causes, classification, and treatment of peripheral neuropathy. N. Engl. J. Med 1982; 307 (5):283-6.
44. Wills ED: Mechanism of lipid peroxide formation in animal tissues. Biochem J 1966; 99: 667–676
45. Ellman GL: Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82: 48670–48677.
46. Green LC, Wagner DA, Glagowski J: Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem1982; 126: 131–138.
47. Lowry OH, Rosenberg NJ, Farr AL, Randall RJ: Protein measurement with the Folin-phenol reagent. J Biol Chem 1951; 193: 265–275.
48. Luck H Catalase. In: Methods of Enzymatic Analysis. Bergmeyer HU, ed. Academic Press, New York, 1971; 885-893.
49. Chung JM, Chung K: Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain 2002; 2:87–97.
50. Seltzer Z, Dubner R, Shir Y: A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990; 43:205–18.
51. Regan RF: The vulnerability of spinal cord neurons to excitotoxic injury: comparison with cortical neurons. Neurosci Lett 1996; 213:9–12.
52. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM: Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59:369–76.
53. Seltzer Z, Dubner R, and Shir Y: A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990; 43: 205–218.
54. Sharma S, Kulkarni SK, Agrewala JN, Chopra K: Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. European Journal of Pharmacology 2006; 536:256–261.
55. Kaur G, Tirkey N, Bharrhan S, Chanana V, Rishi P, Chopra K: Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatoxicity in rodents. Clin Exp Immunol 2006; 145(2):313-21.
56. Subramanian M, Sreejayan, Rao MN, Devasagayam TP, Singh BB: Diminution of singlet oxygen-induced DNA damage by curcumin and related antioxidants. Mutation Res 1994; 311:249–55.
57. Ramsewak RS, DeWitt DL, Nair MG: Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I–III from Curcuma longa. Phytomedicine 2000; 7:303–8.
58. Balasubramanyam M, Kimura M, Aviv A, Gardner J P: Kinetics of calcium transport across the lymphocyte plasma membrane. Am J Physiol 1993; 265:321–327
59. Merhi M, Dusting GJ, Khalil Z: CGRP and nitric oxide of neuronal origin mediate neurogenic vasodilatation in rat skin microvasculature. Br J Pharmacol. 123:863–868.
60. Naik AK, Tandan SK, Dudhgaonkar SP: Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J Pain 2006; 10:573.
61. R.C. Babbedge, S.L. Hart, P.K. Moore: Anti-nociceptive activity of nitric oxide synthase inhibitors in the mouse: dissociation between the effect of L-NAME and L-NMMA. J Pharm Pharmacol 1993; 45:77–79.
Reference ID: PHARMATUTOR-ART-1026









