About Authors:
D.Ramadevi and S.Ganapaty*
Department of Pharmacognosy and Phytochemistry,
College of Pharmaceutical Sciences, Andhra University,
Visakhapatnam -530 003, India
Abstract
The fusidic acid(ent-16α-acetoxy-3β-dihydroxy-4β,8β,14α-trimethyl-18-nor-5β,10α-cholesta-(17z)-17(20),24-dien-21-oic acid) together with known compounds β-sitosterol, 2-methylanthraquinone, scopoletin, betulinic acid, corchoroside –A and cannogenol from the root extract of Corchorus olitorius. Out of these compounds, 2 - methyl anthraquinone and fusidic acids were new to the genus Corchorus and reported for the first time from C.olitorius. The triterpenoid antibiotic fusidic acid was obtained earlier from a fungi (Fusidium coccineum) , is now reported from the plant C.olitorius. Occurance of coumarins in Corchorus genus are rare, but the author could isolate scopoletin, a coumarin from this species.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1171
Introduction
Corchorus olitorius L, belong to the family Tiliaceae is an annual, much-branched herb 90-120 cm tall, leaves 6-10 cm long, flowers are pale yellow. Capsules 3-6.5 cm long1 The Corchorus olitorius is reported to have anti-inflammatory, antiviral, antibacterial and anticancer activities.2-3Earlier, a good number of secondary metabolites were isolated and characterized from this plant. Cardiac glycosides like corchorosides, terpenoids like betulinic acid, kaempferol like flavonoids were reported from this plant.4-59
Plant material
The roots of Corchorus olitorius, were collected from Warangal in September 2007 (2kg) and was authenticated by Prof.V.S. Raju, Department of Botany, KaKatiya University, Warangal. A specimen was deposited in the Herbarium (Voucher specimen number (CO/07) roots were collected from the plant and dried under shade.
Extraction
The roots of Corchorus olitorius (2kg) were air dried and coarsely powdered in a Wiley mill and successively extracted with petroleum ether (3×3 l), chloroform (3×3 l) and methanol (3×3 l) and concentrated under reduced pressure. The petroleum ether, chloroform extracts of C. olitorius roots shown similar spots on TLC (1:1 Benzene: Chloroform) and hence combined and column chromatographed over silica gel (Acme 100 mesh), which afforded three compounds named as COR-1, COR-2, and COR-3. The methanolic extract showed positive Liebermann-Burchard test for terpenoids and Kedde test for cardiac glycosides. On column chromatography the methanolic extract gave four compounds COR-4, COR-5, COR-6 and COR-7.
Characterization of isolated compounds:
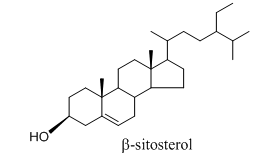
COR-1(β-sitosterol, 30mg)
