{ DOWNLOAD AS PDF }
About Authors:
Rohit Kumar Ahuja*1, Surendra Singh Saurabh1, Poonam Choudhary1, Aniket Singh Chouhan1, Kamal Singh Rathore2
1 Lachoo Memorial College of Science and Technology (Pharmacy Wing) Sector-A, Shastrinagar, Jodhpur (Raj.) 342003, IND.
2 BN Institute of Pharmaceutical Sciences, Udaipur (Raj.) 313002, India
rohitahuja1111@gmail.com
Abstract:
To build up an oral drug delivery system, it is essential to optimize both release rate of drug and residence time of system within gastrointestinal tract. In oral path difference in gastric physiology such as gastric pH and motility display variability on gastric residence time (GRT) and drug delivery actions. Several approaches are currently utilized in the prolongation of the GRT including hydrodynamic balance systems (HBS), swelling and expanding systems, polymeric bioadhesive systems, high-density systems, modified-shape systems and other delayed gastric emptying devices.One such approach is Floating Microspheres (Hollow Microspheres). Floating microspheres are gastro-retentive drug delivery systems based on non-effervescent approach. These microspheres are characteristically free flowing powders made of proteins or synthetic polymers, ideally having a size less than 200 micrometer. Gastro-retentive floating microspheres are low-density systems that have sufficient buoyancy to float over gastric contents and remain in stomach for prolonged period. The drug is released slowly at desired rate resulting in increased gastric retention with reduced fluctuations in plasma drug concentration. Floating microspheres improve patient compliance by decreasing dosing frequency and better therapeutic effect of short half-life drugs can be achieved. Floating microspheres are characterized by their micromeritic properties such as particle size, tapped density, compressibility index, true density and flow properties including angle of repose, scanning electron microscopy, in vitro floatability studies, in vitro drug release studies and stability studies etc.
REFERENCE ID: PHARMATUTOR-ART-2111
Introduction
The endeavor of any drug delivery system is to provide a therapeutic dose at the proper site in the body and then maintain the desired drug concentration. The oral route is mostly used for the delivery of therapeutic agents because the low cost of the therapy and ease of administration lead to high levels of patient compliance1.
Oral administration of controlled release drug delivery system should ideally produce the required plasma levels and maintain it at steady levels for a prolonged period of time. For oral drug delivery system, absorption is highly variable due to physiological variability in gastrointestinal tract such as gastro-retention time, gastro intestinal transit and some drugs release from dosage from after passage from absorption site2.
Hydrodynamically balance system (HBS), floating or gastro-retentive drug delivery system has been developed to overcome the disadvantage of conventional drug delivery system. Gastro retentive drug delivery systems (GRDDS) enhance the absorption of drugs those have limited and narrow absorption window at the upper part of gastro intestine tract or drugs in the gastro duodenum and reduce wastage of drug and improve solubility of drugs that are less soluble in a high pH environment. The approaches for gastric retention drug delivery system includes floating drug delivery system, low density systems, raft systems incorporating alginate gels, bio-adhesive/muco-adhesive system, super porous hydro gels and magnetic systems3.
Floating system was first developed by Davis (1968). Floating systems are the low density systems that have sufficient buoyancy to float and remain in stomach for prolonged period of time4. The floating microspheres not only prolongs the gastric retention time but also controls the space in the stomach by maintaining the delivery system positioned at a steady site and there by properly delivering the drug. The floating microspheres enhance bioavailability and improve pharmacokinetic and pharmacodynamics profiles of the drugs by retaining the drug in stomach. Floating microspheres release the drug in controlled manner to achieve zero-order release kinetics for a prolonged period of time5.
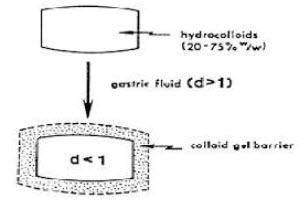
Fig.1: Density of floating tablet
Microspheres can be defined as solid, approximately spherical particles ranging in size from 1 to 1000 micrometer. The Microspheres are characteristically free flowing powders consisting of proteins or synthetic polymers, which are biodegradable in nature. Solid biodegradable microspheres incorporating a drug dispersed or dissolved throughout ratio6.
Floating Drug Delivery Systems5
Floating drug delivery systems (FDDS) or HBS have a bulk density less than gastric fluids and so they float in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents the drug is released slowly at the desired rate from the system. After release of drug, the residual system is emptied from the stomach. This results in an increased GRT and a better control of the fluctuations in plasma drug concentration.
Fig.2: Release of drug from Floating tablets
Approaches to Design Floating Drug Delivery System7, 8, 9
The following approaches have been used for the design of floating dosage forms of single and multi-unit systems.
Classification of FDDS
Based on the mechanism of buoyancy, two distinctly different technologies have been utilized in the development of FDDS, which are:
A.Effervescent system:
Effervescent system includes use of gas generating agents, carbonates (e.g. Sodium bicarbonate) and other organic acids (Citric acid and tartaric acid) to produce carbon dioxide (CO2) gas. It reduces the density of the system to make float on the gastric fluid. The optimal stoichiometric ratio of citric acid and sodium bicarbonate for gas generation is reported to be 0.76:1. Effervescent systems have been further classified into two types:

Fig.3: Effervescent tablet
1. Gas generating system:
a. Intra gastric single layer floating tablets:
These systems are formulatedby intimately mixingCO2 generating agents and the drug along with polymers and compressed as a matrix tablet. These have bulk density lower than gastric fluids and therefore remain floating in the stomach unflattering the gastric emptying rate for a prolonged period. The drug is slowly released at a desired rate from the floating system and after complete release the residual system is expelled from stomach. This leads to an increase in GRT and control fluctuations in plasma drug concentration.
