ABOUT AUTHORS:
*1Charugundla Harini,2 K. Sudheer kumar
1Kakadiya University, Warangal
2Bijupatnaik University of Technology & Sciences, Odisha
charugundlaharini@gmail.com
ABSTRACT
The aim of present research was to develop a fast releasing oral polymeric film, with good mechanical properties, instant disintegration and dissolution, producing an acceptable taste when placed on tongue. Solvent casting method was used to prepare oral films. cetirizine hydrochloride an antihistaminic was incorporated to relieve the symptoms of allergic rhinitis. The polymers selected were HPMC 3cps and PVA. Glycerin was the plasticizer used. Eight batches of films with drug were prepared using different combinations of polymer concentration. The resultant films were evaluated for weight variation, content uniformity, folding endurance, thickness, surface pH, tensile strength, % elongation, % moisture absorption, %moisture loss in vitro disintegration and in vitro dissolution. The optimized films have disintegrated within 28-60sec. The percentage release was varying with concentration of polymer. The films made with HPMC3cps 200 mg released 98.5% of drug in 2min, which was the best release amongst all.
Reference Id: PHARMATUTOR-ART-1974
I.INTRODUCTION
Oral route is the most preferred route for the delivery of the drugs about 60% of all dosage forms available are the oral solid dosage forms till date as it bears various advantages over the other routes of drug administration, but oral drug delivery systems still need some advancements to be made because of their some drawbacks related to particular class of patients which includes geriatric, pediatric and dysphagic patients associated with many medical conditions as they have difficulty in swallowing or chewing solid dosage forms.
Many pediatric and geriatric patients are unwilling to take solid preparations due to fear of choking. Even with fast dissolving tablets there is a fear of choking due to its tablet type appearance. One study showed that 26% of 1576 patients experienced difficulty in swallowing tablets. The most common complaint was tablet size, followed by surface form and taste. The problem of swallowing tablets was more evident in geriatric and pediatric patients, as well as travelling patients who may not have ready access to water.
So, fast-dissolving drug-delivery systems came into existence in the late 1970s as an alternative to tablets, capsules and syrups for pediatric and geriatric patients who experience difficulties in swallowing traditional oral solid-dosage forms. These systems consist of the solid dosage forms that disintegrate and dissolve quickly in the oral cavity without the administration of water. Research and development in the oral drug delivery segment has led to transition of dosage forms from simple conventional tablets or capsules to modified release tablets or capsules to oral disintegrating tablet (ODT) to the recent development of oral fast dissolving films (OFDFs). Amongst the plethora of avenues explored for the rapid drug releasing products, oral strip technology is gaining much attention.
Orally fast-dissolving film is new drug delivery system for the oral delivery of the drugs. It was developed on the basis of technology of the transdermal patch. The delivery system consists of a very thin oral strip, which is simply placed on the patient’s tongue or any oral mucosal tissue, instantly wet by saliva the film rapidly hydrates and adheres on to the site of application. It then rapidly disintegrates and dissolves to release the medication for oro mucosal and intragastric absorption. Technology Catalysts forecasts the market for drug products in oral thin film formulations was valued of $500 million in 20071and could reach $2 billion in 2012. Based on upward global growth trends of the past decade, the fast dissolving dosage market could produce revenues of $13 billion by 2015.
Flow Chart for the Development of Oral Solid Dosage forms
Dissolvable oral thin films (OTFs) are a proven technology2 for the systemic delivery of API. Pharmaceutical companies and consumers, particularly pediatric and geriatric patient populations, have adopted OTFs as a practical alternative to traditional OTC medicines such as liquids, tablets, and capsules, because of the various benefits of the films (fast, accurate dosing, safe, efficacious format, convenience, portability).
The next generation of dissolvable films is being designed to move beyond immediate-release oral delivery into applications such as implantable, topical, sublingual and gastro-retentive platforms for the delivery of both small and large molecules. This work is the direct result of the flexibility in dissolvable film design and manufacture.
Special features of mouth dissolving films
* Thin elegant.
* Available in various size and shapes.
* Unobstructive.
* Fast disintegration.
* Rapid release.
The ideal characteristics of a drug to be selected
- The drug should have pleasant taste.