}The compound was crystalized from petroleum ether as a colorless needles, m.p 136-138°C. It gave green color for sterols with Liebermann-Burchard test. The UV (MeOH) λmax 205 nm; EIMS m/z 414 [M]+(calc. for C29H50O). 1H NMR (CDCI3, 400 MHz): δH3.52 (1H, m, H-3), 5.35 (1H, m, H-6), 0.68 (3H, s, Me-18), 0.98 (3H, s, Me-19), 0.91 (3H, d, J = 6.4 Hz, Me-21), 0.83 (3H, d, J = 6.8 Hz, Me-26), 0.81 (3H, d, J = 6.9 Hz, Me-27), 0.85 (3H, t, J = 7.8 Hz, Me-29). 13CNMR (CDCI3, 100 MHz): δC37.4 (C-1), 31.8 (C-2), 72.0 (C-3), 42.5 (C-4), 140.9 (C-5), 121.9 (C-6), 32.1 (C-7), 29.9 (C-8), 50.3 (C-9), 36.7 (C-10), 21.3 (C-11), 40.0 (C-12), 42.5 (C-13), 56.9 (C-14), 24.5 (C-15), 28.4 (C-16), 56.2 (C-17), 12.0 (C-18), 19.6 (C-19), 36.3 (C-20), 19.0 (C-21), 34.1 (C-22), 26.3 (C-23), 46.0 (C-24), 29.3 (C-25), 20.0 (C-26), 19.2 (C-27), 23.2 (C-28), 12.2 (C-29). Based on the spectral data the compound COR-1 was identified asβ-sitosterol60,61
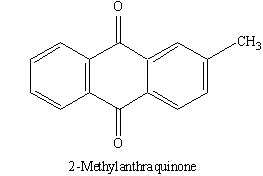
COR-2 (2-methylanthraquinone, 25mg)
It was obtained as an yellow solid, crystallized as needles in methanol: chloroform mixture. mp 170 - 173º C and answered with Kedde’s reagent, negative with LB reaction. Rf 0.72 in CDCl3 : MeOH (19:1) and Rf 0.37 in petroleum ether : acetone : acetic acid (75:25:1.5) and 1HNMR (250 MHz, CDCl3) : 2.47 (3H, s, –CH3), 7.53, (1H,d, J=7.8 Hz, H-3), 7.71–7.73 (2H, m, H-5, 8), 8.04 (1H,s ,H-1), 8.14 (1H, d, J = 7.8 Hz, H-4), 8.23–8.25 (2H, m, H-6, 7). 13 C-NMR: (125 MHz, CDCl3): 127.8 (C-1), 145.5 (C-2), 135.3 (C-3), 127.7 (C-4), 127.4 (C-5), 134.3 (C-6), 134.2 (C-7), 127.5 (C-8), 183.7 (C-9), 183.3 (C-10), 22.1 (CH3). The data corresponded well with that of 2-methylanthraquinone, andfurther confirmed by comparison with an authentic sample by m.m.p and co-TLC.62-69 This is the first report of this compound from C.olitorius and particularly from the genus Corchorus.
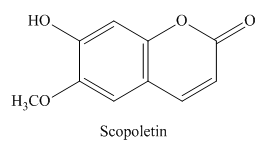
COR-3 (Scopoletin, 80mg)
This compound was obtained as yellow crystal, mp: 202-204 °; IR (KBr) λ max cm-1: 3340, 3106, 3031, 2990, 1710, 1600; UV max (MeOH) nm: 230, 254, 260, 298, 346 ; FAB-MS m/z:193 [M-H]-; 1H-NMR and 13C-NMR spectral data were in accord with the molecular formula C10H8O4. The 1H-NMR spectrum revealed the presence of two doublets at δH 7.90(H-4, J = 6.0 Hz) and 6.25 (H-3, J = 6.0 Hz). The two singlet signals appeared at δ H 6.80 and 7.25 were assigned for the two protons H-5 and H-8, respectively. 1H-NMR: (CD3OD): d 3.90 (3H, s, 6-OCH3), 6.19 (1H, d, J = 9, H-3), 6.75 (1H,s, H-8), 7.09 (1H, s, H-5), 7.84 (1H, d, J = 9, H-4). 13 C-NMR :(CD3OD): d 56.8 (OMe), 104.0 (C-8), 109.9 (C-5), 112.6 (C-3 and C-4a), 146.1 (C-4), 147.1 (C-6), 151.4 (C-8a), 153.0 (C-7), 164.1 (C-2).70-75 Based on the spectral data and chemical tests, the compound was identified as scopoletin.
