ABOUT AUTHORS:
S.R.V.Vivekananda Rao*, K.Sundaramoorthy, T.Vetrichelvan
Department of Pharmaceutics, Adhiparasakthi College of Pharmacy,
Melmaruvathur-603319, Kanchipuram (Dist),
Tamilnadu, India.
*viveksrv1989@gmail.com
ABSTRACT
The work was aim to formulate and evaluate mucoadhesive microspheres of Metronidazole for treatment of Amoebiasis and other protozoal infections by combine the potential advantages of mucoadhesion with sustained drug delivery using various ratio of polymers. Mucoadhesive microspheres were prepared by orifice ionic gelation method. Microspheres were found discrete, spherical and free flowing. The microspheres exhibits good mucoadhesive property in vitro wash off test and showed high drug entrapment efficiency. Metronidazole release from these microspheres was slowed, sustained on the type of polymers used. The formulation f9 was showed consistent drug release up to 10 hr time period. Among all the formulations, f9 containing sodium alginate and methyl cellulose showed the reproducible result with best mucoadhesive profile and good surface morphology. The work has demonstrated that among all the formulations of microspheres, particularly those of formulation f9 are promising candidates for the sustained release of Metronidazole.
REFERENCE ID: PHARMATUTOR-ART-1867
INTRODUCTION
Mucoadhesive drug delivery system is a new system of drug delivery and has recently gained great concern in pharmaceutical sciences. The concept of mucoadhesives was introduced in the early 1980s. Mucoadhesion can be defined as the phenomenon of the attachment of natural or synthetic polymers to a mucosal surface. In general, the process involved in the mucoadhesion phenomenon can be described in three steps: first of all, the wetting and swelling of the polymer should allow an intimate contact with the tissue and secondly, interpenetration of the polymer chains andentanglement between the polymer and the mucin chains should be attained and finally, the formation of weak chemical bonds. Mucus is a viscous and heterogeneous biological product that coats many epithelial surfaces. Mucus-secreting cells are widely spread in different locations in the body, including the nasal, ocular, buccal area and the gastrointestinal, reproductive and respiratory tracts. Mainly, the mucus serves as a lubricant to minimize shear stresses and as a protection barrier against harmful substances. However, mucus can perform other important functions. Goblet cells located in the epithelium are unicellular mucus-Secreting glands. Mucus is stored in large granules in the goblet cell and can be released by exocytosis or exfoliation of the whole cell. Mucus granules are mainly stored in the apical side of the goblet cell, which results in the characteristic balloon shape of these cells. Although the secretion of mucus can vary depending on age, sex, body location and health condition, the average mucus turnover is approximately 6 h. Mucus consists mainly of water (up to 95% weight), inorganic salts (about 1% weight), carbohydrates and lipids (less than 1%) and glycoproteins (no more than 5% weight).
MATERIALS AND METHODS
The following chemicals were obtained from different sources and used as received. Metronidazole was a gift sample from Bindu Pharmaceuticals, Hyderabad, sodium alginate, HPMC, Methylcellulos were obtained from commercial sources. All other chemicals and reagents used were of analytical grade; distilled water was used throughout the experiment. Mucoadhesive microspheres were prepared by orifice ionic gelation method.
EXPERIMENTAL WORK
2.1.1DRUG POLYMER COMPATABILITY
Drug polymer interaction was observed by IR spectroscopy. FTIR study of pure Metronidazole and physical mixture of Metronidazole and polymers were performed by KBr dispersion method.