- The drug to be incorporated should have low dose up to 40 mg.
- The drugs with smaller and moderate molecular weight are preferable.
- The drug should have good stability and solubility in water as well as in saliva.
- It should be partially unionized at the pH of oral cavity.
- It should have the ability to permeate oral mucosal tissue.
Advantage of orally fast dissolving films 3
- Oral dissolving films can be administered without water anywhere any time.
- Due to the presence of larger surface area, films provide rapid disintegrating and dissolution in the oral cavity.
- Oral dissolving films are flexible and portable in nature so they provide ease in transportation, during consumer handling and storage.
- Suitability for geriatric and pediatric patients, patients who experience difficulties in swallowing, mentally ill patients, the developmentally disable and the patients who are un-cooperative.
- Beneficial in cases such as motion sickness, acute pain, episodes of allergic attack or coughing, where an ultra rapid onset of action is required.
- Stability for longer duration of time, since the drug remains in solid dosage form till it is consumed. So, it combines advantage of solid dosage form in terms of stability and liquid dosage form in terms of bioavailability.
- As compared to liquid formulations, precision in the administered dose is ensured from each strip of the film. The oral or buccal mucosa being highly vascularized; drugs can be absorbed directly and can enter the systemic circulation without undergoing first pass hepatic metabolism.
- This advantage can be exploited in preparing products with improved oral bioavailability of molecules that undergo first pass effect.
- The sublingual and buccal delivery of a drug via thin film has the potential to improve the onset of action, lower the dosing, and enhance the efficacy and safety profile of the medicament.
- Provide new business opportunity like product differentiation, product promotion, and patent extension.
- Minimal side effects.
- Delivery can also be terminated relatively easily if required.
- Site specific action and local action.
- Noninvasive.
- No special set up required for the industry.
- Availability of larger surface area that leads to rapid dissolution compared to ODTs.
- Dose accuracy when compared to syrups.
- Rapid onset of action.
Disadvantages4
- High doses cannot be incorporated.
- Dose uniformity is a technical challenge.
Limitations
Drugs with larger doses are difficult to formulate into FDF e.g. Rifampin (600 mg), Ethambutol (1000mg) etc. However, research has proven that the concentration level of active ingredient can be improved up to 50% per dose weight. Novartis Consumer Health's Gas-X® thin strip has a loading of 62.5 mg of simethicone per strip5.
Most bitter drugs should be avoided or taste masking is required. Proteinaceous drugs should be avoided, if used then co-administration of enzyme inhibitors such as aprotinin, puromicin and bile salts required for the inhibition of proteolytic enzymes present in saliva.