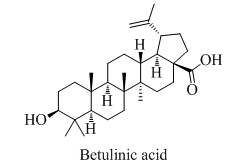
COR-4 (Betulinic acid, 20mg)
It was crystallized from chloroform as white fluffy needles, m.p 276-278°C. It gave positive Liebermann-Burchard test (pink colour) for terpenoids. This observation was supported by ms: m/z 456[M]+, 423, 411, 410, 342, 248, 220, 207, 203, 189, 143, 69 suggested the molecular formula C30H48O3. IR (KBr, cm-1) : 3385 (OH), 3350 (COOH), 1715 cm-1 (C=O); 1H NMR (δ, CDCl3) : 4.56 and 4.68 (=CH2), 1.68 (s, =C-CH3), 2.30 (m, H-19) 3.27 (dd, H-3α), 0.76 (s, 3H), 0.78 (s, 3H), 0.82 (s, 3H), 0.96 (s, 3H), 1.03 (s, 3H) for five tertiary methyl groups; 13C NMR (δ, CDCl3) : 38.7 (C-1), 27.4 (C-2), 78.9 (C-3), 38.8 (C-4), 55.3 (C-5), 18.3 (C-6), 34.3 (C-7), 40.7 (C-8), 50.5 (C-9), 37.2 (C-10), 20.8 (C-11), 25.5 (C-12), 38.4 (C-13), 42.4 (C-14), 30.5 (C-15), 32.1 (C-16), 56.3 (C-17), 46.8 (C-18), 49.2 (C-19), 150.3 (C-20), 29.7 (C-21), 37.0 (C-22), 27.9 (C-23), 15.3 (C-24), 16.0 (C-25), 16.1 (C-26), 14.7 (C-27), 180.5 (C-28), 09.6 (C-29), 19.4 (C-30). The above mentioned spectral data were in close agreement with literature value of betulinic acid. Thus, compound COR-4 was characterized as betulinic acid 76,77
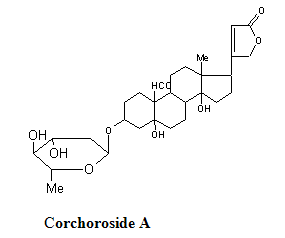
COR-5(Corchoroside A)
The compound was obtained from 20% methanol in chloroform fraction of the columnand was crystallized from methanol-ethyl acetate as colourless prisms. M.p. 164-168oC; [α]D = +19.7o (methanol), it showed positive kedde and legal reactions indicating the cardinolide nature of the compound. This observation was supported by U.V. spectrum (ethanol) which showed maxima at 218 nm and 298 nm confirming the presence of α,β unsaturated γ-lactone group and a carbonyl group. It was analyzed for the formula (C29H42O9) and formed a diacetate.1H NMR : δ 0.84 (3H,s,H3 -18) , 1.22 (3H, d, J=6.5Hz ,boi H3-6), 2.82 (1H, m,H-17), 3.21 (1H,m, boi H-3 ), 4.17 (1H,m, H-3), 4.87 (1H,dd,J=2.0 , 10.0 Hz ,boi H-1). 4.90. 5.02 (each 1H, dd, J=2.0. 18.5 Hz , H2 -21),5.89 (1H,s, H-22), 10.04 (1H, s, H3-19).78-80 Based on the spectral data ,the compound was identified as corchoroside –A.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
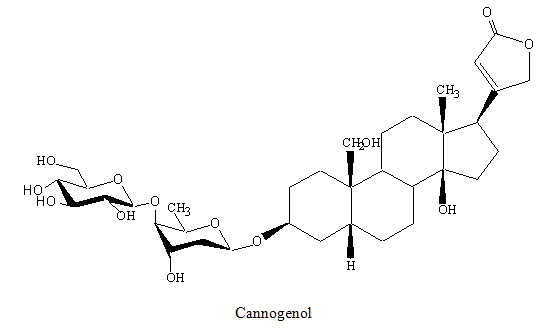
COR -6 (Cannogenol )
It was obtained as white amorphous powder in 30% methanol: chloroform. It showed positive kedde and legal reactions indicating the cardinolide nature of the compound. In the high resolution negative ion FAB mass spectrum , COR-6 showed a [M-H]- ion peak at m/z 681.3436 .The fragment ion peaks of low resolution FABMS,m/z 519 for [M-H-162]- and 389 for [aglycone –H]-, were observed. COR-6 had more mass units from digitoxigenin, and one extra hydroxyl group in the aglycone. The C-19 signal of COR -6was observed at δ 66.0 shifted by +41.7 ppm. The signals of C-1 (δ 24.8, -6.6 ppm), C-5 (δ 30.2 7.7 ppm) and C-10 (δ 40.4,-4.1 ppm) were significantly shifted. (Table-2.1.2) The 1H NMR, H-H COSY and HMQC spectra, two protons at the 19-position were assigned at δ3.41 and 3.81 .These data indicated that the aglycone was cannogenol which had one hydroxyl at C-19 [81,82]. The 1H NMR showed signals as δ 0.88 (3H,s, H3 -18),1.25(3H.d,J=6.5Hz, bio H3 -6),2.82 (1H, m, H-17), 3.41, 3.81 (1H,d,J=11Hz, H2-19 ),3.45 (1H, m, boi H-4 ), 3.65 (1H, dd, J=5.5, 12.0 Hz , glc H-6b), 4.04 (1H, m, H-3 ), 4.15 (1H, br.q, boi H-3), 4.31 (1H, d, J=8.0Hz, glc H-1), 4.88 (1H, dd, J=1.5,18.5Hz, H2-21), 5.89 (1H,s,H-22). Based on the data, the compound was identified as cannogenol.
