About Authors:
Garje V.A1, Nagargoje S.S1, Phanse V.R1, Kshirsagar R.R1
1Dept.of Pharmaceutics. S.V.N.H.T’S College Of B.Pharmacy Rahuri Factory,
Rahuri.413706 MS,India.
*vishnugarje02@gmail.com
ABSTRACT
The objective of present study was to develop an oral sustained release dosage form of diclofenac sodium, with chitosan as an sustained release polymer, and to evaluate the prepared dosage form for physical parameters, like weight uniformity, hardness, friability, and drug content. Chitosan a natural polymer, compatible with physiologic environment, possessing sustained release matrix forming characteristics and binding properties. It was used to prepare sustained release matrix tablets. In the present study, the influence of different concentrations of polymer on erosion of matrix system was studied, with a view to develop sustained release formulation of diclofenac sodium.The diclofenac sodium tablet were prepared by wet granulation method,with a solvent such as distilled water, lactose was used as diluents, talc1% was used as lubricant. The result from in-vitro drug release studies indicated that the formulations batch-2&batch-3,with polymer concentrations 1:1.1&1:1.3 resp. Prepared by wet granulation using distilled water as a solvent, were found to be release the drug at a steady state over an extended period of time upto 24 hrs.
REFERENCE ID: PHARMATUTOR-ART-1651
MATRIX TABLETS
These are the type of controlled drug delivery systems, which release the drug in continuous manner. These release the drug by both dissolution controlled as well as diffusion controlled mechanisms. To control the release of the drugs, which are having different solubility properties, the drug is dispersed in swellable hydrophilic substances, an insoluble matrix of rigid non swellable hydrophobic materials or plastic materials2,3.
Classification of Matrix Tablets
A. On the Basis of Retardant Material Used: Matrix tablets can be divided in to 5 types.
1. Hydrophobic Matrices (Plastic matrices):
The concept of using hydrophobic or inert materials as matrix materials was first introduced in 1959. In this method of obtaining sustained release from an oral dosage form, drug is mixed with an inert or hydrophobic polymer and then compressed in to a tablet. Sustained release is produced due to the fact that the dissolving drug has diffused through a network of channels that exist between compacted polymer particles. Examples of materials that have been used as inert or hydrophobic matrices include polyethylene, polyvinyl chloride, ethyl cellulose and acrylate polymers and their copolymers.The rate-controlling step in these formulations is liquid penetration into the matrix. The possible mechanism of release of drug in such type of tablets is diffusion. Such types of matrix tablets become inert in the presence of water and gastrointestinal fluid.
2. Lipid Matrices:
These matrices prepared by the lipid waxes andrelated materials. Drug release from such matrices occurs through both pore diffusion and erosion. Release characteristics are therefore more sensitive to digestive fluid composition than to totally insoluble polymer matrix. Carnauba wax in combination with stearyl alcohol or stearic acid has been utilized for retardant base for many sustained release formulation.
3. Hydrophilic Matrices:
The formulation of the drugs in gelatinous capsules or more frequently, in tablets, using hydrophilic polymers with high gelling capacities as base exipients is of particular interest in the field of controlled release. Infect a matrix is defined as well mixed composite of one or more drugs with a gelling agent (hydrophilic polymer). These systems are called swellable controlled release systems.
The polymers used in the preparation of hydrophilic matrices are divided in to three broad groups
1.Cellulose derivatives: methylcellulose 400 and 4000 cPs; hydroxyl ethyl cellulose; hydroxypropylmethyl cellulose (HPMC) 25, 100, 4000 and 15000 cPs; and sodium carboxymethylcellulose.
2.Noncellulose natural or semisynthetic polymers: agar-agar; carob gum; alginates; molasses; polysaccharides of mannose and galactose; chitosan and modified starches.
3.Polymers of acrylic acid; corbopol 934, the most used variety.
4. Biodegradable Matrices:
These consist of the polymers which comprised of monomers linked to one another through functional groups and have unstable linkage in the backbone. They are biologically degraded or eroded by enzymes generated by surrounding living cells or by nonenzymetic process in to olegomers and monomers that can be metabolised or excreted.Examples are natural polymers such as proteins and polysaccharides;
modified natural polymers; synthetic polymers such as aliphatic poly (esters) and poly anhydrides.
5. Mineral Matrices:
These consist of polymers which are obtained from various species of seaweeds. Example is Alginic acid which is a hydrophilic carbohydrate obtained from species of brown seaweeds (Phaephyceae) by the use of dilute alkali.
