About Authors:
Priya M. Padalia*, Manthan A. Padalia
Dagon Pharmaceuticals Pvt. Ltd.
*modiyapriya@gmail.com
ABSTRACT
Of the truly abundant polysaccharides in Nature, only glucosamine has yet to find utilization in large quantity. It is the content of exoskeletons of crustaceans and also from cell walls of fungi and insects. The linear β- 1,4 linked polymer of N-acetyl-D-glucosamine (GlcNAc) is known as chitin, whereas chitosan, a copolymer of GlcNAc (~20%) and glucosamine (GlcN, 80%) residues, is a product derived from de-N-acetylation of chitin in the presence of hot alkali. Glucosamine and their modified derivatives find extensive applications in medicine, agriculture, food, and non-food industries as well. Glucosamine derivative have emerged as a new class of physiological materials of highly sophisticated functions. The development of technologies based on the utilization of its derivatives is caused by their polyelectrolite properties, the presence of reactive functional groups, gel-forming ability, high adsorption capacity, biodegradability and bacteriostatic, fungistatic, antitumour influence, anti inflammatory, wound healing property, lubricating material in joints to provide strength. It is having ability to form self assembly nenoparticles. All these are the result of their versatile biological activity, excellent biocompatibility, and complete biodegradability in combination with low toxicity. With more and more useful and specific properties have led to an unlimited R&D efforts on this most versatile amino polysaccharide, to find new applications, which are necessary to realize its full potential. Incidentally, this too has become an environmental priority. No doubt, glucosamine is surely an undisputed biomolecule of great potential.
REFERENCE ID: PHARMATUTOR-ART-1633
INTRODUCTION
Glucosamine (C6H13NO5) is an amino sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids. Glucosamine is part of the structure of the polysaccharides chitosan and chitin, which compose the exoskeletons of crustaceans and other arthropods, cell walls in fungi and many higher organisms. Glucosamine is one of the most abundant monosaccharides. It is produced commercially by the hydrolysis of crustacean exoskeletons or, less commonly by fermentation of a grain such as corn or wheat. In the US it is one of the most common non-vitamin, non-mineral, dietarysupplements used by adults[1].Chitin, a Greek word for ‘envelop’, was discovered in 1811 as a substance occurring in mushrooms. Chitin is very widely distributed, especially in animals, and it also exists in less evolved taxonomic groups such as protozoa[2]. In plants, chitinous cell walls are only found in those forms, such as fungi and molds that like animals find considerable nitrogen in their food. On the contrary, photosynthetic plants utilize nitrogen-free sugars almost exclusively for their supporting structures; chitin is believed to constitute the cell membrane of some lower green plants such as chlorophycea [3].
Chitinous structures are mainly of ectodermal origin in multicellular animals and form the characteristic exoskeleton of most of the invertebrates [3], in contrast to collagenous structures, which almost entirely are of mesodermal origin. Chitin exceptionally constitutes more than half of the total organic matter in chitinous structures. Higher concentrations of up to 85% are found in Arthropoda, which are particularly able to synthesize chitin. The shells of Gastropoda and Lamellibranchia, however, contain only small amounts of chitin. Every year about 100 billion tonnes of chitin are produced by crustaceans, mollusks, insects, and fungi. Chitin is the most underexploited biomass resource available on Earth. Chitinis a linear homopolysaccharide composed of N -acetylglucosamine residues in β1-4linkage figure 2 The only chemical difference from cellulose is the replacement of the hydroxyl group at C-2 with an acetylated amino group.

Figure 1: Cellulose breakdown by wood fungi.
A wood fungus growing on an oak log is shown in figure 1. All wood fungi have the enzyme cellulase, which breaks the (β-1n4) glycosidic bonds in cellulose, such that wood is a source of metabolizable sugar (glucose) for the fungus. The only vertebrates able to use cellulose as food are cattle and other ruminants (sheep, goats, camels, giraffes). The extra stomach compartment (rumen) of a ruminant teems with bacteria and protists that secrete cellulase.
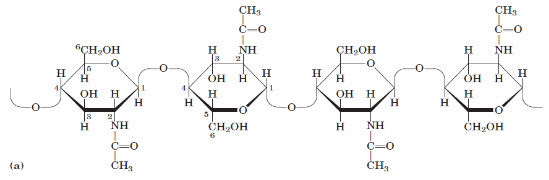
Figure 2:(a) A short segment of chitin, a homopolymer of N-acetyl-D-glucosamine units in (β1-4) linkage.
In addition to simple hexoses such as glucose, galactose,and mannose, there are a number of sugar derivativesin which a hydroxyl group in the parent compound isreplaced with another substituent, or a carbon atom isoxidized to a carboxyl group. In glucosamine,galactosamine, and mannosamine, the hydroxyl at C-2of the parent compound is replaced with an aminogroup. The amino group is nearly always condensed withaceticacid, as in N-acetylglucosamine. This glucosaminederivative is part of many structural polymers, includingthose of the bacterial cell wall. Bacterial cell wallsalso contain a derivative of glucosamine, N-acetylmuramicacid, in which lactic acid (a three-carbon carboxylicacid) is ether-linked to the oxygen at C-3 ofN-acetylglucosamine [4].
CHEMISTRY/MOLECULAR STRUCTURE
Glucosamine was first prepared in 1876 by Georg Ledderhose by the hydrolysis of chitin with concentrated hydrochloric acid. D-Glucosamine is made naturally in the form of glucosamine-6 phosphate, and is the biochemical precursor of all nitrogen-containing sugars [5].Specifically, glucosamine-6-phosphate is synthesized from fructose 6-phosphate and glutamine [6] as the first step of the hexosamine biosynthesis pathway. The end-product of this pathway is UDP-N-acetylglucosamine(UDP-GlcNAc), which is then used for making glycosaminoglycans, proteoglycansand glycolipids.
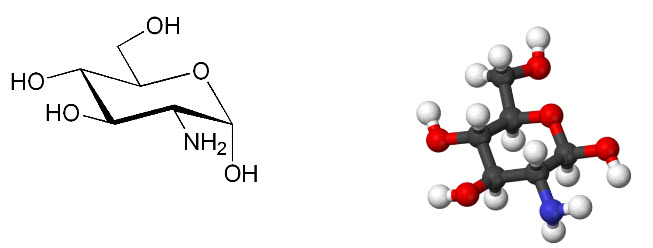
Figure 3: Structure of glucosamine: 2-Amino-2-deoxy-D-glucose chitosamine
IUPAC name: (3R,4R,5S,6R)- 3-Amino-6- (hydroxymethyl)oxane-2,4,5-triol
Chitin is the most abundant natural biopolymer derived from exoskeletons of crustaceans (crab and shrimp) and also from cell walls of fungi and insects. It is a cationic amino polysaccharide, essentially composed of N-acetyl-D-glucosamine (GlcNAc) residues linked β1-4. Chitosan, a copolymer of D-glucosamine (GlcN) (~80%) and N-acetyl D-glucosamine (20%) units, is a product derived from de-N-acetylation of chitin in the presence of hot alkali Figure 4. Chitosan is, in fact, a collective name representing a family of de-N-acetylated chitins, deacetylated to different degrees[7]. The elucidation of the molecular structure of chitin and chitosan literally took several decades to accomplish. The molecular weight of these polysaccharides can be as high as 106 Da. Results of experiments based on lysozyme degradation reactions and NMR spectroscopy have suggested that both GlcN and GlcNAc residues are distributed randomly and not blocked together8. The degree of N-acetylation and the degree of polymerization (dp), which in turn decided the polymer molecular weight, are two important parameters dictating the use of chitosans for various applications. Their influences on the viscosity development of aqueous solutions have significant roles in the biochemical and biopharmacological significance of chitosan. Chitin and chitosan are also known to exhibit polymorphism[8].Generally, the individual chains assume an essentially linear structure, which undergoes one full twist, every 10.1 to 10.5 A along the chain axis. Because each glycosidic unit in the chain is chiral, and all units are connected by an oxygen atom that links C-1 of one glycosidic unit to C-4 of an adjacent unit, a distinct “left” and “right” direction can be assigned to each polymer chain. The most common allomorph exhibited by chitin is known as the α-conformation, in which the unit cell is orthorhombic and the individual chains are arranged in antiparallel fashion[9]. Thus, adjacent chains are oriented in opposite directions. A less common allomorph, known as the β conformation, corresponds to a monoclinic unit cell with the polymer chains arranged in a parallel fashion[10]. A third form, gchitin, has been reported from the stomach lining of Loligo, which is characterized by a three chain unit cell in which two “up” chains are followed by a “down” chain[11]. However, this form has not been subjected to detailed analysis as given to the α- and β-forms and is yet to be established that this is a true third version structure and not a distorted version of α- and β-forms. X-ray diffraction patterns and NMR spectra of these three different allomorphs have been shown to be distinct.