2.1.2.PREPARATION OFMETRONIDAZOLE-CONTAINING MUCOADHESIVE MICROSPHERES:
Orifice ionic gelation method (syringes method)
In this technique cross linking was done with calcium chloride solution to release the drug in a sustained manner. Microspheres were prepared by using the Orife ionic gelation technique in which Sodium alginate, HPMC, Methylcellulose in different ratios as mentioned below was added to 32ml of water with continuous stirring to form homogenous solution. Then sonicating was done for 20 minutes the drug Metronidazole was then added to the above solution to form a clear solution. The drug polymer mixture was poured in 1.5% calcium chloride solution using 22# gauge needle by stirring at 50rpm. The microspheres thus formed were allowed for 30 min Then the calcium chloride solution was decained and The formed mucoadhesive microspheres washed with distilled water and air dried over night at room temperature
Table 1: Formulation of Metronidazole mucoadhesive microspheres
|
S.No. |
Batch.No. |
Drug (g) |
Sodium alginate(%) |
HPMC (%) |
Methyl cellulose(%) |
|
1 |
F1 |
1 |
1.5 |
- |
- |
|
2 |
F2 |
1 |
2.5 |
- |
- |
|
3 |
F3 |
1 |
3.5 |
- |
- |
|
4 |
F4 |
1 |
1.5 |
2 |
- |
|
5 |
F5 |
1 |
2.5 |
2 |
- |
|
6 |
F6 |
1 |
3.5 |
2 |
- |
|
7 |
F7 |
1 |
1.5 |
- |
1 |
|
8 |
F8 |
1 |
2.5 |
- |
1 |
|
9 |
F9 |
1 |
3.5 |
- |
1 |
2.2.EVALUATION AND CHARACTERIZATION OF MICROSPHERES:
2.2.1. ParticleSizeDetermination
Particle size distribution for the microspheres were measured by sieving method analysis, using set of standard sieves was weighed. Particles having size range between 50 and 1500 μm are estimated by sieving method. This method directly gives weight distribution. The sieving method is a useful application in dosage form development of tablets and spheres.
2.2.2. Percentage Yield
The total amount of microspheres obtained were weighed and evaluated for percentage yield.
Practical yield
Percentage yield= ----------------- x 100
Theoretical yield
2.2.3. Drug content estimation andEntrapment efficiency
Metronidazole microspheres (100mg) from each batch were initially stirred in 3 ml sodium citrate solution (1%w/v) until complete dissolution. A quantity of 7 ml of methanol was added to above solution to solubilise the Metronidazole. The filtrate was assayed for drug content by measuring the absorbance at 276 nm after suitable dilution by UV-Visible spectrophotometer and Encapsulation efficiency was calculated using the formula,
Encapsulation efficiency =
Estimated % drug content in microspheres
--------------------------------------------- x 100
Theoretical % drug content in microspheres
2.2.4. Percentage moisture content:
The Metronidazole loaded microspheres was evaluated to determine the percentage moisture content which sharing an idea about its hydrophilic nature. The microspheres weighed (w1) initially kept in desiccator containing Calcium chloride at 37º C for 24 hours. The final weight (w2) was noted when no further change in weight of sample was observed.
W1– W2
Moisture Percentage = ----------- x 100
W2
2.2.5. Scanning electronmicroscopy (SEM):
The microspheres were observed under a Scanning Electron Microscopy. They were mounted directly onto SEM sample stub using double-sided sticking tape and coated with gold film with ion spillter with gold target with resolution 3 nm (30 KV HV Mode),10 nm (30 KV HV Mode), 40 nm (30 LV Mode) and a vacuum system is fitted to it.
2.2.6. In -vitro wash off test for mucoadhesion:
The mucoadhesive property of the metronidazole microspheres was evaluated by an in -vitro adhesion testing method known as the wash – off method. Freshly excised pieces of intestinal mucosa (4×5 cm) from sheep were mounted onto glass slides (3×1inch) with poly cyanoacrylate glue. Two glass slides were connected with a suitable each wet rinsed tissue specimen, and immediately thereafter the support were hung onto the arm of a USP tablet disintegrating test machine. When the disintegrating test machine was operated, the tissue specimen was given a slow, regular up and down movement in the test fluid (400ml) at 37ºC contained in a 1000 ml vessel of the machine. At the end of 1 hr and at hourly interval up to 8 hr, the machine was stopped and the number of microsphere still adhering to the tissue was counted. The test was performed in stomach (pH1.2).