Mechanism of Action
The delivery system is simply placed on a patient’s tongue or any oromucosal tissue. Instantly wet by saliva due to presence of hydrophilic polymer and other excipients, the film rapidly hydrates and dissolves to release the medication for oromucosal absorption.
Materials
|
SI.NO |
DRUG/EXCIPIENT |
SOURCE |
|
1 |
Cetirizine |
Hetero laboratories, Hyderabad |
|
2 |
HPMC3cps |
SD Fine chemical Ltd, Mumbai. |
|
3 |
Poly vinyl alcohol |
SD Fine chemical Ltd, Mumbai |
|
4 |
Glycerin |
Cadila Pharmaceuticals Ltd,Ahmedabad |
|
5 |
Aspartame |
Mahendralabs, Bangalore |
Formulation of HPMC 3cps & PVA films by casting method:
|
SI.no |
ingredients |
Quantity for 16square inch films |
|||
|
F 1 |
F2 |
F3 |
F4 |
||
|
01 |
HPMC 3cps |
500 mg |
400 mg |
300 mg |
200 mg |
|
02 |
PVA |
50 mg |
50 mg |
50 mg |
50 mg |
|
03 |
Glycerin |
300 mg |
300 mg |
300 mg |
300 mg |
|
04 |
Aspartane |
55 mg |
55 mg |
55 mg |
55 mg |
|
05 |
Cetirizine |
40 mg |
40 mg |
40 mg |
40 mg |
|
06 |
Water |
15 ml |
15 ml |
15 ml |
15 ml |
Formulation of HPMC films by casting method:
|
SI.no |
ingredients |
Quantity for 16square inch films |
|||
|
F 5 |
F6 |
F7 |
F8 |
||
|
01 |
HPMC 3cps |
500 mg |
400 mg |
300 mg |
200 mg |
|
02 |
Glycerin |
300 mg |
300 mg |
300 mg |
300 mg |
|
03 |
Aspartane |
55 mg |
55 mg |
55 mg |
55 mg |
|
04 |
Cetirizine |
40 mg |
40 mg |
40 mg |
40 mg |
|
05 |
Water |
15 ml |
15 ml |
15 ml |
15 ml |
VIII. RESULTS AND DISCUSSION
Table 8: Preformulation studies of API
|
SI.No. |
Characteristics |
Results |
|
1. |
Organoleptic evaluation |
white to off-white crystalline powder, odourless |
|
2. |
Solubility analysis |
It is freely soluble in water, practically insoluble in solvents like dichloromethane, acetone |
|
3. |
Melting point |
110-115º |
DRUG EXCIPIENTS COMPATIBILITY STUDY
Physical observation
Compatibility with excipients was confirmed by physical observation. The pure drug and along with its formulation excipients were subjectedto compatibility studies & studies were carried out by mixing definite proportions of drug and excipients and kept in glass vials which are stored at 40oC ±2oC &75± 5%RH for one month. Physical observation of sample was done every week for any color change or lump formation, the results of the physical observation are shown in Table9
Table 9: Results of Compatibility study
|
S.No |
Name of the excipient |
Ratio API: Expt |
Initial observation |
Final observation
|
Conclusion |
|
|
40°C/75% RH |
||||||
|
2nd week |
4th week |
|||||
|
1 |
API (Cetirizine hydrochloride) |
--- |
white |
white |
white |
Compatible |
|
2 |
API+ HPMC3cps |
1 :1 |
white |
white |
white |
Compatible |
|
3 |
API + Polyvinylalcohol |
1 : 1 |
white |
white |
white |
Compatible |
FTIR STUDIES
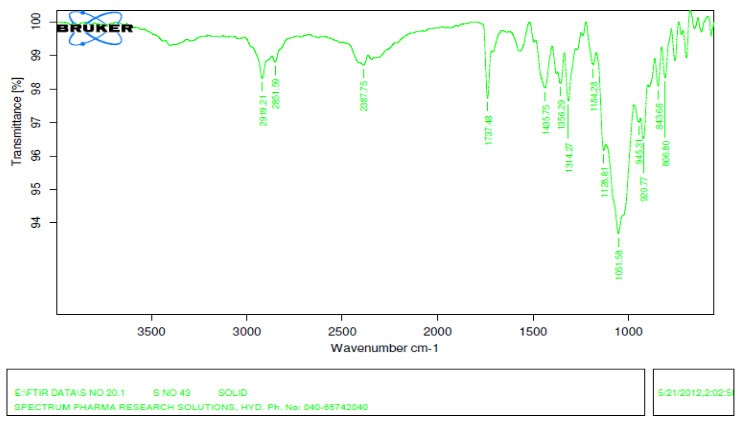