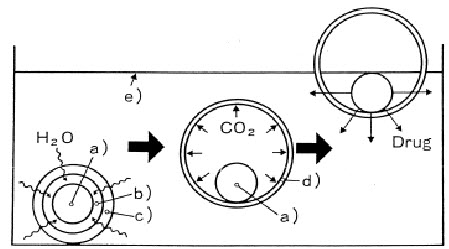
Fig.:4: Floating tablet with CO2 generating agent
b. Intra gastric bilayered floating tablets:
It contains the gas generating agents in hydrocolloid layer and drug in other layer formulated for SR effect.
2. Volatile liquid/Vacuum containing system:
a. Inflatable gastrointestinal delivery system:
In these systems, an inflatable chamber is incorporated, which contains liquid e.g. ether, cyclopentane, that gasifies at body temperature to cause the chamber to inflate in the stomach. These systems are fabricated by loading the inflatable chamber with a drug reservoir, which can be a drug, impregnated polymeric matrix, then encapsulated in a gelatin capsule. After oral administration, the capsule dissolves to release the drug reservoir together with the inflatable chamber. The inflatable chamber automatically inflates and retains the drug reservoir compartment in the stomach. The drug is continuously released from the reservoir into the gastric fluid.
B. Non effervescent systems:The non effervescent FFDDS is based on swelling mechanism of polymer or bioadhesion to mucosal layer in GI tract. The most commonly used excipients in non-effervescent FDDS are gel forming or highly swellable cellulose hydrocolloid, polysaccharides and matrix forming material such as polycarbonates, polyaccrylate and carbopol.
The various type of this system is as follows:
1.Colloidal gel barrier: These systems are able to maintain their low density, while the polymer hydrates and builds a gelled barrier at outer surface. The air trapped by swollen polymer maintains density less than unity and confers buoyancy. The drug is released slowly from swollen matrix.
2.Single layer floating tablets: These are formulated by mixing of drug with a gel forming hydrocolloid which swell in contact with gastric fluid and maintain bulk density less than unity. The air trapped by swollen polymer confers buoyancy to these dosage forms.
3.Alginate beads: Multi-unit floating dosage forms were developed from freeze dried calcium alginate. Spherical beads of approximately 2.5 mm diameter can be prepared by dropping sodium alginate solution into aqueous solution of calcium chloride causing precipitation of calcium alginate leading to formation of porous system, which can maintain a floating force for over 12 hours.
Fig.5: Alginates beads
4.Hollow microsphere: Hollow microspheres loaded with drug in their outer polymer shell were prepared by a novel emulsion solvent diffusion method and spray drying technique.
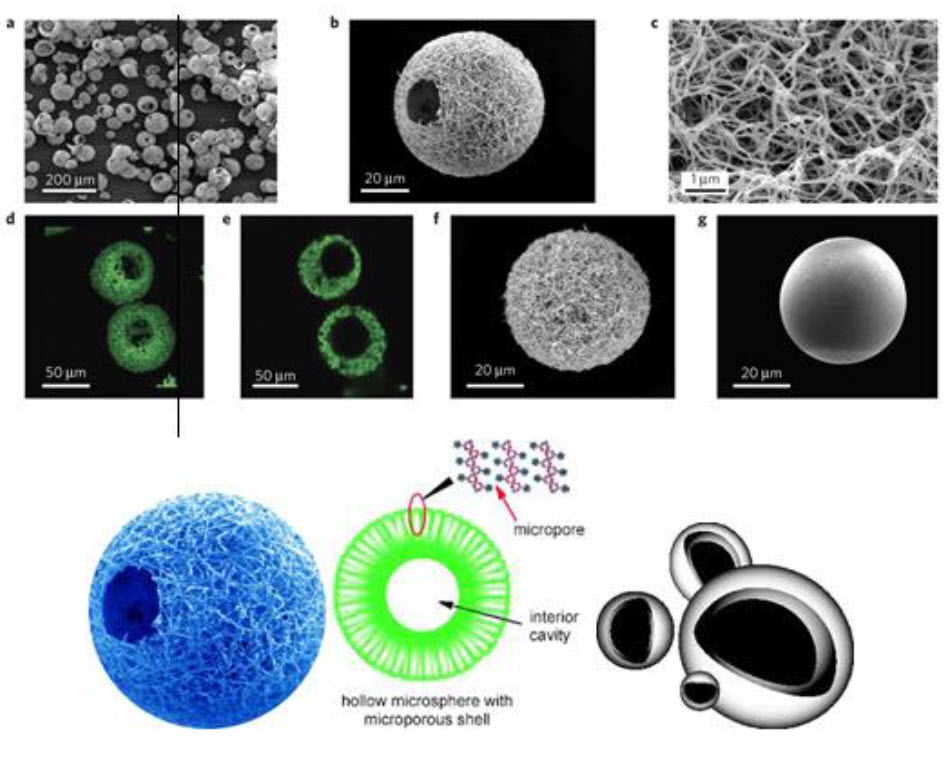
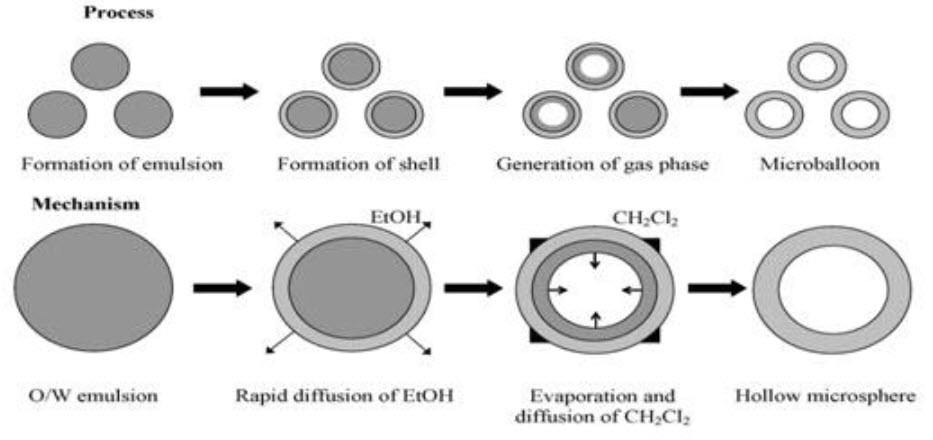
Fig.6: Hollow microspheres
Advantages of hollow microspheres include:10, 11
1.Improves patient compliance by reducing dosing frequency.