2.2.7. In- vitro drug release studies
In-vitro drug release study was carried out in USP dissolution test apparatus. A quantity of microspheres equivalent to 100 mg of Metronidazole microspheres was kept in basket type apparatus and immersed in 900ml of (pH 1.2) in 1000 ml dissolution flask and temperature was maintained at 37 ± 0.5ºC throughout the study. At predetermined time intervals 2 ml of samples was withdrawn by means of a syringe fitted with prefilter and same was replaced into the dissolution flask containing pH 1.2. The absorbance of sample was measured at 276 nm after required dilution with the fresh medium (pH.1.2).All the studies were conducted in triplicate.
2.2.8. Stability study:
The formulation (F9) was stored at accelerated condition (40. C ±2 C at 75% RH ±5%RH) in aluminum foils for 3 months. The samples were withdrawn after end of 1st month, 2nd month and 3rd month. The samples were analyzed for its drug content and in vitro drug release.
3. RESULTS AND DISCUSSION:
Drug estimation
Calibration curve of Metronidazole was done at λ max 276 nm in pH 1.2 with a UV-VIS spectrometer (UV-1700, Shimadzu Corporation). It obeyed Beer’s law. The calibration curve was done in the concentration range of 5-25 μg/ml.
Drug-polymer compatibility
Fourier transform infrared spectroscopy (FT-IR)
IR spectra of individual Metronidazole and the combination of drug with polymers were shown in figures (A-D) An IR spectrum of pure Metronidazole showed the peaks 3648 cm1 (O-H stretching), 3221-3101 cm1 (N-HStretching), 2982-2937cm1 (C-HStretching), 1354-1265cm1 (C-O stretching), 1428-1368cm1 (C-H bending(in plane)), 1340 cm1 (C-C stretching) ,These peaks can be considered as characteristic peaks of Metronidazole and there was no significant change was noticed in IR spectra of Metronidazole along with polymers as shown in the figure (B-D). This indicated that there was no interaction between Metronidazole and polymers.
The main goal of the present investigation efforts was to develop and evaluate new mucoadhesive microspheres comprising a drug- containing mucoadhesive polymeric layer using polymers like sodium alginate, Hydroxy propyl methyl celloulose, and methyl celloulose in various combination.
The nine formulations of metronidazole mucoadhesive microspheres prepared with varying concentration of polymers. These formulations were subjected to various evaluation parameters like percentage yield, particle size, Drug content estimation and Entrapment efficiency, Percentage moisture content, in vitro mucoadhesive wash off test, in vitro drug release, release drug data model fitting and stability studies.
Particle Size Determination:
Average particle size of microspheres was determined for all the formulations by sieving method analysis by using standard sieves. All the values were represented in Table (2). From the values, the formulation f9 had given the less average particle size compared to all other formulations.
Percentage Yield:
The total amount of microspheres obtained were weighed and evaluated for percentage yield was represented in Table (2). From this, formulation F9 showed maximum percentage yield among the formulations were prepared.
Drug content estimation and Entrapment efficiency:
Metronidazole microspheres (100 mg) from each batch were initially stirred in 3 ml sodium citrate solution (1%w/v) until it completely dissolved. 7ml of methanol was added to above solution to gel solubilize calcium alginate and further solubilize Metronidazole. The filtrate was assayed for drug content by measuring the absorbance at 276 nm after suitable dilution. Entrapment efficiency was calculated using the formula. The drug content of microspheres were calculated for all the formulations (F1 to F9) and values represented in Table (2). The formulation F9 was showed maximum drug content among the formulations were prepared.
Percentage moisture content:
The percentage moisture content was calculated for all the formulations (F1 to F9) by using desiccator containing Calcium chloride at 37ºC for 24 hrs. The Final weight was determined and compared to initial weight. The values were represented in Table (2). By comparing all the values of all formulations, formulation F9 was found to be the best one. The formulation F9 showed less moisture content. The order was F9<F8<F6<F7<F5<F4<F3<F2<F1.