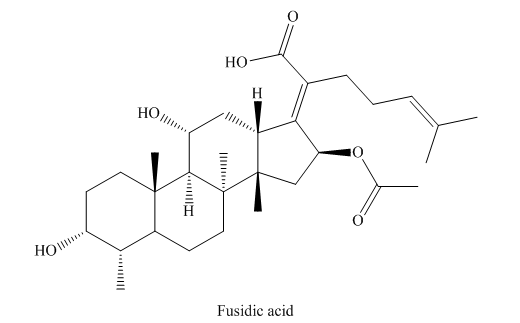
COR-7 (Fusidic acid, 60mg)
It was obtained as colourless substance, m.p.190-1920C . The 1H and 13C NMR spectra of the compound showed signals for five methyl singlets, one methyl doublet, four olefinic carbons and a carbonyl of a carboxylic acid (d, C 173.8), were indicative of a fusidane of the fusidic acid skeleton. Assuming that the methyl doublet was C-28 of the fusidane skeleton, the protons of this group coupled to a methine proton (d,H 1.54, H-4) in the COSY spectrum. H-4 formed part of a spin system with a deshielded methane (d,H 3.73, H-3) and two methylene groups (at C-2 and C-1). In the HMBC spectrum, C-1 was coupled to by the protons of methyl-C19 (d,H 0.95) which showed further couplings to C-10 (2J), C-9 (3J) and C-5 (3J). In the COSY spectrum, H- 5 (d,H 2.31 m) coupled to both protons of a methylene moiety (C-6, d,H 1.45, 1.67), Inspection of the HMBC spectrum showed that the carbon associated with this deshielded proton was coupled to by the protons of a further angular methyl singlet (C-30), which showed additional couplings to a methine carbon (C-9) and two quaternary carbons (C-8, dC 45.6 and C-14, d C 49.6). This completed the resonances for the A and B rings of compound. Inspection of the COSY spectrum showed that the proton associated with C-9 (H-9) formed part of a CH–CH–CH2–CH spin system which allowed identification of positions C-9, C-11, C-12 and C-13, respectively. C-11 was deshielded (d, C 68.7, d,H 4.37) indicating that an oxygen should be placed here. H-13 was also deshielded (d,H 3.05) suggesting that it was allylic and that an olefinic carbon (C-17) should be placed at the neighbouring carbon, which is typical for fusidic acid .The protons of a methyl group (C-18) coupled to C-13 (3J), C-14 (2J) and to a methylene carbon (C-15, 3J). CH2-15 coupled to a deshielded allylic methylene group (d,H 2.68, 2.86 (CH2- 16)) which again was supportive of being alpha to an olefinic carbon (C-17, dC 160.4). This completed rings C and D of 1. H-13 and H2-16 both gave a 2J coupling to C-17 and a 3J coupling to C-20, suggesting a C-17, 20 double bond. In the HMBC spectrum C-17 was also coupled to by the protons of an allylic methylene (C-22, d,H 2.44) which also coupled to a carbonyl carbon of a carboxylic acid group (C-21) and an olefinic methine carbon (C-24, dC 124.0). A further methylene (C-23) could be placed between C-22 and C-24 by couplings observed in the COSY spectrum. Finally, two deshielded geminal methyl groups could be placed on an olefinic carbon (C-25) via their HMBC correlations to this carbon and to the olefinic partner C-24 finalising the C-24–C-25 double bond. These resonances completed the eight carbon chain of the fusidane triterpene skeleton. Chemical shift of the C-21 carbon, a carboxylic acid must be placed at C-21 and this is identical to the fusidic acid. 83-109
Results and Discussion
The chemical examination of the roots of C.olitorius on conventional extraction and a sequence of chromatographic methods afforded seven compounds.. These are characterized as β-sitosterol, 2-methylanthraquinone, scopoletin, betulinic acid, corchoroside –A , cannogenol and fusidic acid. Out of these compounds, 2 - methyl anthraquinone and fusidic acids were new to the genus Corchorus and reported for the first time from C.olitorius. Occurance of coumarins in Corchorus genus are rare, but the author could isolate scopoletin, a coumarin from this species.
References
1. Cochorus L.Germplasm Resources information Network. United states Department of Agriculture.,06,05 (2003). Retrieved,03,13 (2009).