FT-IR spectroscopy is employed to ascertain the compatibility between selected drugs and polymers. The prepared formulation are subjected to FT-IR analysis by KBr pellet method using Fourier transform Infrared spectrophotometer and recorded over the range of 400-4000 cm?¹. Results showed that the drug and the excipients are compatible
FT-IR of pure drug:
Figure 2: Cetirizine:
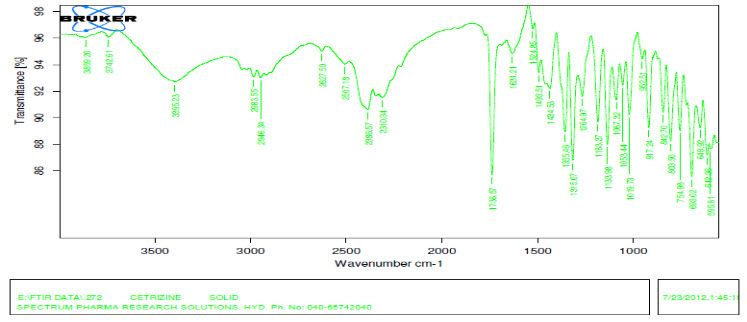
Figure3: FT-IR of optimized formula:
FTIR OF EXCIPIENTS
Figure 4: HPMC 3cps
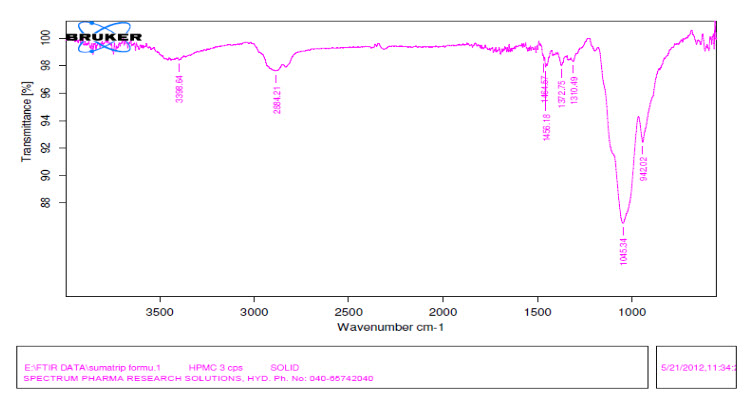
Figure-5 PVA:
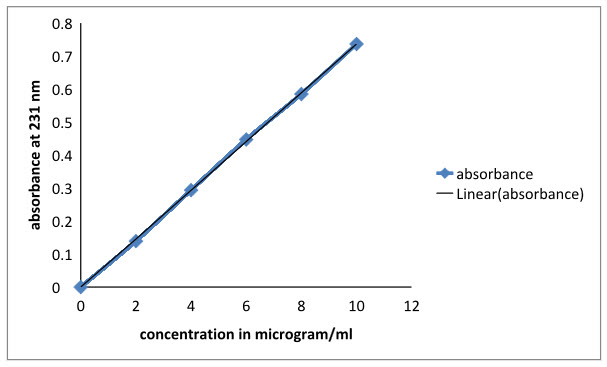
Calibration curve of cetirizine hydrochloride
Development of cetirizine linearity curve by using UV spectrophotometry at λmax 231 nm in distilled water.
Table 10: Calibration curve of Cetirizine
|
Concentration(µg/ml) |
Absorbance (231nm) |
|
0 |
0 |
|
2 |
0.1393 |
|
4 |
0.2938 |
|
6 |
0.4471 |
|
8 |
0.5849 |
|
10 |
0.7365 |
Figure 6: Standard graph of cetirizine hydrochloride
Evaluation of Oral thin films:
Eight formulations were prepared using solvent casting method and dried. Films consist of glycerin as a plasticizer and HPMC 3cps and PVA as polymers. Drug loaded films were flexible and transparent. All surface of the film was smooth, with elegant appearance, good physical properties. Thus these formulations can maintain a smooth and uniform surface when placed on tongue. The prepared films are evaluated for
a) Mechanical properties
Percentage moisture absorption (PMA)
Percentage moisture loss (PML)
Tensile strength
% elongation
Weight variation
Thickness
Surface pH of films
b) Drug content
c) Drug content uniformity
d) Disintegration time
e) In vitro dissolution study
Mechanical Properties
To avoid mechanical failure of the film and to ensure that film can bear the stress during transportation and storage the following mechanical properties such as tensile strength, elastic modules, elongation to break and strain should be measured.
Thickness, tensile strength and % elongation of the films increased by increasing concentration of polymers. Added glycerin alters the physical and mechanical properties by enhancing the mobility of polymers chains of HPMC/PVA. However it was found that glycerin gives the best plasticizer effect for Cetrizine.
Uniformity offilmthickness
The thickness of the films was measured at different points using screw gauge. The average of three readings were measured and presented in Table no11: The thicknesses of the films were increased as the total quantities of the polymers are increased. From these studies the films showed uniformity in their thickness the thickness of the films ranges from 0.18-0.39mm
Folding endurance
The folding endurance was measured manually for the prepared films. A strip of film 2x2cm was cut evenly and repeatedly folded at the same place till it is broken. The number of times the film could be folded at the same place without breaking gives the exact value of folding endurance. The folding endurance of the film was found to between 259-300.