Scanning electronmicroscopy (SEM):
The microspheres were observed under a scanning electron microscopy. The resolution of SEM instrument was 3 nm (30 KV HV Mode), 10 nm (30 KV HV Mode), 40 nm (30 LV Mode) and a vacuum system is fitted to it. The shape of the Metronidazole microspheres was evidenced from the Scanning Electron Microscopy was found to be spherical and uniformly distributed and was shown in Figure (F).
In -vitro wash off test for mucoadhesion:
The wash off test for Mucoadhesion for all formulations (F1 to F9) were represented in Table (3). Microspheres with a coat consisting of alginate and a mucoadhesive polymer exhibited good mucoadhesive properties in the in- vitro wash off test. The wash off test was faster at a pH 1.2 (stomach pH). It was observed that the pH of the medium was critical for the degree of hydration, solubility and mucoadhesion of the polymers.
The results of the wash off test indicated that the formulation F9 had very good mucoadhesive properties with more than 62.18% retention for 8hrsin pH1.2.
In- vitro drug release studies:
In vitro release studies of mucoadhesive microspheres were done by using pH 1.2 as dissolution medium and the drug absorbance measured spectrophotometrically at 276 nm. The drug release was higher in formulation 9. The in- vitro drug release from mucoadhesive microspheres varied with respect to the polymer composition and nature. An increase in drug release from the mucoadhesive microspheres was found with increasing concentration of polymers which were more hydrophilic in nature. The formulation F9 was shown best one among the formulations (F1 to F9) . Formulation F9 in-vitro drug released was 81.76±0.55% over a period of 10 hrs and In vitro drug release of all formulations were showed in the fig (E) and The values were represented in Table (4).Data of the in vitro release were fit to different equations and kinetic models to explain the release kinetics of Metronidazole from these mucoadhesive microspheres. The kinetic models used were zero-order equation, first-order equation, Higuchi release and Korsmeyer&Peppas models. The best fit with the highest correlation coefficient (r) was shown by the Korsmeyer and Peppas (r = 0.9907).“n” value of all the formulations is less than 0.5, It shows that the formulations follow Fickian model of drug release. The values were represented in Table (5).
Stability study:
Optimized selected mucoadhesive microspheres were subjected to short term stability studies. From the stability data it was concluded there was no significant change in drug content and in-vitro drug release, The results were graphically represented in the fig (G) and The values were represented in Table (6).