2.Bioavailability enhances by avoiding first pass effect and fluctuation in plasma drug concentration.
3.Better therapeutic effect of short half-life drugs can be achieved.
4.Gastric retention time is increased because of buoyancy.
5.Drug releases in controlled manner for prolonged period.
6.Site-specific drug delivery to stomach can be achieved.
7.Enhanced absorption of drugs which solubilise only in stomach.
Disadvantages of hollow microsphere
1.Drugs cause gastric irritation is not suitable candidates for FDDS.
2.Drugs which are absorbed along the entire GIT and which undergo first pass metabolism may not be desirable e.g. nifedipine.
3.Drugs having stability or solubility problem in stomach are not suitable candidate for FDDS
4.FDDS require sufficiently high level of fluid in stomach so that the system can float and thus sufficient amount of water (200-250 ml) of water to be administered with FDDS.
Mechanism of drug release from the microspheres13, 14
The mechanism of drug release from microspheres can occur in the following ways:
Diffusion: On contact with aqueous fluids in the gastrointestinal tract (GIT), water diffuses into the interior of the particle. Drug dissolution occurs and the drug solutions diffuse across the release coat to the exterior.
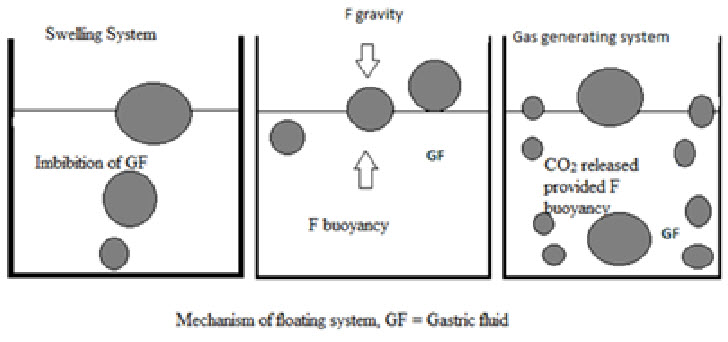
Fig.7: Mechanism of floating system
Erosion: Some coatings can be designed to erode gradually with time, thereby releasing the drug contained within the particle.
Osmosis: In allowing water to enter under the right circumstances, an osmotic pressure can be built up within the interior of the particle. The drug is forced out of the particle into the exterior through the coating.
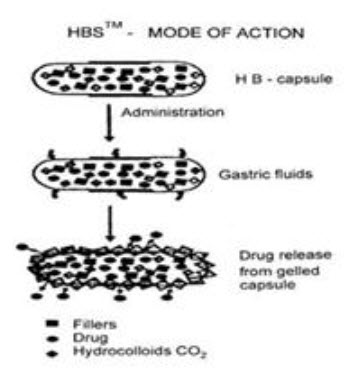
Fig.8: Mode of action of floating system
Materials and Methods:
The polymers used in the preparation of floating microspheres are
1. Synthetic polymers.
2. Natural polymers.
The examples of polymers are given in Table no.1.
Table No.1 Example of polymers
|
Polymer |
Sub Type |
Examples |
|
Synthetic polymer |
Biodegradable |
Lactides, glycosides and their copolymers. Poly alkyl cyanoacrylates, Polyanhydrides. |
|
Non-biodegradable |
Polymethyl methacrylate, Acrolin, Glycidyl methacrylate, Epoxy polymers |
|
|
Natural polymers |
Proteins |
Albumin, Gelatin, Collagen. |
|
Carbohydrates |
Agarose, Carragenan, Chitosan, Starch. |
|
|
Chemically modified carbohydrates. |
Poly dextrans, Poly starch. |
Mechanism behind floating of microspheres8
When microspheres come in contact with gastric fluid the gel formers, polysaccharides, and polymers hydrate to form a colloidal gel barrier that controls the rate of fluid penetration into the device and consequent drug release. As the exterior surface of the dosage form dissolves, the gel layer is maintained by the hydration of the adjacent hydrocolloid layer. The air trapped by the swollen polymer lowers the density and confers buoyancy to the microspheres.