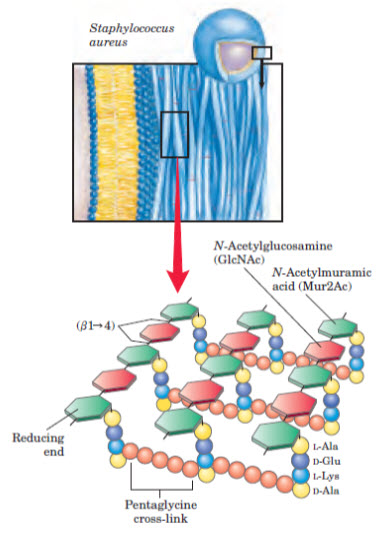
Figure 4: Glucosamine linkage in cell wall synthesis
BIOSYNTHESIS
Glucosamine polymer biosynthesis in crustaceans differs significantly from that of fungi. However, they do share common biosynthetic steps, and the enzymatic machinery exhibit more or less similar catalytic regulation. The nonreducing disaccharide, trehalose is the most common sugar in insects. It is hydrolyzed by trehalase, and the glucose units are converted to uridine di phospho-N-acetylglucosamine (UDP-GlcNAc). The terminal polymerization step has been the contentious issue in chitin biosynthesis in insects for which several explanations and speculation were given. However, based on the available evidence, it is now proposed that the epidermis in insects using UDP-GlcNAc as the precursor synthesizes soluble oligosaccharides (dp 8) that are linked to polyprenol lipids such as dolichol phosphate, transported outside the cell and attached to specific residues, possibly aspargine, on a receptor protein. The resultant primer (attached to receptor protein) is extended by the sequential addition of GlcNAc residues via chitin synthetase[12].
ASSOCIATION WITH OTHER BIOMOLECULES
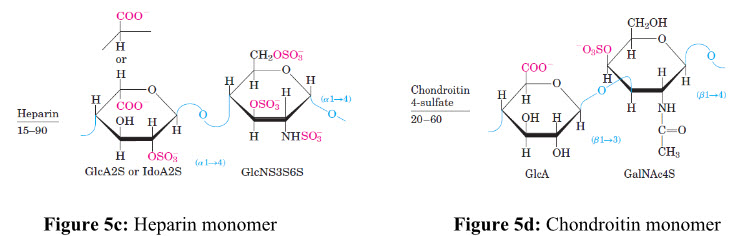
Due to the availability of reactive free amino groups, chitin/chitosan molecules tend to associate in situ with several other (macro-) molecules and give rise to new types of structures and physiochemical characteristics[13]. For example, the chitinous structures are build up of a glycoprotein framework, in which chitin is covalently linked to proteins. At this level of organization, the chitinous structure exhibits high tensile strength but is essentially pliable and flexible, allowing movements and limited expansion. In Figure 5a, 5b, 5c and 5dshows repeating disaccharide units of various glycosaminoglycans and its left side figure indicates number of replicating disaccharide units.
A. Protein Association Experimental evidence of covalent bondsbetween chitin and proteins has been obtained bymany researchers[14]. N-Acetylglucosamine andchitin react with α-amino acids, especially tyrosine,peptides, and cuticular proteins, to givestable complexes, dissociable, however, after pHchanges. Chitin, whether in its α-or β-form iscovalently linked to arthropodins, resilins, andsclerotins to form more or less stable glycoproteinsthrough aspartyl and histidyl residues. Owingto the stability of these complexes in hot alkaliand their instability in hot acids, the linkage couldprobably be as in N-acetylglucosamine which isbetween a carboxyl and the amino group of glucosamine.Other types of bonds have also beenreported. It is shown that predominant amino acidsin the residual chitin after rigorous alkalinehydrolysis are aspartic acid, serine, and glycine,which may be involved in the chitin-protein linkage[15]. In a similar perspective, Gottschalk proposeda linkage in the form of glycosidic esterbetween acetyl glucosamine and β-carboxylgroups of aspartyl residues[16]. Enzymatic studiesand acid hydrolysis of cuticle have also led to theconclusion that a stable linkage exists, proteinsbeing linked to chitin through a nonaromatic aminoacid[17].
B. Carotenoids Association Carotenoids occur in many different speciesof insects, usually as conjugates with proteins and chitin, giving rise to the green color of manyinsects. They include lutein, β-carotein,astaxanthin, and their derivatives. The pink orred color of crustacean shells is due to carotenoids;for example the crab Taliepus nuttallipossesses a red purplish epicuticle[18]. Crabs, likemany other animal species, convert digestedyellow plant carotenoids into oxygenated, andthus more polar, orange or red keto derivativesand in some instances conjugate the latter togive chromoproteins or calcerous esters, like inhydrochoral skeletons. Decalcified chips of cleaned carapace yieldtheir pigments to ethanol or acetone, warm aceticacid, cold formic acid, and a mixture of ammoniumsulfate + sulfuric acid. In view of this experimentalevidence, it was deduced that carotenoidsare combined with chitin amino groups by carbonylamino or Schiff’s base-type linkages[19].
C. Glucan Association With the exception of the lower fungi and mosttrue yeasts, which have cellulose and glucan in thecell wall, respectively, the most important structuralcomponent of the fungal cell wall is chitin.Proteins and polysaccharides such as mannans,glucans, galactans, and some heteropolysaccharidesrepresent the cementing material, which, by joiningthe skeletal components together, provide themacromolecular dimension of the cell wall[20]. In themacromoleculelar network of the cell, glucan chainsare linked to chitin through their reducing ends viaamino acids, particularly lysine[21]. Apart from thelinkages of the two polymers to each other, bothchitin and β-glucan chains are hydrogen bondedamong themselves. The β-glucan chains are solubilizedwhen chitin is depolymerized by chitinaseor by treatment with nitrous acid after deacetylation.The (1-3) -β-glucans and C-6 substituted (1-3)-β-glucan are known to form triple helices and bydoing so they would cross-link the chitin microfibrilsinto a rigid network[22,23].
APPLICATIONS
As characteristic features for use as industrial materials, chitin and chitosan are (1) from natural resource and biologically reproducible; (2) biodegradable and do not pollute natural environment; (3) biocompatible not only in animal but also in plant tissues, (4) almost nontoxic; (5) biologically functional; and (6) changeable in molecular structure. These features provide a stimulus to devising methods of adopting this valuable biopolymer for versatile applications. The multitudes of properties exhibited by glucosamine are listed here which reveals its potential for innumerable applications in several commercial products.
1). WATER PURIFICATION The largest single use of chitosan is the clarificationof waste and effluent water[24]. Better awarenessof the ecological and health problems associatedwith heavy metals and pesticides and theiraccumulation through the food chain has promptedthe demand for the purification of industrial waterprior to their discharge for use. The ability of theNH2 group of chitosan to form coordinate covalentbonds with metal ions is of great interest.