B. On the Basis of Porosity of Matrix
Matrix system can also be classified according to their porosity and consequently, macroporous; microporous and non-porous systems can be identified:
1. Macroporous Systems:
In such systems the diffusion of drug occurs through pores of matrix, which are of size range 0.1 to 1 μm. This pore size is larger than diffusant molecule size.
2. Microporous System:
Diffusion in this type of system occurs essentially through pores. For microporous systems, pore size ranges between 50 – 200 A°, which is slightly larger than diffusant molecules size.
3. Non-porous System:
Non-porous systems have no pores and the molecules diffuse through the network meshes. In this case, only the polymeric phase exists and no pore phase is present.
MECHANISM OF DRUG RELEASE
These systems are described in terms of fronts. The following fronts have been defined, with regard to anomalous release systems:
- The “swelling front”, the erosion front, and the diffusion front. The swelling front separates the rubbery region (swelling polymer area) which has enough water absorbed within the polymer to lower the Tg of the polymer below the respective environmental temperature allowing for macromolecular mobility and swelling, from the non-swelling polymer region (where the polymer exhibits a Tg that is above the respective environmental temperature).
- The “erosion front” separates the matrix from the bulk solution and is the interface between the unstirred layer with polymer concentration gradient and the well stirred medium.
- The “diffusion front” is between the swelling and erosion front and separated the areas of non dissolved drug from the area of dissolved drug.
|
DRUG PROFILE DICLOFENAC SODIUM:- |
||
|
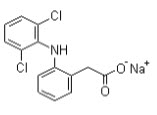
Name- |
Diclofenac sodium |
|
|
Synonyms- |
2-[(2,6-Dichlorophenyl)amino]benzene acetic acid sodium salt |
|
|
Molecular Structure- |
|
|
|
Molecular Formula- |
C14H10Cl2NNaO2 |
|
|
Molecular Weight- |
318.13 |
|
|
CAS Registry Number- |
15307-79-6 |
|
|
EINECS |
239-346-4 |
|
Class: Other Nonsteroidal Anti-inflammatory Agent
Chemical Name: 2-[(2,6-dichlorophenyl)amino] benzene acetic acid, monopotassium salt
Molecular Formula:
C14H11C12NO2•KC14H11C12NO2.NaC20H24Cl2N2O3
CAS Number: 15307-81-0
Table 1 – Preparations and their doses.
|
Preparation |
Dosage |
|
Diclofenac potassium conventional tablets |
100–150 mg daily, given as 50 mg 2 or 3 times daily |
|
Diclofenac sodium delayed-release tablets |
100–150 mg daily, given as 50 mg 2 or 3 times daily or 75 mg twice daily |
|
Diclofenac sodium extended-release tablets |
100 mg once daily |
|
Diclofenac sodium (in fixed combination with misoprostol) |
50 mg 3 times daily |
EXPERIMENTAL
MATERIALS AND METHODS:
Materials:
Diclofenac sodium, hitosan,Lactose ,Magnesium stearate, Talc were purhesed from HILAB chemicals, shrirampur Maharastra state . Other materials used were of analytical grade, and procured from commercial sources.
MFG.FORMULA:-
Table no:-2
|
SR. NO |
INGRADIENTS |
USES |
B1 |
B2 |
B3 |
|
1 |
Diclofenac sod. |
NSAID |
100mg |
100mg |
100mg |
|
2 |
Chitosan |
polymer |
100mg |
110mg |
130mg |
|
3 |
Lactose |
Diluent |
44mg |
34mg |
14mg |
|
4 |
Magnesim stearate |
Lubricat |
4mg |
4mg |
4mg |
|
5 |
Talc |
Glident |
2mg |
2mg |
2mg |
|
6 |
Dist. water |
Granulat-ing agent |
q.s |
q.s |
q.s |
* Method:-
Preparation of sustained release matrix tablets
Tablet formulation was prepared by wet granulation technique. All the powder ingredients were passed through BSS-80 mesh. Required quantities of Diclofenac sodium and other polymers (chitosan) were mixed separately and thoroughly and a sufficient volume of granulating agent (water) was added slowly. After enough cohesiveness was obtained, the mass was sieved through NO: 10 mesh. The granules were dried at 40°c for 30 mins and then were passed through BSS-16 mesh. Talc and magnesium stearate were finally added as glidant and lubricant for each batch of granules. The tablets were compressed (9 mm diameter, standard flat punches) using 8 station double rotary compression machine
EVALUATION TESTS :-
INSTRUMENTS:-
Table No-03
|
Sr. No |
Instruments |
Mech. |
Model |
|
01 |
Hardness |
------ |
Monsanto |
|
02 |
Friability |
------ |
Cat No-2094d |
|
03 |
Disintegrator |
Intelli series |
|
|
04 |
U.V Spectroscopy |
Systonics |
Double beam & Graphic LCD |
|
05 |
Dissolution Apparatus |
Intelli Series |
|
|
06 |
Digital Balance |
Wensar |
------ |
|
07 |
Punching machine |
8 station double rotary compression machine |
EVALUATION OF GRANULES
* Angle of repose
The angle of repose of granules was determined by the funnel method. The granules were allowed to flow through the funnel freely onto the surface. The diameter of the powder cone was measured and angle of repose was calculated using the following equation1,7,8,9.