2. S. Negm, O.EI-Shabrawy, M.Arbid and Radwan, A.S.Zeitsc. Ernaehrungsw. 19(1),28-32 (1980).
3. A. Sharaf, S.H. Kamel, A. Salama and M.S. Arbid, Egyptian J.Vet.,Med.,14 (2):87-93(1979).
4. K.Azuma Nakayama, M. Koshioka ,M. Ippoushi ,K. Yamaguchi, Y. Kohata Yuji Yamauchi ,K. Ito, H. Higashio, H. J. Agric. Food Chem, 47, 3963–3966 (1999)
5. K .K. Mukherjee, S. K. Mitra and S. N. Ganguly, Natural Product Science, 4, 51–52,(1998) .
6. M. Yoshikawa, M. Murakani H. Shimada, N. Fukada, H. Matsuda, Y. Sashid, and Yamahara .,J, Heterocycles, 48, 869–873 (1998)
7. A.Z. Tulio,J.r. Ose, K. Chachin, Y.Ueda., Postharvest Biology and Technology, 26, 329-338 (2002)
8. A.H.S Abou Zeid., Food Chemistry, 76, 187-195 (2002).
9. H. Matsufuji , S. Sakai, M. Chino, Y. Goda, M. Toyoda, M. Takeda., Journal of Health Science, 47(2) 89-93(2001).
10. M. Gupta, U.K. Mazumder, D.K. Pal, S. Bhattacharya., Journal of Ethnopharmacology, 89, 55-59 (2003).
11. D.K. Pal , M. Mandal, G.P Senthilkumar and A. Padhiari., Fitoterapia, 77(7-8), 589-91(2006).
12. T. Nakamura, Y.Goda, S. Sakai, K. Kondo, H. Akiyama, M. Toyoda, Phytochemistry, 49, 2097-2101(1998)
13. S .B. Mahato, N. P. S. K. Sahu, Roy and, B. K. Pramanik J. Chem. Soc. Perkin Trans, I, 2065–2068(1989).
14. V.A Maslennikova and N. K. Abubakirov Khim. Prir. Soedin, 11, 525–526 (1975).
15. M. T. Turakhozhaev , L. P. Zubkova , M .I. Shamsutdinov G. L. Genkina, and T .T. Shakirov. Khim. Prir. Soedin, 8, 81–83 (1972).
16. E. V. Rao, K .N .Rao , and D. V. Rao, Curr. Sci, 42, 731–732 (1973).
17. T. Nakamura, Y. Goda, S. Sakai, K. Kondo, H. Akiyama and M.Toyoda, Phytochemistry, 49, 2097–2101 (1998).
18. Y. Goda, S. Sakai, T. Nakamura, K. Kondo, H. Akiyama and M. Toyoda ., J. Food Hyg. Soc, 39, 415–420 (1998).
19. Y. Goda, S. Sakai, T. Nakamura , H. Akiyama and Toyoda, M., J. Food Hyg. Soc, 39, 256–265 (1998).
20. M. Yoshikawa , T. Murakami, H. Shimada, N. Fukuda , H. Matsuda , Y Sashida and Yamahara., J, Heterocycles, 48, 869–873 (1998).