Chitosan powder and dried films have more potential use in metal ion complexing because it will release most of its free amino groups above the pKa. The use of commercially available chitosan for potable water purification has been approved by the United State Environmental Protection Agency (USEPA) up to a maximum level of 10 mg L–1.15 Chitosan, carboxymethyl chitosan, and cross-linked chitosan have been shown to be effective in the removal of Pb2+, Cu2+, and Cd2+ from drinking water[24,25]. Chitosan is highly efficient and effective than activated charcoal for purification of polychlorinated biphenyls from contaminated water.
2). AGRICULTURE Chitosan is still an underutilized biopolymerin various applications, for example, in agriculture.Research at Washington State University hasshown that the coating of wheat seeds with chitosanresults in increased crop yields, a practice (allowedby the USEPA) that has been adopted bymany so far[26]. The resistance response incited bychitosan results in an enhanced synthesis of plantRNA species. There are at least 20 proteins selectivelysynthesized in this resistance response. Finally, in this chitosan-induced resistance response there is a resultant increase in the activity of enzymes in major secondary pathways for the production of the antifungal phytoalexin, pisatin, as well as hydrolytic enzymes such as chitinase and glucanase [27]. Chitosan is also used as a controlled release agent for pesticides and herbicides. The incorporation of chitinous biomass into soil stimulates natural microbes that provide protection to certain crops [28].
3). PHARMACEUTICAL Increasingly over the last decade, chitosanhas been examined by the pharmaceutical industryfor possible biomedical applications[29]. Its polymeric cationic character along with its possession of a potentially reactive functional group have given it unique possibilities for utilization in controlled drug release technologies[30,31]. The drug is either physically blended or covalently linked to the amino groups of chitosan, generally is released from the chitosan matrix after contact with body fluids. Chitosan membranes have been proposed as artificial kidney membranes, possessing high mechanical strength in addition to permeability to urea and creatinine. Because they are impermeable to serum proteins and they probably might be unique in offering the advantage of preventingimmission of toxic metals into the bloodstream, as it currently happens with other membranes. N- and O-sulfated chitosans have been found to possess 15 to 45% of the anticoagulant activity of heparan in vitro[32]. The introduction ofuronic carboxyl groups has shown to increase the anticoagulant activity of sulfated chitosan. Chitosan at concentrations ranging from 6 to 50 neq m1–1 has been shown to aggregate L-1210 leukemia cells in vitro[33,34]. Therefore, the growth and invasive movement of cancer cells could be specifically inhibited by chitosan. Chitosan also has immunostimulating property, due partly to the stimulation of TNF-α, IL-1, and IL-6 production from monocytes, which could be very useful in the treatment of sepsis and viral diseases[35]. Chitosan has been shown to facilitate wound healing[36]. Chitosan is shown to be an effective and adequate hemostatic agent even under the mostsevere conditions of anticoagulation. It is a good substitute for blood during graft hemostasis[37].
4). BIOLOGICAL Cell and enzyme immobilization is a methodto keep them confined in a distinct phase separatedfrom the bulk phase while allowing exchange betweenthese phases. Cells and enzymes can easilybe trapped in ionotropic chitosan gels formed bymixing chitosan solutions with solutions of anionicpolymers (sodium alginate, carrageenan or pectin)[38]. Alternatively, enzymes can be covalentlylinked to chitosan. The immobilization of enzymes,namely, α-amylase, β-amylase, glucose isomerase,and amyloglucosidase on krill chitin activated byformaldehyde, was shown to be initiated by thegeneration of the hydrated form of formaldehydethat condenses with free amino groups of chitin,forming Schiff’s bases and dihydroxymethyl derivativesof aldehydes[39]. The former might be responsiblefor immobilization by reacting with variousfunctional groups of the enzymes, thus formingmethylene bridges[40]. A similar study reported 20%activity retention of crude seal gastric proteaseafter immobilization on glutraldehyde-treatedchitin.[41] The immobilization of penicillin acylaseon different physical forms of chitosan, namely,beads, particles, and powder was studied by Braunet al.,[42] who observed activity retention of 40, 95,and 100%, respectively. Microbial preparations,that contain enzymes, typically cultures or cells ofyeast, molds, and bacteria, can also be immobilized.The immobilization of cultured plant andmicrobial cells was studied by Knorr[43] and Bhat etal.,[44] who suggested that chitosan concurrently canserve as an effective immobilizing and permeabilizingagent for Ameranthus tricolor cellsand permeabilized yeast cells, respectively.
5). COSMETICS Depolymerized chitosan and carboxymethylchitinare being used as active ingredients of hair shampoo,conditioner, and treatment, because their aqueoussolutions are viscous, film forming, and moisture retaining, and give hair and skin softness[45].
6). FOODS Although the use of chitin and chitosan inprocessed food is currently limited, the conversionof processing discards into valuablebyproducts and alternative specialty materials hasbeen identified as a timely challenge for foodresearch and development associated with numerousapplications of chitinous polymers[46]. Thesebiopolymers offer a wide range of unique applications,including bioconversion for the productionof value-added food products, preservation offoods from microbial deterioration, formation ofbiodegradable films, recovery of waste materialfrom food processing discards, and clarificationand deacidification of fruit juices.
7). ANTIMICROBIAL ACTIVITY The growing demand for foods without chemicalpreservatives has focussed efforts in the discoveryof new natural antimicrobials[47]. In thiscontext, the unusual antimicrobial activity ofchitin, chitosan, and their derivatives against differentgroups of microorganisms, such as bacteria, yeast, and fungi, has received considerableattention in recent years[48].Because of the positive charge on C-2 of theglucosamine monomer at below pH 6, chitosan ismore soluble and has a better antimicrobial activitythan chitin[49]. The exact mechanism of theirantimicrobial action is still unknown. It is possiblethat the permeabilization of microbial cellmembrane, binding of trace metals, water bindingand the activation of several defense processes inhost tissue may inhibit microbial growth. These variations weresuggested to be due to the existing differences inthe dp and degree of acetylation of chitosan;chitosan with lower dp and with a degree of acetylationof ~7.5 was more effective[50]. Chitosan also has been shown to reduce the invitro growth of numerous fungi with the exceptionof zygomycetes, that is, the fungi containingchitosan as a major component of cell walls. Inaddition to the formation of gas permeable films, chitosan has a dual function, viz. its direct interferenceof fungal growth and activation of severa defense processes, such as the accumulation ofchitinase, synthesis of proteinase inhibitors, lignification,and induction of callous synthesis[51]. The antifungal effect of chitosan on in vitroand in vivo growth of common postharvest fungalpathogens in strawberry fruits showed that chitosanwith an –NH2 content of 7.5% markedly reducesthe radial growth of Botrytis cinerea and Rhiozopusstolonifer.[52] A 2% chitosan coating was also shownto inhibit sclerotina rot of carrot. N-Carboxymethylchitosan reduced aflatoxin production inA. flavus and A. parasiticus by more than 90%,while fungal growth was reduced to less thanhalf [53]. These results suggest that coating fruitsmay have some positive advantages for their longtermstorage.
8). PACKAGING FILMS The use of edible films and composite coatingsto extend the shelf life and improve the qualityof fresh, frozen, and fabricated foods has beenexamined during the last few years[54-56]. Due totheir film-forming properties, chitin and chitosanhave been used as food wraps. The use of N,Ocarboxymethylchitin films to preserve fruits overa long period has been approved in several countries.Due to its ability to form semipermeablefilm, chitosan can be expected to modify the internalatmosphere as well as decrease the transpirationloss and delay the ripening in fruits[56].The preparation of chitosan films and chitosanlaminatedfilms with other polysaccharides hasbeen reported by various authors[54-57]. Chitosan films are tough, long lasting, flexible, and difficult to tear. Most of their mechanical properties are comparable to those of many commercial polymers films. We have shown that chitosan films have moderate water vapor permeability and exhibit good barrier to permeation of oxygen. The extension of the storage life and better control of the decay of peaches, Japanese pears, kiwifruits, cucumbers, bell peppers, strawberries, and tomatoes with the application of chitosan film has been documented[58]. Similarly, we have also shown that banana and mango could be stored for long periods after coating with chitosan and its N,Ocarboxymethyl derivative[59]. These results are mainly attributed to decrease respiration rate, delayed ripening due to the reduction of ethylene and carbon dioxide evolution, and inhibition of fungal development.