Tan θ = h/r
Where h and r are the height and radius of the powder cone.
* Bulk Density
Both loose bulk density and tapped bulk density were determined and calculated by using the following formulas.
LBD = weight of the powder / volume of the packing
TBD = weight of the powder / tapped volume of the packing1,7,8,9.
* Compressibility Index
The compressibility index of the granules was determined by Carr's compressibility index1,7,8,9 .
Carr's index (%) = [TBD-LBD] X 100 / TBD
EVALUATION OF TABLETS:
All the marketed tablets were evaluated for various evaluation parameters like weight variation, Hardness tests, friability test, Dissolution test and Assay for that bth types of tablet as shown in table.
Table No-04
|
TABLET |
DOSE |
MFG. BY |
|
Reactin 100SR |
100 mg |
Cipla |
|
Sample tab |
100 mg |
….. |
* Weight variation test :-
The weight variation test would be a satisfactory method of determining the drug content uniformity of tablets if the tablets were all or essentially all 95 to 105 active ingredient , or if the uniformity of the drug distribution in the granulation of powder form which the tablet were made were perfect for tablets such as aspirin which are usually 90 % or more active ingredient the (±) 5 % weight variation should come close to defining true potency and content uniformity (95 to 105 % of the label strength.).
Procedure:-
The USP weight variation test is run weighing 20 tablets individually calculation the average with and comparing individual tablet weight to the average. The tablets meet UPS test if no more than 2 tablets are outside the percentage limit and if no tablet differs by more than 2 times the percentage limit. The weight variation tolerance for uncoated tablets differs depending on average tablet weight1,7,8,9 .
* Hardness tests : -
Tablets require certain amount of strength, or hardness and resistance to friability to withstand mechanical shock of handling if Mfg packaging and shipping in addition, tablet should able to withstand reasonable abuse when in hand of costumer such as bouncing about women’s purse in a partially fill prescription bottle.
The hardness is define as force require to breakdown tablets, the tab require certain strength to withstand mechanical shocks of handling1,7,8,9.
1. Monsanto tester
2. Pfizer
3. Erweka tester
4. Schuleuniger tester
Limit– the tablets pass the test if the hardness in 3- 5 Kg/cm2
* Friability test:-
The test is carried out to ensure the tab can withstand mechanical shock of handling shipping friabilator consist that revolves at 25 rpm dropping the tab at a distance of 6 inches with each revolution for per weighed tab sample is place in friablator which is then operator for 100 revolution the tab are then dedusted and re-weight finally calculated the % friability by using the formula1,7,8,9 .
% Friability = (1 - W2 / W1) x 100
Where;
W1 = initial wt of 10 tab
W2 = weight of 10 tab
Standard limit: - The % friability should NMT 1 % as per USP
* Dissolution test:-
Bioavaiblity measurement of release of drug form tablets is called as dissolution study the % release of drug is studied in dissolution test of tablets.
Objective:
The rate of drug release is uniform batch and is same as the release rate from dose bathes prove to be bioavailability and clinical effective.
Apparatus:-
1. Paddle Type
Cylindrical vessel made up of borosilicate glass the motor with speed regulator fitted with the drive shaft and blade forming a paddle water bath set to maintain dissolution media at 36.37.50 C
2. Basket type :
He assembly is same apparatus first except in the stirring assembly paddle is replaced by basket.