Figure(D)FTIR spectra of Metronidazole with methyl cellulose
Table:2 Evaluations of Mucoadhesive Microspheres of Metronidazole
|
S.No |
Formulation code |
Average |
Percentage |
Meandrug content |
Entrapment efficiency(%) |
Percentagemoisture content (%±S.D) |
|
1 |
F1 |
649.38±1.14 |
75.19 |
42.32±0.62 |
70.69 |
8.723±0.144 |
|
2 |
F2 |
693.18±0.44 |
77.40 |
36.34±1.46 |
71.96 |
7.637±0.078 |
|
3 |
F3 |
720.64±2.54 |
73.20 |
38.82±1.56 |
80.75 |
5.876±0.080 |
|
4 |
F4 |
738.28±0.81 |
82.20 |
40.62±1.38 |
83.66 |
4.158±0.121 |
|
5 |
F5 |
760.58±1.36 |
82.00 |
41.89±0.49 |
78.32 |
3.529±0.050 |
|
6 |
F6 |
794.56±1.94 |
71.60 |
39.64±2.26 |
84.68 |
3.069±0.132 |
|
7 |
F7 |
660.85±2.77 |
65.60 |
34.06±0.72 |
70.92 |
2.516±0.040 |
|
8 |
F8 |
690.48±0.34 |
87.95 |
32.92±1.86 |
77.62 |
1.944±0.130 |
|
9 |
F9 |
642.38±1.14 |
88.65 |
46.96±0.29 |
87.21 |
1.116±0.138 |
Table:3 Data of in- vitro wash off test to assess mucoadhesive properties of microspheres
|
S.No |
Formulations |
1 hr |
2 hr |
4 hr |
6 hr |
8 hr |
|
1 |
F1 |
96.07 |
88.79 |
68.34 |
56.45 |
40.08 |
|
2 |
F2 |
94.74 |
84.21 |
78.92 |
60.96 |
41.16 |
|
3 |
F3 |
98.19 |
80.32 |
76.49 |
66.46 |
46.36 |
|
4 |
F4 |
98.52 |
86.41 |
72.37 |
58.37 |
44.29 |
|
5 |
F5 |
96.63 |
82.62 |
76.59 |
54.38 |
52.56 |
|
6 |
F6 |
97.42 |
90.35 |
82.72 |
66.74 |
54.84 |
|
7 |
F7 |
98.27 |
88.53 |
82.49 |
64.36 |
44.19 |
|
8 |
F8 |
96.86 |
90.81 |
78.67 |
58.49 |
42.28 |
|
9 |
F9 |
99.84 |
97.68 |
92.48 |
84.39 |
62.18 |
Table:4 Data of Invitro drug release profile of Metronidazole mucoadhesive microspheres
Table:4 Data of In vitro drug release profile of Metronidazole mucoadhesive microspheres
|
Time in hrs |
Formulations |
||||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
|
1 |
13.89±0.11 |
10.68±1.54 |
17.42±1.04 |
11.58±1.54 |
15.46±1.56 |
12.65±0.45 |
18.35±2.78 |
16.93±0.45 |
19.84±1.54 |
|
2 |
23.36±1.23 |
16.98±1.89 |
21.54±2.66 |
17.29±0.61 |
19.75±1.05 |
20.67±0.15 |
25.90±1.96 |
22.76±0.55 |
26.72±1.45 |
|
3 |
29.07±1.54 |
25.37±0.54 |
27.65±0.20 |
21.45±0.02 |
23.67±1.51 |
26.74±1.63 |
30.05±0.12 |
28.23±0.56 |
31.36±1.52 |
|
4 |
36.64±1.56 |
32.46±1.65 |
31.56±1.53 |
28.87±0.91 |
29.56±1.55 |
33.45±1.55 |
39.45±0.56 |
35.98±0.22 |
38.43±0.01 |
|
5 |
42.34±0.57 |
45.36±0.22 |
39.78±1.01 |
35.47±0.78 |
34.78±0.45 |
39.87±1.23 |
44.89±1.56 |
41.87±1.51 |
46.64±0.12 |
|
6 |
49.07±0.23 |
47.48±0.55 |
48.97±0.50 |
41.68±0.51 |
40.56±0.61 |
43.75±1.25 |
52.98±3.45 |
48.24±1.02 |
51.28±0.65 |
|
7 |
53.03±1.54 |
50.67±0.23 |
51.87±1.21 |
48.89±0.05 |
46.78±0.49 |
49.56±0.61 |