9). CONTROL OF ENZYMATIC BROWNING IN FRUITS Phenolic compounds, together with the activityof polyphenol oxidase, are responsible forbrowning, which affects the color, taste, and nutritionalvalue of fruits and vegetables[60,61]. Theeffect of the application of a chitosan-coated filmon enzymatic browning of litchi fruit delayedchanges in the contents of anthocyanins, flavonoids,and total phenolics. It was also shown to delay an increase in polyphenol oxidase activityand partially inhibit the increase in peroxidaseactivity[62].
10). CLARIFICATION AND DEACIDIFICATION OF FRUIT JUICES Chitosan salts, which carry a strong positivecharge, have been shown to be effective asdehazing agents, and they can also be used tocontrol acidity in fruit juices[63]. Chitosan has beenshown to be a good clarifying agent for grapefruitand apple juice either with or without pectinasetreatment, which gives zero turbidity productswith 0.8 kg m–3 of chitosan[64]. Chitosan has also agood affinity for polyphenolic compounds suchas catechin, proanthocyanidines, cinnamic acid,and other derivatives that can change the initial straw-yellow color of white wine into a deepgolden yellow color[65].
11). ANTIOXIDANT PROPERTIES Muscle food products are highly susceptibleto off-flavor and rancidity development causedby the oxidation of their highly unsaturated lipids.The effectiveness of chitosan treatment on oxidativestability showed that the addition of chitosanat 1% resulted in a decrease of 70% in2-thiobarbituric acid (TBA) values of meat after3 days of storage at 4?C. A 93% inhibition ofTBA and a 99% reduction in hexanal content wasreported in meat after treatment with N-carboxymethylchitosan[66]. N,O-Carboxymethyl chitosan(NOCC) and its lactate and acetate salts weresimilarly effective in controlling the oxidationand flavor deterioration of cooked meat over a9-day storage at refrigerated temperatures[67]. Themechanism by which this inhibition takes place isthought to be related to chelation of free iron,which is released from hemoproteins of meatduring heat processing. This would, in turn, inhibitthe catalytic activity of iron ions.
12). NUTRITIONAL EFFECTS Multiple actions of chitin and chitosan in foodsystems relate to their effects as dietary fiber andas a functional food ingredient. The United StatesFood and Drug Administration has approvedchitosan as a feed additive[68]. Chitosan is also used in the food industry as a food quality enhancer incertain countries like Japan, Italy, and Norway.The nutritional significance of chitinous polymersin animals as a feed additive has shownlower cholesterol and triacylglycerol values inrabbits, hens, and broilers fed on chitin andchitosan at < 1.4 g kg–1 body weight without anyadverse effect on normal growth pattern[69]. Thelowering of cholesterol was attributed to enhancedreverse cholesterol transport in response to intestinallosses of dietary fats[70].The effect of chitin as a feed additive on the enhanced growth of bifidobateria in the guts of chickens[71] Bifidobacteria, in addition to inhibiting the growth of other types of microorganisms, also generate the lactase and galactokinase, which are required for the digestion of milk lactose and galactose metabolism. This is of significance for humans with lactose intolerance and galactose accumulation in old age. The use of chitin as a functional food ingredient in dough fermentation for bread has been reported[72,73]. The loaf volume of wheat bread increased when up to 2% microcrystalline chitin was added. Chitin shows most of the criteria of dietary fiber and has a highly characteristic property in relation to other plant dietary fibres. The nondigestibility in the upper gastrointestinal tract, polymeric nature and high water-binding properties leading to high viscosity are responsible for the hypocholesterolemic potential of chitin/chitosan[74]. Due to the ability of forming ionic bonds at low pH, it can bind in vitro t o different types of anions, such as bile acids or free fatty acids[75]. Large proportions of these escape hydrolysis by lipase, promoting the excretion of fatty materials, including cholesterol, sterols, and triacylglycerols[76]. Inside the digestive tract, chitosan forms micelles with cholesterol, both endogenous and from dietary sources, in the alkaline fluids in the upper part of the intestine, resulting in a decrease in the absorption of dietary cholesterol and circulation of cholic acid to the liver. Because of the decreased cholic acid level, cholic acid synthesis from cholesterol in the liver tends to decrease blood cholesterol concentration. In the stomach chitosan is solubilized to form an emulsion with intragastric oil droplets, which later precipitates out at pH 6 to 6.5 in the small intestine. With the aggregation of polymeric chains, the oil droplets are entrapped in their matrices, thereby passing through the lumen and emptying into feces. Being an excellent polyelectrolyte, the use of chitosan in polymer batteries has been recently studied. The availability of several polar groups (OH and NH2) in its structure can act as electron donors and interact with inorganic salts[77]. Such interactions have been proven by X-ray photoelectron spectroscopy.
13). ENVIRONMENTAL-RESPONSIVE CHITOSAN-BASED NANOPARTICLES BY SELF-ASSEMBLY METHOD Environmental-responsive nanoparticles of chitosan-graft-poly(N-isopropylacrylamide) copolymers (CS-g-PNIPAAm) were prepared by the self-assembly method. The copolymer was first synthesized through polymerization of NIPAAm monomer in the presence of CS in an aqueous solution using cerium ammonium nitrate as the initiator. Then, the CS-g-PNIPAAm solution was diluted by deionized water and heated to a proper temperature for CS-g-PNIPAAm to undergo self-assembly. Micelles of CS-g-PNIPAAm were formed, and glutaraldehyde was added to reinforce the micelle structure to form nanoparticles. TEM images showed that a porous or hollow structure of nanoparticles was developed. The synthesized nanoparticles carried positive charges on the surface and their mean diameter could be manipulated by changing the temperature of environment. These nanoparticles with environmentally sensitive properties are expected to be utilized in hydrophilic drug delivery system.As shown in figure 6, CS-PNIPAAm nanoparticles were successfully prepared by using self-assembly method. At an appropriate concentration and temperature, CS-g-PNIPAAm self-assembled to form micelles. After crosslinking, the CS-PNIPAAm nanoparticles were obtained. Structure and morphology of nanoparticles were investigated. From TEM observation, we found a porous or hollow structure for nanoparticles of CS-PNIPAAm. The synthesized nanoparticles were found to be environmentally responsive, in which their particle size could be manipulated by changing the temperature of the medium. Because of their thermo- sensitive behavior, the CS-PNIPAAm nanoparticles have potential to be applied in hydrophilic drug delivery and long-time controlled release vehicle[78].