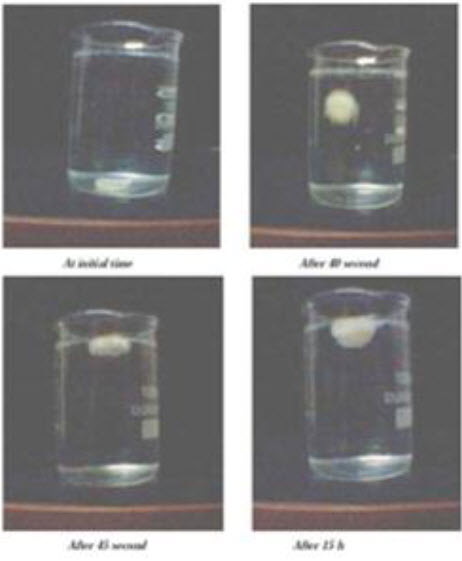
Fig.9: Mechanism of floating tablets
Methods of Preparation
These floating systems are prepared by using low density and Swellable polymers, gel forming agents. When these systems come in contact with the gastric fluid they form barrier and releases the drug in controlled manner. The following methods are used for the preparation of floating microspheres.
1. Emulsion Solvent Evaporation Technique20
This technique is widely used to control release of drug. This technique involves the emulsification of an organic solvent containing dissolved polymer and dispersed drug in an excess amount of aqueous continuous phase, with the aid of an agitator. The concentration of the emulsifier present in the aqueous phase affects the particle size and shape. When the desired emulsion droplet size is formed, the stirring rate is reduced. Subsequent evaporation of the dispersed phase solvent under reduced pressure at an appropriate temperature yields solid polymeric microparticles entrapping the drug. The solid microparticles are recovered from the suspension by filtration, centrifugation, or lyophilisation.
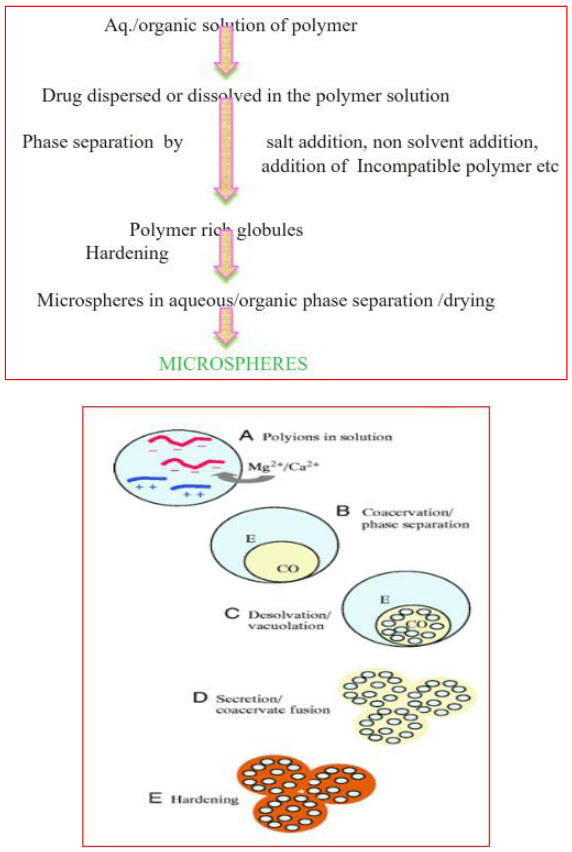
Fig.10: Various techniques of microsphere preparation
2. Emulsion Cross Linking Technique21
The micro particulate carrier of natural polymers like proteins and carbohydrates are prepared by using this method. In this technique, The drug is dissolved in aqueous gelatin solution which is previously heated for 1hr. at 40°C and prepared solution is added drop wise to liquid paraffin while stirring the mixture at 1500 rpm for 10 min at 35°C, which results in w/o emulsion further stirring is done for 10min at 15°C. Then the microspheres are washed with acetone and isopropyl alcohol. The cross linking is achieved either by means of heat or by using chemical cross linkers. Further air dried and dispersed in 5ml of aqueous glutaraldehyde saturated toluene solution at room temperature for 3 hrs for cross linking and treated with 100ml of 10Mm glycine solution containing 0.1%w/v of between 80 at 37°C for 10 min to block unreacted glutaraldehyde. The chemical cross linking agents used are glutaraldehyde, formaldehyde and acid chloride. The heat as cross linking agent is not suitable for thermolabile substances.
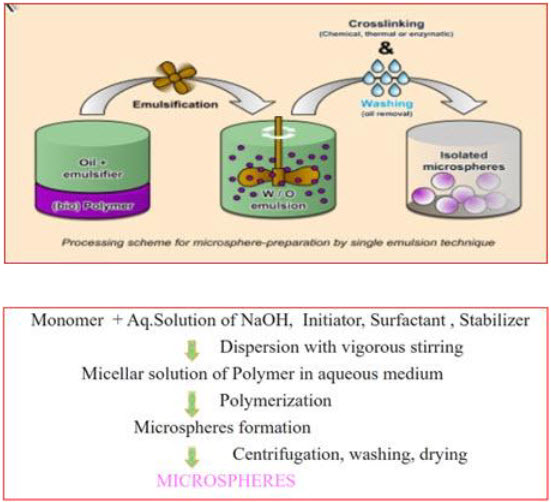
Fig.11: Cross-linking technique
3. Emulsion-Solvent Diffusion Technique22
This method is widely used to prepare floating microsphere. In this emulsion solvent diffusion technique, first the drug is dissolved in suitable polymer solution. This drug polymer mixture is dissolved in a mixture of ethanol and dichloromethane (1:1) then the mixture is added drop wise to sodium lauryl sulphate (SLS) solution. The solution is stirred with propeller type agitator at room temperature at 150 rpm for 1 hour, washed and dried in desiccator at room temperature.