Basket consist of top and bottom the bottom is removable after the intervals examination the distance between inside bottom of the vessel and the basket is exaintained at 23-37 m during the test.
These dissolution medium specified in the individual monograph these.
Procedure:-
1. the required amount of dissolution medium is placed in the method in apparatus
2. warm the medium to maintain temperature between 36.5-37.5 0 C
3. When the apparatus used then the bottom allowed to sink to the bottom of the vessel prior to rotation.
4. When apparatus second the tablets is paced in a dry basket into position before rotation.
5. Operate the apparatus to specify of rotation with draw the sample at specified time interval.
6. Perform the analysis as given in individual monograph for each tablets. Calculate the amount of dissolved active ingredient in the medium as the rate % of the studied above as the % release of the drug1,7,8,9 .
* Assay of diclofenac sodium tablet
20 tablet was weighed and accurate quantity of powder weight which is equivalent to 50 mg diclofenac sodium was taken.shaked with 60 ml of methanol in 200 ml volumetric flask and diluted to volume with methanol. Diluted 5.0 ml of this solution to 100 ml with methanol, and measured the absorbance of resulting solution at the maximum at about 285 nm.
Calculated the content of the Diclofenac sodium from the absorbance obtained by repeating the procedure using diclofenac sodium in place of the substance being examined and from the deleared the content of diclofenac sodium in diclofenac sodium RS7.
RESULT AND INTERPRETATION
Evaluation of granules:-
Table no:-05-Evaluation of Granules
Observations:-
|
SR NO |
SAMPLE NO |
ANGLE OF REPOSE |
BULK DENSITY (gm/ml) |
CARR’S INDEX (%) |
|
01 |
B1 |
320 |
0.75 |
23 |
|
02 |
B2 |
31.770 |
0.66 |
22.34 |
|
03 |
B3 |
27.440 |
0.5 |
21 |
Evaluation of Tablet:-
X:-Reactin 100 SR 100 mg
Y:-Tablet prepared from chitosan polymers.
Observations:-
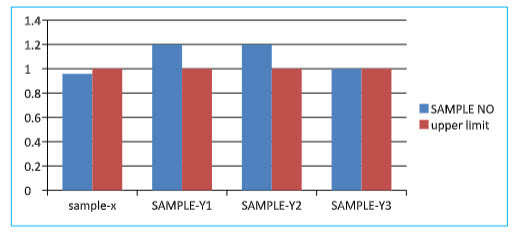
* Friability Test:-
Table no:-06
|
SR NO |
SAMPLE NAME |
UPPER LIMIT (%) |
FRIABILITY(%) |
|
1 |
X |
1 |
0.9852 |
|
2 |
Y1 |
1 |
1.2 |
|
3 |
Y2 |
1 |
1.2 |
|
4 |
Y3 |
1 |
1 |
Limit:-The % friability should NMT 1% as per USP.
GRAPH:-
* Graph no:-01- Friability Test:-
* Weight variation test:-
Table no:-07
Table no:-07
|
SR NO |
SAMPLE NAME |
INDIVISUAL WEIGHT (mm) |
||||
|
1 |
Sample X
Gr.weight ( 251.5 mg) |
260 |
250 |
260 |
250 |
250 |
|
250 |
250 |
245 |
255 |
255 |
||
|
240 |
260 |
265 |