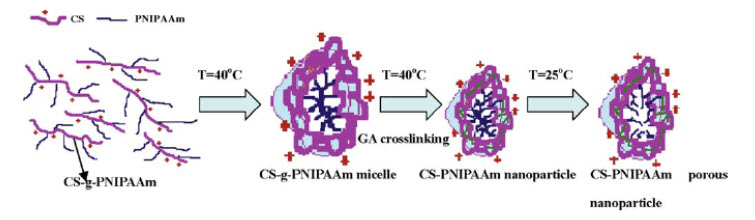
Figure 6: Nanoparticles self-assembly synthesis
14). EFFECT ON WOUND HEALINGChitin and chitosan have many useful and advantageous biological properties in the application as a wound dressing, namely biocompatibility, biodegradability, hemostatic activity, anti-infectional activity, and a property to accelerate wound healing. We studied the effects of chitin and chitosan on wound healing, and found that these materials induced the activation of a complement system, polymorphonuclear cells fibroblasts and vascular endothelial cells. When chitin and chitosan are applied in the body, these materials are biodegraded by some enzymes such as chitinase and chitosanase, and subsequently become to their oligomers and monomers. Our previous results indicated that not only chitin and chitosan but also their oligomers and monomers influenced fibroblasts and endothelial cells migration. This suggests that their oligomers and monomers influence wound healing in vivo. In addition to chitin/chitosan, their oligomers/ monomers were found to enhance wound healing acceleration. Wound break strength and collagenase activity of the chitosan group (D-glucosamine (GlcN), chito-oligosaccharide (COS), chitosan) were higher than the chitin group (N-acetyl-D-glucosamine (GlcNAc), chiti-oligosaccharide (NACOS), chitin). In histological findings, collagen fibers run perpendicular against the incisional line in the oligosaccharide group (NACOS, COS) and many activated fibroblasts were observed around the wound in the chitosan group. In figure 7deacetylation, the higher the degree of deacetylation was, the stronger the break strength and more activated fibroblasts were observed[79].
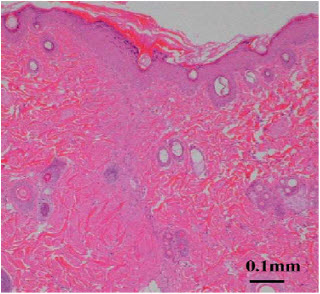
Figure 7: More activated fibroblasts were observed in the wound.
15). PRODUCTION OF SUPERABSORBENTS FROM CARBOXYMETHYL CHITOSAN Superabsorbent polymers (SAP) are highly hydrophilic polymer networks which are three-dimensionally crosslinked and able to swell, absorb and retain water up to a 100 times their dry weight. They are nowadays widely used in personal care and hygienic Products Crosslinked polymers of acrylic acid are the most common materials for production of SAP, resulting in a high water-binding capacity (WBC) and water-binding rate (WBR), even under load. these materials do not have good WBC compared to polyacrylates. Therefore, it is necessary to convert them to more hydrophilic materials by e.g. carboxymethylation. Carboxymethyl cellulose and carboxymethyl starch have been successfully used for production of superabsorbents. Carboxymethyl chitosan (CMCS) is a well-known water-soluble derivative of chitosan[80].
16). ANALGESIC EFFECT The analgesic effects of chitin and chitosan on inflammatory pain were evaluated using the acetic-acid-induced writhing test in mice.When chitin and chitosan suspensions were mixed with the 0.5% acetic acid solution (chitin-AC and chitosan-AC, respectively) andadministered intraperitoneally in mice, both agents induced a dose-dependent decrease in the number of the abnormal behaviors (writhing) due to pain, including extension of the hind legs, abdominal rigidity, and abdominal torsion. This effect was greater in the animals administered the chitosan-AC than in those administered the chitin-AC. In vitro study indicated that addition of the chitin or chitosan suspension increased the pH of the AC, and that this effect was greater in the chitosan than the chitin. Furthermore, the level of bradykinin in the peritoneal lavage in the animals administered the chitin-AC was lower than in the animals administered the chitosan-AC. In vitro study showed that the chitin particles absorbed bradykinin more extensively than the chitosan particles. These results suggest that the main analgesic effect of chitosan is the absorption of proton ions released in the inflammatory site, while that of chitin is the absorption of bradykinin[81].
17). EFFECT ON BLOOD COAGULATION The effects of chitin and chitosan on blood coagulation and platelet aggregation using canine blood were evaluated. Whole blood was mixed with chitin and chitosan suspensions (0.0001–1.0 mg/ml), and then the blood coagulation time (BCT) was measured using the modified Ree-White method. Chitin and chitosan reduced BCT in a dose-dependent manner. Platelet rich plasma (PRP) was mixed with chitin and chitosan suspensions, and then platelet aggregation (PA) was measured using a Dual aggregometer. The PA level induced by chitin was the strongest in all samples including chitosan, cellulose, and latex. When the washed platelet was used, the PA level induced by chitin was similar to that of chitosan, while the rate of coagulation was lower than that of PRP. Chitin and chitosan enhanced the release of the platelet derived growth factor-AB (PDGF-AB) and the transforming growth factor-b1(TGF-b1) from the platelets, particularly, more with chitosan. The platelets were found to adhere strongly to chitin and chitosan particles. Furthermore, this effect enhanced in the presence of plasma. This result is figure 8consistent with the data of PA. Chitin and chitosan have amino residue in their chemical structure, but cellulose did not although another chemical structure is the same. This suggests that amino residue is important in platelets aggregation.
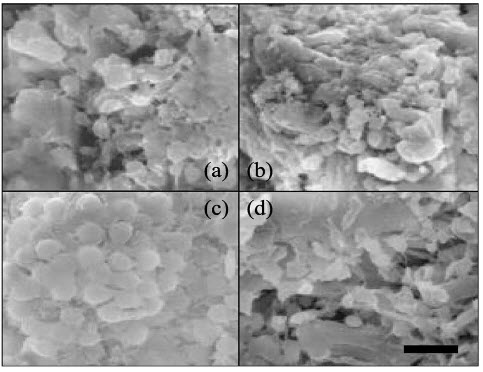
Figure 8: Morphology of platelet aggregation.
(a) Chitin (6.9 mm), (b) chitosan (6.2 mm), (c) latex, (d) cellulose. After measurement of platelet aggregation (PA)
18). CHITIN AND CHITOSAN BASED NANOMATERIALS Chitin and chitosan are biopolymers having immense structural possibilities for chemical and mechanical modifications to generate novel properties, functions and applications especially in biomedical area. Chitin and chitosan are effective materials for biomedical applications because of their biocompatibility, biodegradability and non-toxicity, apart from their antimicrobial activity and low immunogenicity, which clearly points to an immense potential for future development. These candidate biopolymers can be easily processed into gels, sponges, membranes, beads and scaffolds forms. This review emphasizes recent research on different aspects of chitin and chitosan based nanomaterials, including the preparation and applications of chitin and chitosan based nanofibers, nanoparticles and nanocomposite scaffolds for tissue engineering, wound dressing, drug delivery and cancer diagnosis. The novel applications for which these natural biopolymers can be put to use in a variety of nanostructural forms and sizes. Nanostructured composite scaffolds can be developed as promising tissue engineered constructs or for wound healing. Multifunctional use of chitin and chitosan based nanomaterials have been proved to aid simultaneous cancer targeting and drug delivery. We expect that this review will provide insights on the use of these important of chitin and chitosan nanomaterials for researchers working in nanobiotechnology[82].
19). ANTIINFLMMATORY ACTION Glucosamine, a naturally occurring amino monosaccharide, is present in the connective and cartilage tissues as a component of glycosaminoglycans. Thus, glucosamine has been widely used to treat osteoarthritis, a joint disease characterized by cartilage degeneration, in humans. In addition, glucosamine is expected to exert an anti-inflammatory action, since glucosamine suppresses inflammatory cell activation. To further extend the anti-inflammatory actions of glucosamine, we investigated the effects of glucosamine on synovial cells, endothelial cells and intestinal epithelial cells using in vitro and in vivo systems. glucosamine administration repressed the formation of atherosclerotic lesion and infiltration of inflammatory cells into the lesion in spontaneously hyperlipidemic mice. glucosamine can function as not only a chondroprotective agent but also an anti-inflammatory molecule in the body[83].
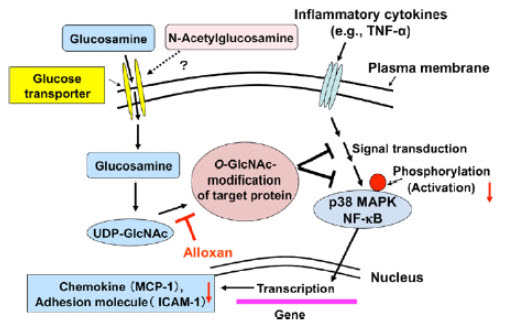
Figure 9: Possible mechanism for the O-GlcNAc modification-mediated suppression of endothelial cell activation by glucosamine.