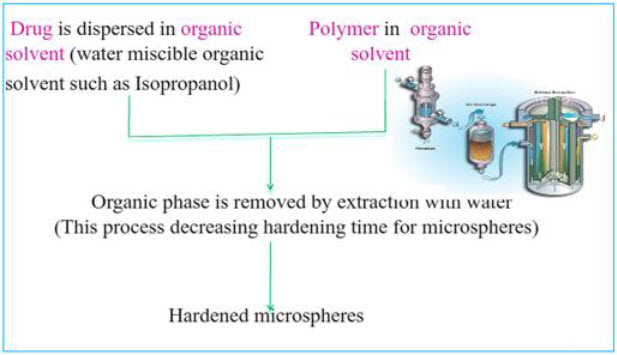
Fig.: 12:Emulsion-Solvent Diffusion Technique
4. Multiple Emulsion Method23
This method involves the formation of the multiple emulsions or the double emulsion such as w/o/w. This emulsion is then subjected to solvent removal by solvent evaporation.This method can be used with the natural as well as synthetic polymers.
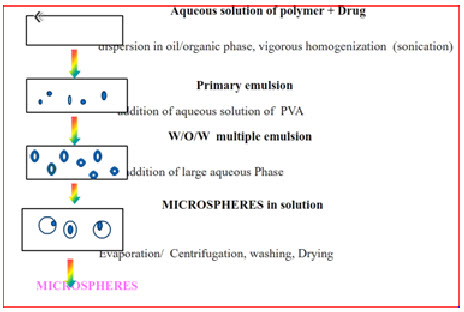
Fig.:13:Multiple Emulsion Method
5. Spray Drying Technique24
This method is based on the drying of the mist of the polymer and drug in the air. In this technique, the polymer is first dissolved in a suitable volatile organic solvent such as dichloromethane, acetone, etc. The drug in the solid form is then dispersed in the polymer solution under high speed homogenization. This dispersion is then atomized in a stream of hot air. The atomization leads to the formation of the small droplets or the fine mist from which the solvent evaporates instantaneously leading the formation of the microspheres in a size range 1-100 μm.
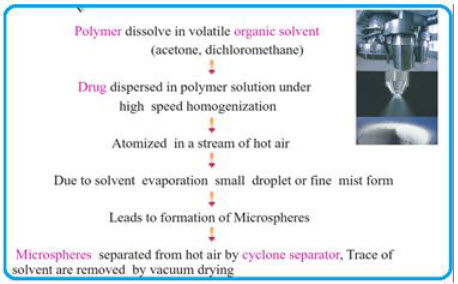
Fig.14:Spray Drying Technique
6. Ionic-Gelation Tecchnique25
The ionotropic gelation technique is successfully used for the preparation microspheres by using low density polymers and gas generating agents like tartaric acid, citric acid etc. In this technique low density polymer is dissolved in purified water to form a homogeneous polymer solution. The core material or drug as fine powder passed through mesh no.120 is added to the polymer solution and mixed to form a smooth viscous dispersion. This dispersion is added drop wise into 10%w/v CaCl2 solution through a syringe with a needle of diameter 0.55mm. The added droplets are retained in CaCl2 solution and allowed to cure for 20 minutes at 200 rpm to produce spherical rigid microsphere. Finally the microspheres are collected and dried in an oven at a temperature 45°C for 12 hrs.
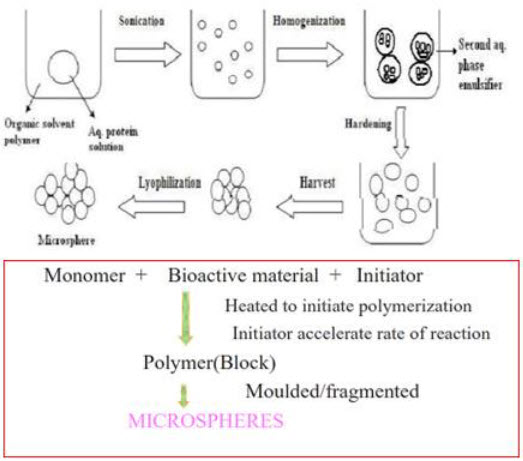
Fig.15: Ionic-Gelation Tecchnique
Characterizations of Floating Microsphere
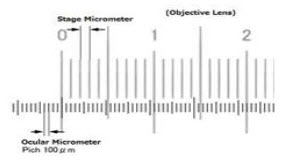
1. Particle size determination26
The particle size can be determined by using an optical microscope under regular polarized light, and mean particle size was calculated by measuring 200-300 particles with the help of a calibrated oculometer.
2. Bulk density27
It is the ratio of weight of the blend to bulk volume. It was measured by pouring microspheres in measuring cylinder and measuring the volume occupied by microspheres.
Bulk density = (Weight of microspheres)/(Bulk volume)
3. Tapped density
It is the ratio of weight of the blend to tapped volume. It was measured by digital tap densitometer by measuring the volume occupied by microsphere after 100 standard tapping.
Tapped density = (Weight of microspheres)/(Tapped volume)
4. Carr’s (compressibility) index
Compressibility index (C.I.) or Carr's index value of micro particles was computed according to the following equation.
% compressibility = [(Tapped density – Bulk density )/(Tapped density)] X 100
The value below 15% indicates a powder with good flow property, whereas above 25% indicate poor flow property.