59.56±1.56 |
52.76±2.55 |
57.14±0.54 |
|
8 |
61.96±1.45 |
56.89±1.55 |
58.16±0.54 |
52.98±0.07 |
54.81±0.61 |
53.23±0.74 |
64.87±1.26 |
59.56±2.16 |
66.49±1.55 |
|
9 |
66.76±0.77 |
61.87±0.54 |
64.89±0.26 |
59.89±0.07 |
63.46±1.54 |
65.56±0.07 |
69.90±0.16 |
67.87±2.55 |
72.38±0.65 |
|
10 |
69.26±0.54 |
65.08±1.54 |
71.08±0.50 |
63.47±0.52 |
67.89±0.91 |
70.45±0.21 |
74.13±1.51 |
76.12±1.16 |
81.76±0.55 |
*All values are expressed as mean ± S.D. n=3
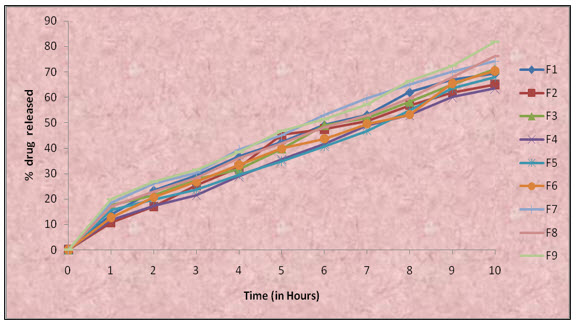
Figure-(E) comprehensive In-vitro release profile of formulations F1-F9
Scanning electron microscopy (SEM):
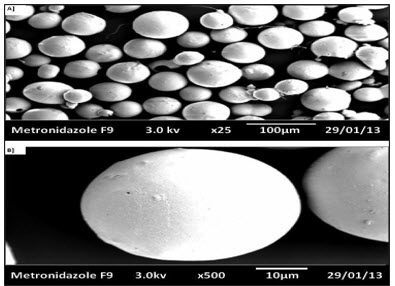
Figure-(F) Scanning electron microscopy of Metronidazole loaded sodium alginate and Methyl cellulose
Table : 5 In vitro drug released kinetics studies of all formulations
|
Formulation code |
Zero order R2 |
First order R2 |
Higuchi
R2 |
Korresmayer Peppas |
Best fit model |
|
|
R2 |
n |
|||||
|
F1 |
0.9621 |
0.9501 |
0.9723 |
0.9908 |
0.432 |
Peppas |
|
F2 |
0.9650 |
0.9705 |
0.9663 |
0.9909 |
0.456 |
Peppas |
|
F3 |
0.9900 |
0.9471 |
0.9530 |
0.9985 |
0.402 |
Peppas |
|
F4 |
0.9503 |
0.8439 |
0.9782 |
0.9908 |
0.485 |
Peppas |
|
F5 |
0.9592 |
0.8811 |
0.9729 |
0.9903 |
0.365 |
Peppas |
|
F6 |
0.9622 |
0.9307 |
0.9684 |
0.9903 |
0.462 |
Peppas |
|
F7 |
0.9540 |
0.8611 |
0.9770 |
0.9901 |
0.398 |
Peppas |
|
F8 |
0.9597 |
0.9162 |
0.9756 |
0.9907 |
0.441 |
Peppas |
|
F9 |
0.9647 |
0.9609 |
0.9663 |
0.9907 |
0.356 |
Peppas |
Table:6 Data of stability studies of formulation F9
|
Characteristics |
Initials* |
1 month* |
2 month* |
3 month* |
|
Drug content (%) |
46.96±0.29 |
46.54±0.18 |
46.34±0.38 |
45.99±0.07 |
|
In-vitro drug released |
81.76 |
81.26 |
81.12 |
81.04 |
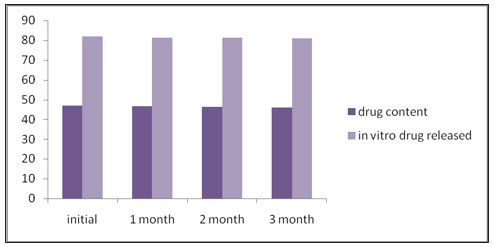
Figure (G): Graphical representation of stability data
CONCLUSION:
Mucoadhesive microspheres of Metronidazole using polymers like sodium alginate, HPMC, and methyl cellulose in various proportions and combinations showed satisfactory physicomechanical characteristics. The proportional amounts of various hydrophilic polymers in various formulations have influence on permeation and drug release from these formulated Mucoadhesive microspheres of Metronidazole. From the present investigation, it can be concluded that such Mucoadhesive microspheres of Metronidazole may provide sustained drug delivery for prolonged periods in the management of Amoebiasis. The formulation F9 was concluded best formulation among the formulations were prepared.
ACKNOWLEDGEMENT: The author are thankful to the management ,Adhiparasakthi College of pharmacy, for providing all necessary facilities.