Surface pH of Films
The surface pH of fast dissolving oral thin films was determined in order to investigate the possibility of any side effects in vivo. As an acidic or alkaline pH may cause irritation to the oral mucosa, it was determined to keep the surface pH as close to neutral as possible. A combined pH electrode was used for this purpose. Oral strip was slightly wet with the help of water. The pH was measured by bringing the electrode in contact with the surface of the film. The pH of all the films were found to be around 6.8 that is close to the neutral pH
% Drug content
The drug content was analyzed by spectrophotometrically at 231nm for cetirizine and the data is given in the Table11. The formulations exhibited uniform drug content and minimum batch variability. The drug content analysis of the formulations have showed that the process employed to prepare films in this study was capable of giving films with uniform drug content and minimum batch variability. The drug content of the films ranges from 93.6-99.8%
Uniformity ofweight
Three different films of the individual batch are weighed and the average weight was calculated. The dried films were weighed on digital balance. The films exhibited uniform weight. The data of the individual weights are shown in the Table11. From this studies the films exhibited uniform weight and there was no deviation in the weight of any formulation.
Tensile strength and percent elongation (%elongation)
Tensile strength of the films was determined using Hounse field universal testing machine. The data is shown in the Table from this studies The tensile strength increases with increasing the concentration of polymer. The tensile strength results obtained in formulations indicate the risk of film cracking. The films have shown reasonable tensile strength and no sign of cracking in the films observed, which may be attributed to addition of plasticizer.
Percent elongation of films gives information of how much a specimen can elongate before it breaks. It was carried out by Hounse field universal testing machine. The percentage elongation at break point is measured on scale and the data of the percentage elongation is presented in the Table12. from this studies the films have shown moderate % elongation and exhibited satisfactory elongation.
Percentage Moisture Absorption (PMA)
The moisture absorption studies carried out at 75% relative humidity. % moisture up take increases when PVA and HPMC concentration increases, because of hydrophilic nature of those polymers. The % moisture absorbance is maximum for F1 because the concentration of the polymer is more
Percentage Moisture Loss (PML)
The moisture loss studies carried in a desiccator containing anhydrous calcium chloride. All the films showed least percentage moisture loss.The percentage moisture loss is maximum for F1 because the concentration of the polymers is more.
In vitro disintegration time
Disintegration test was performed in the USP disintegration time testing apparatus. Distilled water was used as medium. The films were placed in the tube of the container and disintegration time was recorded. The disintegration time for the formulation F8 was found to be less that is 26seconds.
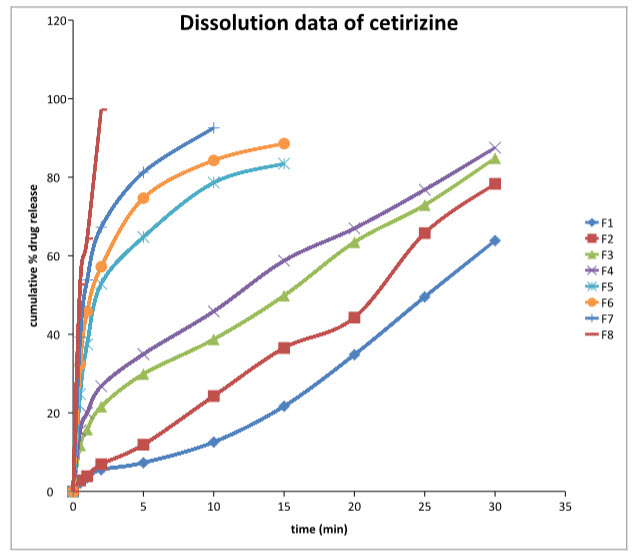
In vitro dissolution study:
The dissolution was carried out for different experimental trials. The various results that are obtained are tabulated below. Dissolution studies are carried out in the following media.