5. Hausner’s ratio
Hausner’s ratio of microspheres was determined by comparing tapped density to bulk density using the equation.
Hausner’s ratio = (Tapped density)/(Bulk density)
6. Angle of repose 28
Angle of repose (θ) of the microspheres was measured using funnel method. The microspheres were poured through a funnel that can be raised vertically until a maximum cone of height was obtained.
The radius of heap was measured and angle of repose was calculated.
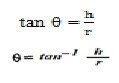
Where, θ = Angle of repose,
h = Height of granules above the flat surface,
r = radius of the circle formed by the granule heap.
7. Percentage yield29
The percentage yield of the floating microspheres was determined for drug and was calculated using the following equation
Actual weight of floating microspheres
Percentage yield = ---------------------------------------- X 100
Total weight of excipients and drug
8. Drug entrapment efficiency30
The amount of drug entrapped was estimated by crushing the microspheres and extracting with aliquots of 0.1N HCl repeatedly. The extract was transferred to a 100 ml volumetric flask and the volume was made up using 0.1N HCl kept for 24 hours. The solution was filtered and the absorbance is measured by Spectrophotometer against appropriate blank. The amount of drug entrapped in the microspheres was calculated by the following formula:
Estimated percent drug content
% Entrapment efficiency = -------------------------------- X 100
Therotical percent drug content
9. Scanning Electron Microscopy29
Dry microspheres are placed on an electron microscope brass stub a coated with gold in anion sputter. Then picture of microsphere were taken by spectro random scanning of the stub. The microspheres are viewed at an accelerating voltage of 20KV.
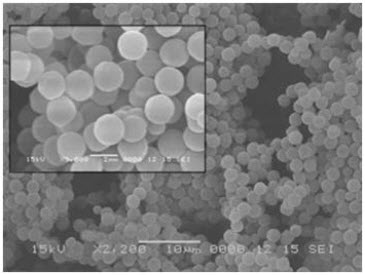
Fig.16: SEM of floating micrcosphere
10. In vitrobuoyancy31
Microspheres (300mg) were spread over the surface of a USP XXXVI dissolution apparatus type II filled with 900 ml of 0.1 N hydrochloric acid containing 0.02% Tween-80. The medium was agitated with a paddle rotating at 100 rpm for 12 hrs. The floating and the settled portions of micro spheres were recovered separately. The microspheres were dried and weighed. Buoyancy percentage was calculated as the ratio of the mass of the microspheres that remained floating and the total mass of the microspheres.
Wf
Buoyancy (%)= --------- X 100
Wf + Ws
Where Wf and Ws are the weight of floating and settled microsphere respectively.
11. In-Vitrorelease studies31
The In-vitro release rate of floating Microspheres was determined in a United States Pharmacopoeia (USP) XXIII basket type dissolution apparatus. A weighed amount of floating microspheres equivalent to dose of drug placed in the basket of dissolution rate apparatus. Nine hundred milliliters of the SGF containing 0.02% w/v of Tween 20 was used as the dissolution medium. The dissolution fluid was maintained at 37 ± 1°C at a rotation speed of 100rpm. Perfect sink conditions prevailed during the drug release study. 5ml samples were withdrawn at each 30 min interval, passed through a 0.25 μm membrane filter (Millipore), and analyzed using UV spectrophotometer to determine the concentration present in the dissolution medium. The initial volume of the dissolution fluid was maintained by adding 5 ml of fresh dissolution fluid after each withdrawal. All experiments were run in triplicate.
12. In-Vivostudies32, 33
The in vivo gastric retentivity of a floating dosage form is usually determined by scintigraphy or roentgenography.
Applications of floating microsphere13, 34
1. Floating microspheres are very effective approach in delivery of drugs that have poor bioavailability because of their limited absorption in the upper GIT. These systems efficiently maximize their absorption and improve the bioavailability of several drugs. e.g. furosemide, riboflavin etc.
2. These microparticulate systems provide sustained drug release behavior and release the drug over a prolonged period of time. Hollow microspheres of tranilast are fabricated as a floating controlled drug delivery system.
3. Hollow microspheres of non-steroidal anti-inflammatory drugs are very effective for controlled release, and reduce the major side effect of gastric irritation. For example, floating microspheres of indomethacin are quite beneficial for rheumatic patients.
4. Floating microspheres can be used as carriers for drugs with so-called absorption windows, these substances, for example antiviral, antifungal and antibiotic agents (sulphonamides, quinolones, penicillins, cephalosporins, aminoglycosides and tetracyclines) are taken up only from very specific sites of the GI mucosa.
5. Hollow microspheres can greatly improve the pharmacotherapy of the stomach through local drug release, leading to high drug concentrations at the gastric mucosa, thus eradicating Helicobacter pylori from the sub-mucosal tissue of the stomach and making it possible to treat stomach and duodenal ulcers, gastritis, oesophagitis etc.
6. These systems provide an easy way of maintaining constant blood level with an ease of administration and better patient compliance.