21. G .Soliman and W. Saleh., J Chem Soc, 2198-2200 (1950).
22. A.Alam , A. Khalique and M. Ahmed., Curr Sci, 23,332 (1954).
23. N .K. Sen and J .K. Chakravarti, Sci and Cult, 19,102(1953).
24. J .K. Chakravarti and N .K.Sen, J Am Chem Soc, 76, 2390-2392(1954).
25. V. K. Orlov and G .V. Lazu, The Zhur Khim, 27,3-10 (1957)
26. V. K. Orlov, The Zhur Khim, 1,123-128 (1958)
27. M. Frerejacque, and M, Durgeat Compt. Rend, 238,507-509 (1954)
28. N. K. Abubakirov, V. A. Maslennikova and M .B. Gorovits, Zhur Obshchei Khim, , 28, 2279-2283(1958).
29. N .K. Abubakirov , V. A .Maslennikova, and , M. B. Gorovits Chem Abstr, 53, 6290 (1959).
30. N .K. Abubakirov, V .A .Maslennikova, and M. B. Gorovits , Zhur Obshchei Khim, 29,1235-1240 (1959).
31. N. K. Abubakirov, Zh Biol Khim Absts, 3F 833 (1965).
32. R .D .Samilova, and T .A. Lagodich , Vrach Delo, 1,27-31(1977).
33. P .M. Loshkarev , Aromat Rast, 15,618-628 (1969).
34. G. Ferrari, and C. Casagrande, Chem Abstr, 70,38053(1969).
35. E .V. Rao, K. N. Rao and D .V. Rao, Indian J Pharm, 34,168(1972)
36. G. Yukihiro , N. Takatoshi, S. Shinobu, M. Hiroshi , K. Kazunari , Hiroshi, and A. Masatake, T. Tennen Yuki Kagobutsa Toronkai Koen Yoshishu, 40, 371-376 (1998).
37. N. Takatoshi , G. Yukihiro, S. Shinobu, K. Kazunari, A. Hiroshi , and T. Masatake, Phytochemistry, 49,2097-2101(1998).
38. M. Yoshikawa, T. Murakami, H. Shimada , N. Fukada , H. Matsuda, Y. Sashida , .and Yamahara .,J, Heterocycles, 48,869-873(1998).
39. G. Yukhihiro, S. Shinobu , N. Takatoshi, A. Hiroshi , and T. Masatake , J Food Hyg Soc Japan, 39,256-265(1998).
40. D. V. Rao, and E.V. Rao, Phytochemistry, 14, 533-537 (1975).
41. E .V. Rao, D. V. Rao, S. K. Pavanaram , E .J . Von. and T. Reichstein, Helv Chim Acta, 54,1960-1968(1971).
42. A. Qudrate- i- Khuda, M. Khalique and D .C. Das , Sci Res, 2,152-159(1965).
43. K. Ohtani, , K. Okai U. Yamashita, I. Yuasa and, A. Misaki Biosci Biotechnol Biochem, 59,378-381 (1995).
44. H. Kohda, S. Tanaka, Y. Yamaoka, S. Moringa , O. Yhara , Nat Med, 48,213- 214 (1994).
45. M. Yoshikawa, H. Shimada, M. Saka , S. Yoshizumi, J. Yamahara and H. Matsuda, Chem Pharm Bull, 45,464-469 (1997).
46. K. Azuma, K. Nakayama , M. Koshioka, K. Ippoushi, Y. Yamaguchi, K. Kohata, Y. Yamauchi , H. Ito and H.Higashio , J AgricFood Chem, 47, 3963-3966 (1999).
47. K. K. Mukherjee, S .K. Mitra, and S. N. Ganguly, Nat Prod Sci, 4,51-52 (1998).
48. G .Soliman, and W. Saleh, J. Chem Soc, 1506-1508(1954).
49. A . Sen Gupta, and U. K. Mazumder, J Sci Food and Agr, 24,1391-1395(1973).
50. M. Yoshikawa, T. Murakami , H. Shimada , S. Yoshizumi, M. Saka , Yamahara, J , Chem Pharm Bull, 46,1008-1014 (1988).
51. P. Schumersahl, Arch Pharm Ber Deutsch Pharm Ges, 304,307-314(1971).
52. L. A. Leshchinskii , G. V. Karbasnikova, O. M. Zebelyan, and A. A. Vasilkova , Kardiologiya, 18,76-83 (1978)
53. S. Khatib, A. Alkofahi, M. Hasan , N. Najib, and A.El- oqlah , Biomedical Letters, 57,147-152 (1998).
54. S. Laskar , S. G. Majumder, B .Basak, and C. D. Dey, Physiol Bohemoslov, 35,86-89 (1986).
55. S. Innami, K. Tabata, J. Shimizu, K. Kusunoki, H, Ishida M. Matsuguma, Wada , N .Sugiyama and M. Kondo. Plant foods Hum Nutr, 52,55-65 (1998).