20). CARDIOPROTECTIVE ACTION The modification of serine and threonine residues of nuclear and cytoplasmic proteins by O-linked β-Nacetylglucosamine (O-GlcNAc) has emerged as a highly dynamic post-translational modification that plays a critical role in regulating numerous biological processes. Much of our understanding of the mechanisms underlying the role of O-GlcNAc on cellular function has been in the context of its adverse effects in mediating a range of chronic disease processes, including diabetes, cancer and neurodegenerative diseases. However, at the cellular level it has been shown that O-GlcNAc levels are increased in response to stress; augmentation of this response improved cell survival while attenuation decreased cell viability. Thus, it has become apparent that strategies that augment O-GlcNAc levels are pro-survival, whereas those that reduce O-GlcNAc levels decrease cell survival. There is a long history demonstrating the effectiveness of acute glucose–insulin–potassium (GIK) treatment and to a lesser extent glutamine in protecting against a range of stresses, including myocardial ischemia. A common feature of these approaches for metabolic cardioprotection is that they both have the potential to stimulate O-GlcNAc synthesis. Consequently, here we examine the links between metabolic cardioprotection with the ischemic cardioprotection associated with acute increases in O-GlcNAc levels. Some of the protective mechanisms associated with activation of O-GlcNAcylation appearto be transcriptionally mediated; however, there is also strong evidence to suggest that transcriptionally independent mechanisms also play a critical role. In this context we discuss the potential link between OGlcNAcylation and cardiomyocyte calcium homeostasis including the role of non-voltage gated, capacitativecalcium entry as a potential mechanism contributing to this protection[84].
CHITOOLIGOMERS
Research on the preparation and physiological activities of chitooligomers has continuously attracted much attention in the food, pharmaceutical, and agricultural fields due to their versatile antitumor activity, immunoenhancing effects, protective effects against some infectious pathogens in mice, antifungal, and antimicrobial activities[85]. In plants, chitooligomers are thought to elicit the formation of fungal cell wall degrading enzymes such as β 1,3-glucanase and chitinase. Oligomers with six or more residues show strong physiological activities. Studies have shown the antitumorigenic properties of chitin and chitosan oligomers in the inhibition of the growth of tumor cells via an immuno-enhancing effect[86]. N-Acetylchitooligosaccharides, from (GlcNAc)4 to (GlcNAc)7 display a strong attracting response to peritoneal exudate cells in BALB/C mice, whereas (GlcN)2 to (GlcN)6 did not exhibit such an effect. Chitin and chitosan oligomers, (GlcNAc)6 and (GlcN)6, also show a tumor growth-inhibitory effect in allogenic and syngeneic mouse system, including Sarcoma 180 and MM 46 solid tumor.Further, it was concluded that the effect was host mediated and not by direct cytocidal action on the tumor cells[87]. The two oligosaccharides (GlcNAc)6 and (GlcN)6 exhibiting growth-inhibitory effect against Meth-A solid tumor transplanted into BALB/C mice was assumed to involve the increased production of lymphokines, including interleukin 1 and 2, sequentially leading to the manifestation of antitumor effect through proliferation of cytolytic T-lymphocytes. A significant antimetastatic effect for (GlcNAc)6 in mice bearing Lewis lung carcinoma has also been reported[88]. The mechanism of action is assumed to involve the activation of phagocytic cells attracted to the peritoneal cavity from blood and lymphoid fluid. Chitooligomers were responsible for enhancing protective effects against infection with some pathogens in mice. The protective effect of chitin oligomers in mice infected with Listeria monocytogenes was based on the fact that interferon-gand interleukin 2 were able to enhance the growth-inhibitory effect by (GlcNAc)6-treated macrophages. (GlcNAc)6 induced phytoalexin formation has been shown in suspension-cultured rice cells[49]. GlcNAc oligomers smaller than trimers and a series of deacetylated oligomers had almost no activity. Chitooligomers of DP 3 to 7 are shown to elicit the formation of pisatin in immature pea pods, and those of DP 4 to 6 induce the synthesis of protease inhibitor in tomato leaves49. A hexamer of GlcNAc as well as GlcN is a growth inhibitory substance against Sarcoma 180 in mice[53]. Chitosan oligomers as well as chitosan have been shown to inhibit the growth of several fungi and bacteria, especially pathogens. The relationship between the dp of chitosan and the inhibition effect has been studied. It is shown that chitosan oligomers (dp 2 to 8) as well as low-molecularweight chitosan (LMWC) possessed stronger growth inhibitory effect than high-molecularweight chitosan against several pathogens, including Fusarium oxyporum, Phomopsis fukushi, Alternaria alternata, among others.102 The oligomers with higher molecular weight are slightly hydrolyzed with chitosanase and were more active in both antifungal and antibacterial activities than native chitosan[89]. Of the three oligomers produced by enzymatic reactor system, the highest molecular weight oligomers (MW 5000 to 10,000 Da) had the strongest bactericidal and fungicidal activities. LMWC as well as chitooligomers also have been shown to possess hypocholesterolemic and hypolipidemic properties[90]. Ikeda et al. have studied the relationship between the molecular weight and hypocholesterolemic effect113. They showed that chitosan hydrolyzates with water like viscosity and molecular weight of 5000 to 20,000 were more effective than high-viscous chitosans with molecular weights of 50,000 or above. The beneficial lowering effect on plasma cholesterol may play an important role in the prevention and treatment of cardiovascular diseases.
FUTURE PROSPECTUS
The most plentiful natural biomolecule chitin has kindled increasing attention by all partly because of the recent understanding of its multifaceted functionality in biology, biotechnology, and medicine. Its application versatility is a great challenge to scientific community and to industry. The commercial availability of high-purity forms of chitin and chitosan and the continuous appearance of new types of chitin/chitosan derivatives with more and more useful and specific properties have led to unlimited R&D efforts on this most versatile amino polysaccharide, chitin. The efficient utilization of marine biomass resource not only augers in converting wastes from marine food-processing industries into useful products, but it has become an environmental priority too. It is anticipated that its additional specific applications will be realized in the near future.
REFERENCES
1. Horton D, Wander JD. The Carbohydrates. New York: Academic Press. 1980; Vol IB: 727–8.
2. Ruiz-Herrera J. The distribution and quantitative importance of chitin in fungi. In Proc. First Internal. Conf. Chitin and Chitosan. Muzzarelli R A A. and Priser J. (Ed), MIT Sea Grant Report. 1978; 78(7):11–21.
3. Jeuniaux C. Chitinous structures. In, Comprehensive Biochemistry. Florkin and Stotz (Eds.), Elsevier (Amsterdam). 1971;26C, 595–632,
4. Lehninger, Nelson, Cox. Carbohydrates and Glycobiology. In, Albert L Lehninger (Ed). Principle of biochemistry. USA, Worth publishers, 1993; 243.
5. Roseman S, Reflections on glycobiology. J Biol Chem. 2001; 276 (45): 41527–42.
6. Ghosh S, Blumenthal H J, Davidson E, Roseman S. Glucosamine metabolism. V. Enzymatic synthesis of glucosamine 6-phosphate. J Biol Chem. 1960; 235(5):1265.
7. Muzzarelli R A A. Chitin, Pergamon Press Ltd., NY, 1977.
8. Braek G S, Anthonsen T and Sandford P. (Eds.). Chitin and Chitosan. Elsevier Applied Science, NY,1989.
9. Ramakrishnan C and Prasad N. Rigid-body refinement and conformations of α-chitin. Biochem. Biophys. Acta. 1972; 261:123–35.