7. The higher dose of drugs can be reduced due to increase in gastric retention time which led to low dose frequency.
List of drugs formulated as HBS floating microsphere
1. Repaglinide35
2. Cimetidine36
3. Rosiglitazone37
4. Nitrendipine38
5. Acyclovir39
6. Ranitidine HCl40
7. Misoprostol
8. Metformin41
9. Aceclofenac42
10. Diltiazem
11. L-Dopa and beneseragide
12. Flourouracil
Marketed Products
Table: 2 some of the marketed formulations are listed as follows:
|
Marketed Products of Gaestro-retentive tablets |
|||
|
Brand name |
Delivery system |
Drug (dose) |
Company name |
|
Valrelease® |
Floating capsule |
Diazepam (15mg) |
Hoffmann-LaRoche, USA |
|
Madopar® HBS (Prolopa® HBS) |
Floating, CR capsule |
Benserazide (25mg) and L-Dopa (100mg) |
Roche Products, USA |
|
Liquid Gaviscon® |
Effervescent Floating liquid alginate preparations |
Al hydroxide (95 mg), Mg Carbonate (358 mg) |
GlaxoSmithkline, India |
|
Topalkan® |
Floating liquid alginate preparation |
Al – Mg antacid |
Pierre Fabre Drug, France |
|
Almagate Flot coat® |
Floating dosage form |
Al – Mg antacid |
----------- |
|
Conviron® |
Colloidal gel forming FDDS |
Ferrous sulphate |
Ranbaxy, India |
|
Cytotech® |
Bilayer floating capsule |
Misoprostol (100µg/200µg) |
Pharmacia, USA |
|
Cifran OD® |
Gas-generating floating form |
Ciprofloxacin (1gm) |
Ranbaxy, India |
Conclusion
Floating microspheres as gastro retentive HBS dosage form precisely controls the release rate of target drug to a specific site and facilitates an enormous impact on health care. Floating microspheres provide several all the advantages including greater flexibility and adaptability of microparticulate dosage forms which gives clinicians and those engaged in product development powerful new tools to optimize therapy.
Abbreviations
HBS: Hydrodynamically balance system, GRT: gastric residence time, FDDS: floating drug delivery systems,GRDDS: Gastro retentive drug delivery systems, SLS: sodium lauryl sulphate, CI: Compressibility index, SR: Sustained release, GIT: gastro intestinal tract.
Acknowledgements
Authors are profusely thankful to Lachho Memorial College of Science and Technology, Jodhpur and BN Institution of Pharmaceutical Sciences, Udaipur staff for their constant and perennial support and friends Jigar Pathak, Rekha Singh Saurabh and Arpana Singh.
References
1.Kawatra M., Jain U. and Ramana J. Recent Advances in Floating Microspheres as Gastro-Retentive Drug Delivery System: A Review. International journal of recent dvances in pharmaceutical research July 2012; 2(3): 5-23.
2.Dutta P., Sruti J., Patra N. and Rao M. E. Floating microsphere recent trends in the development of gastroretentive floating drug delivery system. Internatonal journal of pharmaceutical sciences and nanotechnology 2011; 4(1)
3.Bhoyar P. K., Baheti J. R. and Burde V. V. An overview of a gastro-retentive floating drug delivery system. World journal of pharmaceutical research 2012; 1(2): 22-40.
4..Sivanarayana P., Saikishore V., Pramamala R. and Veda P. A review on novel approach in gastro retentive floating drug delivery: Floating microsphere. Research journal of pharmaceutical, biological & chemical sciences 2012; 3(3): 1281-1286.
5.Kumar N., Niranjan S. K., Irchhaiya R. et al. Novel approaches of floating drug delivery system: A review. International journal for pharmaceutical research scholar 2012; 1(4): 96-111.
6.Chein YW. Novel drug delivery systems. Marcel Dekker, New York; 1992 ; 2 : 185- 210.
7.Sivanarayana P., Saikishore V., Pramamala R. and Veda P. A review on novel approach in gastro retentive floating drug delivery: Floating microsphere. Research journal of pharmaceutical, biological & chemical sciences 2012; 3(3): 1281-1286.
8.Narang N. An updated review on: floating drug delivery system. International journal of applied pharmaceutics 2011; 3(1): 1-7.
9.Kaushal A., Patel R., Jain A. and Hardenia S. S. Floating drug delivery systems: A review. Asian journal of pharmacy and life science 2011; 1(3): 284-294.
10.Shukla s., Patidar A., Agarwal S. and Choukse R. A review on: Recent advancement of stomach specific floating drug delivery system. International journal of pharmaceutical & biological archives 2011; 2(6): 1561-1568.
11.Gaba P., gaba M., Garg R. and Gupta G. Floating Microsphere: A review. Pharmainfo.net 2008; 2(5): 5-9.
12.Tripathi P., Ubaidulla U. and Khar R. K. Floating drug delivery system. International journal of research and development in pharmacy and life sciences 2012; 1(1): 1-10.
13.Pujara N. D., Patel N. V., Thacker A. P. et al. Floating microspheres: A novel approach for gastro retention. World journal of pharmacy and pharmaceutical sciences 2012; 1(3): 872-895.
14.Vyas SP, Khar RK. Targeted & Controlled Drug Delivery. New Delhi: CBS Publishers and Distributers; 2002:417-457.
15.Gattani Y. S. Floating multiparticulate drug delivery systems: An overview. International journal pf pharma and bio science 2010; 1(2): 1-9.
16.Goud S, R., Reddy E. R., Adavi L. S., Floating microsphere: a novel approach in drug delivery. Journal of drug research 2012; 1(4): 1-7.
17.Sharma S, Prashar M, Sahu R, “Floating Drug Delivery System: Incredible Revolution” Pharmacology online 3: 2011; 1039-1054
18.Soppimath KS , Kulkarni AR, Aminabhavi TM. In vitro and in vivo evaluation of ranitidine hydrochloride ethyl cellulose floating microparticles, Drug Dev. Ind. Pharm, 2001; 27(6): 507-515.