56. U .K. Mazumder , M. Gupta , D. K Pal , and D. K Bhattacharya , Acta Pol Pharm, , 60, 317-323 (2003).
57. M. Gupta, U. K. Mazumder , D .K. Pal , S. Bhattacharya, and S. Chakrabarty, Acta Pol Pharm, 60,207-210 (2003).
58. M. Gupta, U. K. Mazumder, D. K Pal, and S. Bhattacharya, J Ethnopharmacol, 89, 55-59 (2003).
59. M. Gupta, U .K. Mazumder, D. K. Pal, and S. Bhattacharya, Ind J Exp Biol, 41, 641-644 (2003).
60. M. A Faizi, R. Saleem, S. Bibi . Magnetic Resononance in Chemistry, 39, 399-405 (2001).
61. G.Arunachalam , B. Paromita and D, Chattopadhyay, Journal of Pharmacognosy and Phytotherapy 1(1), 001-013,(2009).
62. M. Leonor Arrebola, Therese Ringbom, Robert Verpoorte., Phytochemistry, 52,1283-1286 (1999).
63. Rui Liu, Yanbin Lu, Tianxing Wu, Yuanjiang Pan, Chromatographia, 68, 95-99, (2008).
64. H. Itokawa, K. Mihara, K. Takeya , Chem Pharm Bull, 31,2353–2358 (1983)
65. E. Okuyama , K. Sato, K. Yoshihira , Phytochemistry, 29,3973–3974 (1990).
66. J. Koyama , T. Ogura , K. Tagahara, T. Konoshima, M. Kozuka, Phytochemistry , 31,2907–2908 (1992).
67. A. Tai-Shun Lin Beverly, Teicher, and Alan C Sartorelli., Journal of Medicinal Chemistry, 23(11), 1239 (1980)
68. D. P . Chakraborty, A. Islam, S. Roy, Phytochemistry, 17,(11), 2043 (1978).
69. O.E.Kenneth, N.F.Gabriel, K. Victor, P .B. Veronique, K.Karsten , Hidayat, H.Augustin, E.N. Michael, S.Salem, R.S,Achim, Phytochemistry,67(6),605-609 (2006)
70. A. L. B. Soad , G. R. Michael , R. B. John, S. B. Ian , Phytochemistry, 71, 5-6, 598-604 (2010)
71. Mizuo Mizuno, Hiroyuki Kojima, Munekazu Iinuma, Toshiyuki Tanaka, Kiyoto Goto. , Phytochemistry, 31,(2), 717-719 (1992)
72. Emma Maldonado, Marisela Bello, José Luis Villaseñor, Alfredo Ortega., Phytochemistry, 49 (4),1115-1118 (1998)
73. V. Derek, Banthorpe, Geoffrey ,D. Brown, Phytochemistry, 28(11),3003-3007(1989)
74. Y. N. Shukla, Anil Srivastava, Sunil Kumar, Sushil Kumar., Journal of Ethnopharmacology, 67 (2),241-245 (1999).
75. L.Jørgen, P. C. Lars , T. Tove , Phytochemistry, 31 (8), 2881-2882 (1992).
76. S. Pallavi , K. G. Yogesh, M .C .Sharma, and M. P. Dobhal, Indian Journal of Chemistry, 49, 374-378,(2010)
77. L.B. De Silva, and, U. L. L. De SilvaJ.Natn.Coun. Sri Lanka 7(1), 1-3 (1979).
78. S.B. Mahato, N.P. Sahu, S.K. Roy, and B.N. Pramanik, Journal of Chemical Society Perkin Trans I, ,2065 (1989).
79. V.A.Maslennikov, and N.K. Abubakirov, Khimiya Prirodnykh Soedinenii. 11,525(1975).
80. G.L.Turakhozhaev, M.i. Zubkina , and T.T. Shakirov, Khimiya Prirodnykh Soedinenii, 8,81 (1972).
81. E.V. Rao, K.N. Rao and D.V. Rao , Current Sciences, ,42,731 (1973).
82 P.Schmersahl, Tetrahedron Letters , 10,789 (1969).
83. E. Okuyama , K. Sato, K. Yoshihira , Phytochemistry, 29,3973–3974 (1990).
84. J. Koyama , T. Ogura, K. Tagahara , T. Konoshima, M. Kozuka , Phytochemistry , 31,2907–2908 (1992)
85. A. Tai-Shun Lin Beverly , Teicher, and Alan C Sartorelli., Journal of Medicinal Chemistry, 23,(11), 1239 (1980)
86. D. P. A Chakraborty, S .Islam, Phytochemistry, 17,(11), 2043 (1978).
87. O. E. Kenneth, N. F. Gabriel, K. Victor , P. B. Veronique, K. Karsten, H.Hidayat, E. N. Augustin , S. Michael, R. S Salem, H. Achim, Phytochemistry, 67(6),605-609 (2006).