10. Winterowd J G and Sandford P A. Chitin and Chitosan. In, Food Polysaccharides and Their Applications, Stephen A M (Ed.). Marcel Dekker, Inc., NY, 1995; 441–462.
11. Rudall K M. Skeletal structures in insects. Biochem. Soc. Symp. 1965; 25: 83–87.
12. Cabib E. The synthesis and degradation of chitin. Adv. Enzymol. 1987; 59: 59–101.
13. Andrey A K and Vladimir A G. Glycoproteins with a chitin-like carboydrate moiety in insect cells. In, Chitin and Chitosan. Elsevier Applied Science, NY.1989; 793–801.
14. Sromova D and Lysek H. Visualization of chitinprotein layer formation in Ascaris lumbricoides eggshells. Folia Parasitol. (Praha). 1990; 37: 77–80.
15. Brine C J and Austin P R. Chitin isolates: species variation in residual amino acids. Comp. Biochem. Physiol. 1981; 70B: 173–8.
16. Gottshalk A, Murphy W H and Graham E R B. Carbohydrate-peptide linkages in glycoproteins and methods for their elucidation. Nature, 1962; 194: 1051– 53.
17. Lipke H and Geoghegan T. The composition of peptidochitodextrins from sarcophagial purparial cases. Biochem. J., 1971; 125: 703–16.
18. Deuchi K, Kanauchi O, Shizukushi M and Kobayashi E. Continuous and massive intake of chitosan affects mineral and fat soluble vitamin status in rats fed on high fat diet, Biosci. Biotechnol. Biochem. 1995; 59: 1211–6.
19. Fox D L. Chitin-bound keto-carotenoids in a crustacean carapace. Comp. Biochem. Physiol., 1973; 44B: 953– 62.
20. Muzzarelli R A A, Tanfani F, and Scarpini G. Chelating, film forming and coagulating ability of chitosan-glucan complex from Aspergillus niger industrial waste. Biotechnol. Bioeng., 1980; 22: 885–96.
21. Sietsma J H, and Wessels J G H. Solubility of (1-3)- β-D/(1-6)-β-D-glucan in fungal walls: Importance of presumed linkage between glucan and chitin. J. Gen. Microbiol., 1981; 125: 209–121.
22. Jelsma J and Kregar D R. Ultrastructural observations on (1-3)-β-D-glucan from fungal cell walls. Carbohydr. Res. 1975; 43: 200–03.
23. Marchessault R H and Deslandes Y. Fine structure of (1-3)-β-D-glucans: Curdlan and paramylon. Carbohydr. Res., 1979; 75: 231–42.
24. Kurita K Ishiguro M and Kitajima T. Studies on chitin. 17. Introduction of long alkylidene groups and their influence on the properties. Int. J. Biol. Macromol., 1988; 10: 124–25.
25. Hirano S, Ohe Y and Ono H. Selective N-acetylation of chitosan. Carbohydr. Res., 1976; 47: 315–20. |26. Muzzarelli R A A. Amphoteric derivatives of chitosan and their biological significance, In Chitin and Chitosan, Braek G S, Anthonsen T and Sandford P (Eds.), Elsevier Applied Science, NY, 1989; 87–99. 27. Hadwiger L A, Fristensky B, and Riggleman R C. in Chitin, Chitosan and Related Enzymes. Zikakis J P. (Ed.), Academic Press, NY, 1984; 291. 28. Walker Simmons M, Hadwiger L and Ryan R A. Chitosan and pectic polysaccharides both induce the accumulation of the antifungal phytoalexin pisatin in pea pods and antinutrient proteinase inhibitors in tomato leaves. Biochem. Biophys. Res. Comm., 1983; 110: 194–99. 29. Struszezyk H, Pospieszmy H and Kotlinski S. Some new applications of chitosan in agriculture, in Chitin and Chitosan. Braek G S, Anthonsen T, and Sandford P. (Eds.), Elsevier Applied Science, NY, 1989; 733–42.
30. Shu X Z, Zhu K J, and Song W. Novel pH sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm., 2001; 212: 19–28.
31. Nagai T, Sawayangi Y, and Nambu N. In, Chitin, Chitosan and Related Enzymes. Zikakix J P. (Ed.) Academic Press, NY, 1984; 21.
32. Baba S, Uraki Y, Miura Y and Tokura S. Controlled release and hydrolysis of prodrug using carboxymethylchitin as a drug carrier. In, Chitin and Chitosan, Brack G S, Anthonsen T, and Sandford P. (Eds.), Elsevier Applied Science, NY, 1989; 703–74.
33. Whistler R and Kosik M. Anticoagulant activity of oxidized and N- and O-sulfated chitosan. Arch. Biochem. Biophys. 1971; 142: 106-110.
34. Nishinari K. and Doi E. (Eds.). Food Hydrocolloids. Plenum Press, NY, 1993. 35. Sirica A E and Woodman R J. Selective aggregation of L1210 Leukemia cells by the polycation chitosan. J. Natl. Cancer Inst., 1971; 47: 377–88.
36. Suzuki S, Okawa Y, Okura Y, Hashimoto K and Suzuki M. Immunoadjuvant effect on chitin and chitosan, Proc. 2nd Internet Conf. Chitin and Chitosan, Jap. Soc. of Chitin and Chitosan, Tottori, Japan, 1982.
37. Malette W G and Quigley H J. Methods for the Therapeutic Occlusion of Blood Vessels. U.S. Pat. 4,452,785, June 5, 1984. 38. Itoi H, Komiyama N, Sano H and Mandai H. Pharmaceutical Bandages. Japanese Patent Kokai. 60–142927, July 29, 1985.
39. Protan Laboratories, Inc., Chitosan for Cell Immobilization, PL1–004, Redmond, WA, 1987. 40. Protan Laboratories, Inc., Sea CureTM Chitosan for Immobilization of Enzymes, PL1–003, Redmond, WA, 1987. 41. Synowiecki J, Sikirsk Z E, Naczk M N and Piotrzjiwsjam G. Immobilization of enzymes on krill chitin activated by formaldehyde. Biotechnol. Bioeng., 1982; 24: 1871–6. 42. Han X and Shahidi F. Extraction of harp seal gastric proteases and their immobilization on chitin. Food Chem., 1995; 52: 71–6. 43. Brann J, Chanu P I and Goffic F L. The immobilization of penicillin G acylase on chitosan. Biotechnol. Bioeng., 33, 242–246, 1989.
44. Knoor E. Chitosan gels for entrapment of cultured plant cells. In, Chitin in Nature and Technology. Muzzarelli R A A, Jennianz C, and Gooday G W. (Eds.), Plenum Press, NY, 1986; 428–31. 45. Bhat S G, Nagajyothi Upadya R, Shailasree S, Tharanathan R N and Kittur F S. A Process for the Preparation of an Immobilized Biocatalyst. Indian Patent 533/DEL/00. 46. Gross P, Konrad E and mager H. Chitin-chitosan, Proc. 2nd Internet Conf. Chitin and Chitosan, Jap. Soc. of Chitin and Chitosan, Tottori, Japan, 1982; 205–9. 47. Ouattar B, Simard R E, Piett G, Begin A and Hollye R A. Inhibition of surface spoilage bacteria in processed meats by application of antimicrobial films prepared with chitosan. Int. J. Food Microbiol., 2000; 62: 139–48.