19. Dr. Jose GR, Omidian H, Shah K. Formulation And Evaluation Of Capcitabine Microspheres, Pharm. Tech, 2003; pp. 152-154
20.Tiwar S. and Verma P. Microencapsulation by solvent evaporation technique. International journal of pharmacy and life science 2011; 2(8): 998-1005.
21.Samal H. B., Dey S., Kumar D., and Kumar S. Formulation, characterization and in-vitro evaluation of floating microsphere of nateglinide. International journal of pharma and bio science 2011; 2(1): 148-157.
22.Najamudin M., Shelar S., Ali A. et al. Formulation and in- vitro evaluation of microsphere of ketoprofen by emulsion solvent diffusion method. International journal of pharmaceutics 2010; 2(1): 13-17.
23.Wagh D. D., Mule M. S. and Jain D. S. Gastroretentive floating microspheres: A review. International journal of pharmacy and technology 2011; 3(4): 1783-1799.
24.Mane R. B., Bhingar C., Bhalekar M.R.,”Preparation and evaluation of carvediol microsphere by spray drying technique: effect of process parameter on formulation”, International Journal of Pharmaceutical Quality Assurance” 2013; vol.4(1): 4-12.
25.Kaushal A., Patel R., Jain A. and Hardenia S. S. Floating drug delivery systems: A review. Asian journal of pharmacy and life science 2011; 1(3): 284-294.
26..Kannan.K., Karar.K.P., Manavalan.R., Formulation and Evaluation of Sustained Release Microspheres of Acetazolamide by Solvent Evaporation Technique, J.Pharm.Sci & Res. 2009; 1 (1):36-39.
27. Subramanyam C.V.S., Textbook of Physical Pharmaceutics. New delhi: MK Jain for vallabh prakashan: 2009; 210-228.
28.Shoufeng L., Senshang L., Chein YW, Daggy BP, Mirchandani HL. Statistical optimization of gastric gloating system for oral controlled delivery of calcium. AAPS Pharm. Sci. Tech. 2001;
29.Tanwar Y.S., Ojha G.R., Development and evaluation of floating microsphere of verapamil hydrochloride. Brazilian Journal of Pharmaceutical Sciences 2007; 43(4): 529-534.
30.Jain A., Jain C.P., Formulation, Characterization and In vitro evaluation of floating microsphere of famotidine as a gastro retentive dosage form. Asian Journal of Pharmaceutics 2009; 222-224.
31.Nadicroti J., Dharani S. and Yamsevi M. R., Formulation & evaluation floating microparticles of metoprolol succinate. Asian Journal of Pharmaceutics & Clinical Research, 2011; vol.4: 132-135.
32.Timmermans J, Gansbeke BV, Moes A.J., Assessing by gamma scintigraphy the in vivo buoyancy of dosage forms having known size and floating force profiles as a function of time, Proc. 5th Int. Conf. Pharm. Technol, APGI, Paris, 1989; 1: 42–51.
33.Kawashima Y, Niwa T, Takeuchi H, Hino T, Ito Y. Microspheres As Floating Drug- Delivery Systems To Increase Gastric Retention Of Drugs, J. Control. Release, 1991; 16: 279– 290.
34.Dixit N., Floating drug delivery system. Journal of Current Pharmaceutical Research, 2011; 7(1): 6-20.
35.Jain S.K., Awasthi A.M., Jain N.K., Agarwal G.P., Calcium silicate based microspheres of repaglinide for gastroretentive floating drug delivery: Preparation and in vitro characterization. J. Control. Release. 2005; v.107: 300- 309.
36.Srivastva A.K., Ridurkar D.N., Wadhwa S., Floating microspheres of cimetidine: Formulation characterization and in vitro evaluation. Acta Pharm.2005; v.55: 277-285.
37.Kamila M.M., Mondal N., Ghosh L.K., Gupta B.K., Multiunit floating drug delivery system of rosiglitazone maleate: development, characterization, statistical optimization of drug release and in vivo evaluation. AAPS. Pharm. Sci. Tech. 2009; v.10: 887-899.
38.Cui F., Wang J., Shi K. Yang L., Wang S., Zhang L., In vivo evaluation of a sustained-release multiple-unit floating system containing nitrendipine. Asian J. Pharm. Sci. 2008; v.3: 151-157.
39.Patil M.P., Patil H.S., Bharat W.T., Patil V.R., Formulation and in-vitro evaluation of floating microspheres of acyclovir. Arch. Pharm. Sci. Res. 2009; v.1: 194-198.
40.Wei Y., Zhao, L., In vitro and in vivo evaluation of ranitidine hydrochloride loaded hollow microspheres in rabbits. Arch. Pharm. Res. 2009; v.31: 1369-1377.
41.Maina Chouhan, Kamal S Rathore, Floating microspheres of metformin, thesis M.Pharm., RUHS, BN institute of Pharmaceutical Sciences, Udaipur, 2012.
42.Pinky Gupta, Kamal S Rathore, Floating microspheres of aceclofenac, thesis M.Pharm., RUHS, BN institute of Pharmaceutical Sciences, Udaipur, 2012.
43.Davis SS. Formulation strategies for absorption windows. DDT 2005; 10:249.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 2 Received On: 14/01/2014; Accepted On: 22/01/2014; Published On: 10/02/2014 How to cite this article: RK Ahuja, SS Saurabh, P Choudhary, AS Chouhan, KS Rathore, Microspheres as Hydrodynamically Balance System, PharmaTutor, 2014, 2(2), 52-68 |