88. Ivan Addae-Mensah, Reiner Waibel, Hans Achenbach, Gichuru Muriuki, Clive Pearce, Jeremy K.M. Sanders, Phytochemistry, 28,(10), 2759-2761 (1989).
89. Incent Castola, Ange Bighelli, Serge Rezzi, Giovanni Melloni, Serafino Gladiali, Jean-Marie Desjobert, Joseph Casanova , Industrial Crops and Products, 15(1),15-22(2002).
90. B. Hashi, P. Mahato, Asish, Kundu , Phytochemistry, 37(6),1517-1575(1994).
91. Y. H. Bang , C. Hee-Byung , B. S. K. Leonardus, R. Soedarsono , R. F. Norman, A. C. Geoffrey , M. P. John , A. D. Kinghorn, Phytochemistry,62(2), 197-201(2003).
92. M. Rosane , Aguiar, Juceni, P. David, Jorge, M. David., Phytochemistry, 66(19),2388-2392 (2005).
93. A. Hymavathi, Suresh Babu, K. V. G .M. Naidu, Rama Krishna, S. Prakash, V. Diwan, J. Madhusudana Rao., Bioorganic and Medicinal Chemistry Letters, 19,5727-5731(2009).
94. W. Stöcklin, L .B. De Silva, T .A. Geissman, Phytochemistry, 8,(8) 1565-1569(1969).
95. A. L .B.Soad , G. R. Michael, R. B. John, S. B. Ian , Phytochemistry, 71, 5-6, 598-604 (2010).
96. Mizuo Mizuno, Hiroyuki Kojima, Munekazu Iinuma, Toshiyuki Tanaka, Kiyoto Goto, Phytochemistry, 31( 2) 717-719 (1992).
97. Emma Maldonado, Marisela Bello, José Luis Villaseñor, Alfredo Ortega., Phytochemistry, 49(4),1115-1118 (1998)
98. V. Derek, Banthorpe, Geoffrey, D. Brown., Phytochemistry, 28(11),3003-3007 (1989).
99. Y. N. Shukla , Anil Srivastava, Sunil Kumar, Sushil Kumar., Journal of Ethnopharmacology, 67(2), 241-245 (1999).
100. L. Jorgen, P. C. Lars , T. Tove, Phytochemistry, 31(8), 2881-2882 (1992)
101. Tian-Shung Wu, Shiow-Chyn Huang, Jeng-Shiow Lai, Che-Ming Teng, Feng-Nien Ko, Chang-Sheng Kuoh., Phytochemistry, 32 (2),449-451(1993).
102. Jin Dong-Zhe, Min Zhi-Da, Geroge C Y, Chiou, Munekazu Iinuma, Toshiyuki Tanaka,Phytochemistry, 41 (2) 545-547 (1996).
103. Liam Evans, John, N. Hedger, David Brayford, Michael Stavri, Eileen Smith, Gemma O’Donnell, Alexander I. Gray, Gareth W. Griffith, Simon Gibbons., Phytochemistry, 67(19), 2110-2114 (2006)
104. Riisom, T. Jakobsen ,H .J. Rastrup-Andersen ,N .and Lorck, H., Tetrahedron Letters, 15(26),2247-2250 (1974).
105. Martin Laurberg, Ole Kristensen, Kirill Martemyanov, Anatoly T Gudkov, Ivan Nagaev, Diarmaid Hughes, Anders Liljas, Journal of Molecular Biology, 303(4), 593-603 (2000).
106. A .K. Tatyana , F. S. Olga , S. A. Shamil , V .P. Mikhail, A. D. Vladimir, and B. E. George, Biochemical Systematics and Ecology,29(8),873-874(2001)
107. W. O. Godtfredsen, W. S. von Daehne, A. Vangedal, Marquet and, D. Arigoni, A. Melera, Tetrahedron, 21(12), 3505-3530 (1965).
108. W. O .Godtfredsen and S. Vangedal Tetrahedron, 18( 9), 1029-1048 (1962)
109. Tore Duvold, Anne Jørgensen, Niels, R. Andersen, Anne S. Henriksen, Morten Dahl Sørensen, Fredrik Björkling, Bioorganic and Medicinal Chemistry Letters, 12(24),3569-3572 (2002)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