250 |
245 |
||
|
245 |
250 |
250 |
250 |
250 |
||
|
2 |
Sample Y1
Gr.weight (255.7 mg) |
250 |
255 |
260 |
260 |
260 |
|
240 |
255 |
255 |
255 |
260 |
||
|
260 |
245 |
255 |
245 |
265 |
||
|
250 |
255 |
260 |
260 |
265 |
||
|
3 |
Sample Y2
Gr.weight (254.5 mg) |
245 |
260 |
255 |
250 |
265 |
|
250 |
270 |
255 |
240 |
265 |
||
|
250 |
265 |
255 |
250 |
265 |
||
|
240 |
250 |
250 |
240 |
270 |
||
|
4 |
Sample Y3
Gr.weight (255.7 mg) |
250 |
240 |
240 |
250 |
250 |
|
265 |
250 |
240 |
255 |
265 |
||
|
250 |
260 |
245 |
250 |
240 |
||
|
260 |
255 |
245 |
260 |
255 |
||
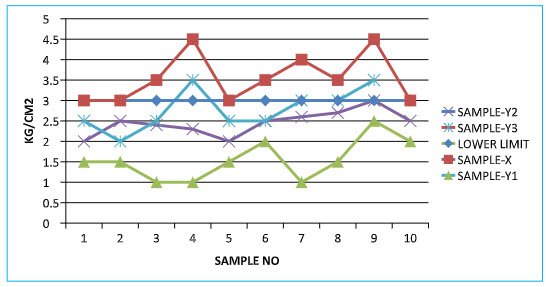
Hardness Test :-
Table no:-8 Unit-Kg/cm2 Limit:-3-5 Kg/cm2s
|
SR NO |
SAMPLE NO |
LOWER LIMIT |
SAMPLES |
|||
|
X |
Y |
|||||
|
Y1 |
Y2 |
Y3 |
||||
|
1 |
1 |
3 |
3 |
1.5 |
2 |
2.5 |
|
2 |
2 |
3 |
3 |
1.5 |
2.5 |
2 |
|
3 |
3 |
3 |
3.5 |
1 |
2.4 |
2.5 |
|
4 |
4 |
3 |
4.5 |
1 |
2.3 |
3.5 |
|
5 |
5 |
3 |
3 |
1.5 |
2 |
2.5 |
|
6 |
6 |
3 |
3.5 |
2 |
2.5 |
2.5 |
|
7 |
7 |
3 |
4 |
1 |
2.6 |
3 |
|
8 |
8 |
3 |
3.5 |
1.5 |
2.7 |
3 |
|
9 |
9 |
3 |
4.5 |
2.5 |
3 |
3.5 |
|
10 |
10 |
3 |
3 |
2 |
2.5 |
2.5 |
|
11 |
MAX |
3 |
4.5 |
2.5 |
3 |
3.5 |
|
12 |
MIN |
3 |
3 |
1 |
2 |
2 |
|
13 |
AVG |
3 |
3.55 |
1.55 |
2.45 |
3.1 |
GRAPH:- Graph no:-02- Hardness Test :-
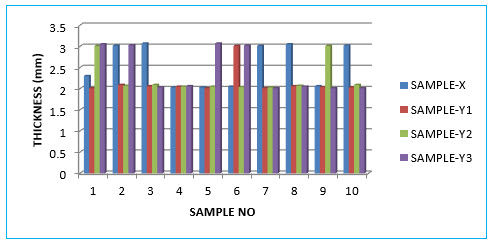
Thickness Test:-
Table no:-09( In mm)
|
SRNO |
SAMPLE NO |
SAMPLE |
||||
|
X |
Y |
|||||
|
Y1 |
Y2 |
Y3 |
||||
|
1 |
1 |
2.03 |
2.01 |
3.01 |
3.05 |
|
|
2 |
2 |
3.02 |
2.09 |
2.07 |
3.03 |
|
|
3 |
3 |
3.07 |
2.06 |
2.09 |
2.03 |
|
|
4 |
4 |
2.03 |
2.05 |
2.05 |
2.06 |
|
|
5 |
5 |
2.03 |
|
2.01 |
2.05 |
3.07 |
|
6 |
6 |
2.05 |
|
3.01 |
2.04 |
3.02 |
|
7 |
7 |
3.01 |
|
2.01 |
2.03 |
2.01 |
|
8 |
8 |
3.05 |
|
2.06 |
2.07 |
2.05 |
|
9 |
9 |
2.06 |
|
2.04 |
3.01 |
2.01 |
|
10 |
10 |
3.07 |
|
2.03 |
2.09 |
2.01 |
|
11 |
MAX |
3.07 |
|
3.01 |
3.01 |
3.07 |
|
12 |
MIN |
2.07 |
|
2.01 |
2.03 |
2.01 |
|
13 |
AVG |
2.55 |
|
2.13 |
2.251 |
2.233 |
GRAPH:-
Graph no:-03- Thickness Test:-
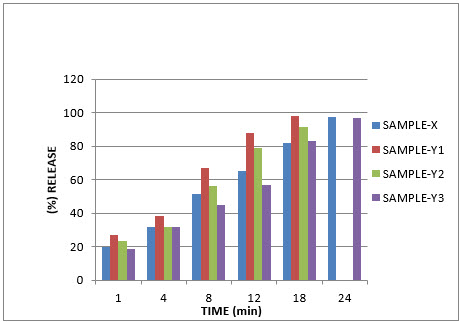
Dissolution Rate:-
Table no:-10
|
SR NO |
TIME IN HOUR |
% RELEASE |
|||
|
SAMPLES |
|||||
|
X |
Y |
||||
|
Y1 |
Y2 |
Y3 |
|||
|
1 |
1 |
20 |
27 |
23.5 |
18.5 |
|
2 |
4 |
32 |
38.15 |
32 |
31.7 |
|
3 |
8 |
51.2 |
66.79 |
56 |
45 |
|
4 |
12 |
65 |
88 |
79.5 |
57 |
|
5 |
18 |
82 |
98.2 |
91.44 |
83 |
|
6 |
24 |
97.50 |
- |
- |
96.95 |
GRAPH:-Graph no:-04- Dissolution Rate:-
Assay:-
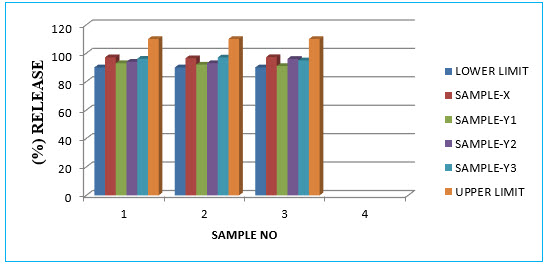
Table no:-11
|
SR NO |
SAMPLE NO |