48. Yalpani M, Johnson F and Rotinson L E. Adv. Chitin and Chitosan, Brine C J, Sandford P A and Zikakis J P. (Eds.) Elsevier Applied Science, London, 1992; 543. 49. Rinaudo M and Domard A. Solution properties of chitosan, in Chitin and Chitosan, Braek C J, Sandford P A, and Zikakis J P. (Eds.), Elsevier Applied Science, London, 1989; 71–76. 50. Chen C, Lian W and Isai G. Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J. Food Protec., 1998; 61: 1124–8.51. Schlumbaum A, Mauch F, Vogel U and Boller T. Chitinase are potent inhibitors of fungal growth. Nature, 1986; 324: 365–7. 52. EI Ghaonth A, Arul J, Asselin, A., and Benhamon N. Antifungal activity of chitosan on post-harvest pathogens: induction of morphological and cytological variations on Rhizopur stolonfier. Mycol. Res., 1992; 96: 769–79. 25 53. Cuero R G, Osufi G and Washington A. NCarboxymethylchitosan inhibition of aflatoxin production: Role of zinc. Biotechnol. Lett., 13, 441–444,1991. 54. Kester J J and Fennema O R. Edible films and coatings: a review. Food Technol., 1986; 40: 47–59. 55. Lobuza T P and Breene W M. Application of active packaging for improvement of shelf life and nutritional availability of fresh and extended shelf life in foods. J. Food Proc. Preserv., 1989; 13: 1–69. 56. Kittur F S, Kuman K R and Tharanathan R N. Functional packaging properties of chitosan films. Z. Lebensen-unters. Forsch. A, 1998; 206: 44–7. 57. Chen M, Yeh G H and Chiang B. Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. J. Food Proc. Preserv. 1996; 20: 379–90. 58. EI Ghaouth A, Arul J, Ponnampalam R and Boulet A. Chitosan coating effect on storability and quality of fresh strawberries. J. Food Sci., 1991; 56: 1618–1620. 59. Kittur F S, Saroja N, Habibunnisa and Tharanathan R N. Polysaccharide based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol., 2001; 213: 306–311. 60. Huanpu M, Blake P S, Browning G and Taylor J M. Metabolism of gibberlins A1 and A3 in fruits and shoots of Prunus airum. Phytochem. 2001; 56: 67–76. 61. Kader A. Biochemical and physiological basis for effects of controlled and modified atmosphere on fruits and vegetables. Food Technol., May, 1986; 99–104.
62. Sapers G M and Douglass F W, Jr. Measurement of enzymatic browning at cut surfaces and in juice of raw apple and pear fruits. J. Food Sci., 1987; 52: 1258– 62.
63. Imeri A G and Knoor D. Effect of chitosan on yield and compositional data of carrot and apple juice. J. Food Sci., 1998; 53: 1707–09.
64. Soto-Perlata N V, Muller H, and Knoor, D. Effect of chitosan treatment on the clarity and color of apple juice. J. Food Sci., 1999; 54: 495–6.
65. Spagna G, Piffer P G, Rangoni C, Mattivi F, Nicollni G and Palmonari, R. The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res. Int., 29, 241–248, 1996. 26 66. St. Angelo, A.J. and Vercellotti, J.R. Inhibition of Warmed-Over Flavour and Preserving of Uncured Meat Containing Materials. US patent, 4,871,556, 1989. 67. Xie W, Xu P and Liu Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett., , 2001; 11: 1699–701. 68. Knoor D. Nutritional quality, food processing and biotechnological aspects of chitin and chitosan: A review. Process Biochem., 1986; 6: 90–92. 69. Hirano S. Production and application of chitin and chitosan in Japan. In Chitin and Chitosan, Braek G S, Anthonsen T and Sandford P. (Eds.), Elsevier Applied Science, NY, 1989; 37–43. 70. Wuolijoki E, Hirvela T and Ylitalo P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods Exp. Clin. Pharmacol., 1999; 21: 357–61.
71. Austin P R, Brine C J, Castle J E and Zikakis J P. Chitin: new facets of research. Science, 1981; 212: 749–53. 72. Knoor D and Betschart A A. The relative effect of an inert substance and protein concentration of loaf volumes of breads. Trends Food Sci. Technol., 1978; 11: 198–205. 73. Knoor D and Betschart A A. Water absorption and loaf volume of protein fortified breads. Trends Food Sci. Technol., 1981; 14: 306–16. 74. Chiang M T, Yao H T and Chen H C. Effect of dietary chitosans with different viscosity on plasma lipids and lipid peroxidation in rats fed on a diet enriched with cholesterol. Biosci. Biotechnol. Biochem., 2000; 64: 965–971. 75. Muzzarelli R A A. Chitosan-based dietary foods. Carbohydr. Polymers. 1996; 29: 309- 16. 76. Zacour A C, Silva M E, Cecon P R, Bumbirra E A and Vieira E C. Effect of dietary chitin on cholesterol adsorption and metabolism in rats. J. Nutr. Sci. Vitaminol., 1992; 38: 309–16. 77. Osman Z, Ibrahim Z A and Arof A K. Conductivity enhancement due to ion dissociation in plasticized chitosan based polymer electrolytes. Carbohydr. Polymers, 2001; 44: 167–73.
78. Chung-Yang Chuang, Trong-Ming Don, Wen-Yen Chiu Preparation of environmental- responsive chitosan-based nanoparticles by self-assembly method, journal of Carbohydrate Polymers, Elsevier Ltd. CARP-4632, 2010 27 79. Tatsuya Minagawa, Yasuhiko Okamura, Yoshihiro Shigemasa, Saburo Minami, Yoshiharu Okamoto, Effects of molecular weight and deacetylation degree of chitin/chitosan on wound healing, journal of Carbohydrate Polymers, Elsevier Ltd. 2007; 67: 640–44.
80. Akram Zamani, Dag Henriksson, Mohammad J Taherzadeh, A new foaming technique for production of superabsorbents from carboxymethyl chitosan, journal of Carbohydrate Polymers, Elsevier Ltd., 2010; 80: 1091–101.
81. Y Okamoto, K Kawakami, K Miyatake, M Morimoto, Y Shigemasa, S Minami. Analgesic effects of chitin and chitosan, journal of Carbohydrate Polymers, Elsevier Ltd. 2002; 49: 249-52.
82. R Jayakumar, Deepthy Menon, K Manzoor, S V Nair, H Tamura. Biomedical applications of chitin and chitosan based nanomaterials, journal of Carbohydrate Polymers, Elsevier Ltd. 2010; 82: 227–32. 83. I Nagaokaa, M Igarashia, J Huaa, Y Jua, S Yomogidaa, K Sakamotob. Recent aspects of the anti-inflammatory actions of glucosamine, journal of Carbohydrate Polymers, Elsevier Ltd. CARP-4762, 2010
84. John C Chatham, Richard B Marchase, The role of protein O-linked β-N- acetylglucosamine in mediating cardiac stress responses, Biochimica et Biophysica Acta, Elsevier Ltd. 2010; 1800: 57–66. 85. Yamada A, Shibuya N, Kodama O and Akatsuka T. Induction of phytoalexin formation in suspension cultured rice cells by N-acetyl chitooligosaccharides. Biosci. Biotech. Biochem., 1993; 57: 405–09.
86. Pae H O, Seo W G, Kim N Y, Oh G S, Kim G E, Kim, Y H, et al. Induction of granulocytic differentiation in acute promyclocytic leukaemia cells (HL-60) by water-soluble chitosan oligomers. Leuk. Res., 2001; 25: 339–46. 87. Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S and Suzuki M. Antitumor effect of hexa- N-acetylchitohexaose and chitohexaose. Carbohydr. Res., 1986; 151: 403–8. 88. Tsukada K, Matsumoto T, Aizawa K, Tokora A, Naruse R, Suzuki S et al. Antimetastatic and growth-inhibitory effects of N-acetyl chitohexaose in mice bearing lewis lung carcinoma. Jpn. J. Cancer Res., 1990; 81: 259–65. 89. Felt O, Carel A, Baehni P, Buri P and Gurny R. Chitosan as tear substitute: a wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther., 2000; 16: 261–70.
90. Jeon Y J, Park P J and Kim S K. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polymers, 2001; 44: 71–76.
91. Barker S A, Foster A B, Stacey M and Webber J M. Isolation and properties of oligosaccharides obtained by controlled fragmentation of chitin. J. Chem. Soc., 1958; 2218–21.