REFERENCES
REFERENCES
1. Akanksha garud., et al. Metronidazole microcpasules with a coat consisting of alginate and natural cationic polymers, Journal of Pharmacy Research, 2010, 271-2996.
2. Amit Alexandar., Ajazuddin., Tripathi D. K., Swarna and JyothiMaurya. Mechanism responsible for mucoadheson delivery review, International journal of Biological and Chemical Sciences, 2011, 2(1), 323-367.
3. Anonymous, IndianPharmacopoeia. The controller of publication, Govt. of India, Ministry of Health and Family Welfare, New Delhi. Vol I and II, 2007,134, 143-162,475-480, 143-162, 685-688.
4. Anonymous, The United States Pharmacopoeia, XXVII, NF XXII. 27thedn, Vol-3, The United States Pharmacopoeial Convention Inc, Rockvilley, 2009, 3842-3843.
5. Aulton M.E. Eds. Pharmaceutics, The science of dosage form design, 2ndedn, Churchill Livingstone, New York, 2002, 270-278.
6. habaniNayak S. HarshadParma., Sunil BakliwalNayan and Gujarathi SuniPawar. Different methods of formulation and evaluationof mucoadhesive microspheres, Acta Pharmaceutica Sinica, 2009, 1(3), 1157-1168.
7. Bhatt.J H., et al. Controlled release of Metronidazole mucoadhesive microspheres, International Journal of Pharma Tech Research, 2009, 2(4), 2575-2595.
8. Chein Y. W. Novel drug delivery systems, 1st edn, Informa healthcare, New York, 2009,50, 139-199.
9. Chowdary K. P. R. Mucoadhesive microsphere for controlled drug delivery, 2004,1717-1724.
10. Devraj., et al. oral dosage form proposed for the attainment of timed release drug delivery of Metronidazole, Journal of Pharmacy Research, 2010, 211-219
11. Gupta A K., Garg SK., Pal SK., Development and characterization of SRM microspheres of repaglinide, Journal of Drug Delivery and Therapeutics, 2011, 71-77.
12. Jain N. K., Progress in controlled and novel drug delivery system, 1stedn.CBS Publisher and distributers, New Delhi, 2004, 353-376.
13. Khar R. K. and Vyas S. P. Controlled drug delivery and advances, 1st edn. CBS Publishers and Distributors, New Delhi, 2002, 174-176, 257-263.
14. Lachman L. Lieberman H. A and Kanig J. L. The theory and practice of industrial pharmacy, 3rdedn, Varghese Publishing House, Mumbai, 1991, 67-71, 183-184.
15. Madhavi Boddupalli B., Naga Madhumudium and David Banji., Formulation and Evaluation of Mucoadhesive Microspheres of Venlafaxine Hydrochloride, Journal of Pharmacy Research, 2010, 2597-2600.
16. Mahendra singh and Vinayvijay., Formulation and characterization of mucoadhesive microspheres of verapamil Hydrochloride as a model drug, International Journal of Pharmaceutical Sciences, 2011, 2(12), 3260-3268.
17. Malay Das K., Divya P and Maurya. Evaluation of diltiazem hydrochloride loaded mucoadhesives microspheres prepared by emulsification internal gelation method, Acta Poloniae Pharmaceutica-Drug Research, 2008, 65(2), 249-259.
18. Manavalan R. and Ramasamy S. Physical Pharmaceutics, Accelerated Stability Testing, 1stedn, Vignesh Publisher, Chennai, 2004, 288-299.
19. Moffat A. C. Osselton M. D. and Widdop B. In Clarke’s analysis of drugs poisons, 3rd edn, Pharmaceutical Press. 2004. Vol-2, 1692-1693.
20. Nagda Chirag., Chotai Narendra., Patel Sandip., Patel Upendra and AhirKeyur. Design and characterization of bioadhesive microspheres prepared by double eulsion solvent evaporation method, Acta Polonica. 2009, 261-270