% RELEASE |
|||||
|
LOWER LIMIT (%) |
SMPLES |
UPPER LIMIT |
|||||
|
X |
Y |
||||||
|
Y1 |
Y2 |
Y3 |
|||||
|
1 |
1 |
90.0 |
97.14 |
93 |
94 |
96.15 |
110 |
|
2 |
2 |
90.0 |
96.50 |
92.5 |
93.5 |
97 |
110 |
|
3 |
3 |
90.0 |
97.25 |
91 |
96.7 |
95 |
110 |
|
4 |
MAX |
90.0 |
97.25 |
93 |
96.7 |
97 |
110 |
|
5 |
MIN |
90.0 |
97.14 |
91 |
93.5 |
95 |
110 |
|
6 |
AVG |
90.0 |
97.19 |
92 |
95.1 |
96 |
110 |
GRAPH:-
Graph no:05- Assay:-
CONCLUSION
As per the above study it is found that the Batch-3 shows the best results of in-vitro test of friability,hardness,thickness,and dissolusion characteristics. Batch-3 formulation shows the prolong dissolution upto 24 hrs due to natural polymer Chitosan. Chitosan prolongs the drug release when used in concentration more than the 50% of total weight of tablet,and weight of chitosan is increases prolongs the drug release by forming the matrix structure.
ACKNOWLEDMENT: The Author Expresses The Sinceorly Thanks To Mr.Prasad Tanpure Precident Of S.V.N.H.T.’S College Of B.Pharmacy Rahuri Factory Rahuri. Ahmednagar.
REFERENCES
Remington, the science and practice of pharmacy,21 st edition, vol 1st page no- 940.
www.wikypedia.com.
Theory and practice of industrial pharmacy ,By-Leon lachman et al,ediion 2009.
Essential of medicinal chemistry, K.D Tripath, Fifth edition, Jaypee publication page no- 176 177 184.
Pharmaceutical dosage form and disperse system, seventh edition Howard.C.Ansel, G. popovich B.I. Publication page no- 197,198,199,200.
Remington: - The science and practices of pharmacy Volume-I, 20th edition Mack Publishing Company. Page No-858 870.
Indian pharmacopoeia 1996, volume-I Published by controller of publication, Delhi. Page Nos- 387 388
British Pharmacopoeia 2003, Volume-I, Published By council of European introduction, General Notice Monograph, Medicinal & pharmaceutical. Page No- 971,972
USP/NF 2004. Asian Edition National Formulary, Published By Board Of Trustees Page no- 953
Integrated pharmacology, By cartis, Sutter, Waikef, Hoffman Second edition, published by Elsevier Mosby Page No- 337, 444, 445
Remington, the science and practice of pharmacy,21 st edition, vol 1st page no- 940.
www.wikypedia.com.
Theory and practice of industrial pharmacy ,By-Leon lachman et al,ediion 2009.
Essential of medicinal chemistry, K.D Tripath, Fifth edition, Jaypee publication page no- 176 177 184.
Pharmaceutical dosage form and disperse system, seventh edition Howard.C.Ansel, G. popovich B.I. Publication page no- 197,198,199,200.