Medium: Distilled water
Type of apparatus: USP - II (paddle type)
RPM: 50 rpm
Volume: 150 ml
Temperature: 37ºC± 0.50C
Maximum drug release was observed in F8 that is 98.54%
Evaluation for weight variation, Thickness, Folding endurance, Surface pH, % Drug content , Disintegration time of oral thin film formulation of cetirizine
Evaluation for moisture loss, moisture absorption, % Elongation, tensile strength of oral thin films of cetirizine
In-vitro dissolution data for oral thin films formulation of cetirizine
In-vitro dissolution graph of cetirizine hydrochloride
Discussion
The main aim of this work was to develop oral films to release the drug at oral cavity for immediate release. HPMC 3cps and PVA were selected as film forming polymers
Drug Content and Physical Evaluation:
The assayed drug content in various formulations varied between 93 and 99% (96.48%) average weight of the film was found to be between 859mg and 1050 mg (mean 934 mg) and thickness of the films for all the formulations was found to be between 0.18mm and 0.39 mm with average of 0.26mm.
The oral films of cetirizine containing varying proportions of polymers were determined with an insight to develop the films without any irritation and other problems. The reason for such findings might be ionization of HPMC 3cps at salivary pH which leads to improved attachment of the device to oral cavity. Film of formulation F1 containing high amounts of HPMC 3cps and PVA showed more time for drug release than films of all other formulations, which might be due to high viscosity of the PVA. All the films are disintegrated within 60 seconds and less than that. Tensile strength was found to be increase d with increasing polymer concentration.
In-vitroDrug Release Studies
In-vitrodrug release studies revealed that the release of cetirizine from different formulations varies with characteristics and composition of film forming polymers as shown in Figure 15. The release rate of Cetirizine increased with decreasing concentration of HPMC 3cps as in F7 and F8 respectively. These findings are in compliance with the ability of HPMC 3cps to form complex which leads to immediate release of drug from the device. HPMC 3cps is more hydrophilic and it can swell rapidly, therefore decrease of HPMC 3cps content improves the drug release in F8.
The maximum cumulative percent release of cetirizine from formulation F8 could be attributed due to ionization of HPMC 3cps at pH environment of the dissolution medium. Ionization of HPMC 3cps leads to the development of negative charges along the backbone of the polymer. Repulsion of like charges uncoils the polymer into an extended structure. The counter ion diffusion inside the gel creates an additional osmotic pressure difference across the gel leading to the high water uptake. This water uptake leads to the considerable swelling of the polymer. The continued swelling of polymer matrix causes the drug to diffuse out from the formulation at a faster rate.
Formulation F8 showed relatively high rate of release of cetirizine which is due to rapid swelling and erosion of HPMC 3cps. Further, the increase in rate of drug release could be explained by the ability of the hydrophilic polymers to absorb water, thereby promoting the dissolution, and hence the release, of the highly water soluble drug. Moreover, the hydrophilic polymers would leach out and hence, create more pores and channels for the drug to diffuse out of the device. Formulation F1 which contains high amounts of HPMC3cps and PVA gets eroded slowly. Thus higher concentration of PVA cannot be incorporated into such formulations for immediate drug release.
CONCLUSION
The main objective of the study was to formulate and evaluate mouth dissolving film containing cetirizine hydrochloride. HPMC & PVA films were prepared by solvent casting method.
Compatibility of cetirizine with polymers was confirmed by FT-IR studies. Eight films were evaluated for weight variation and thickness showed satisfactory results. Tensile strength, percentage elongation and folding endurance of the films were increased with increase in the concentration of polymer due to increase in the elasticity nature of the polymer. Mouth dissolving time and disintegration time of the films were increased with increase in the concentration of the polymer, as more fluid is required to wet the film in the mouth. Content uniformity study showed that the drug is uniformly distributed in the film. Present study reveals that all the eight formulated films showed satisfactory film parameters.
It can be concluded that, Mouth dissolving film-containing cetirizine can be prepared by casting method. Formulation containing 200mg of HPMC 3cps film exhibited required tensile strength, folding endurance and percentage elongation and in-vitro drug release of 98.54% in 2min. From the present investigation it can be concluded that mouth dissolving film formulation can be a potential novel drug dosage form for pediatric, geriatric and also for general conditions which require a faster relief i.e. quicker onset of action.
REFERENCES
1. Technology catalysts International Corporation accessed on Jun. 15th 2011 Available from Technology catalysts.Com.
2. Oral Thin Films,” in Orally Disintegrating Tablet and Film Technologies, 4th ed. (Technology Catalysts International, Falls Church, VA, 2006), pp: 18-31.
3. Suresh, B., D. Halloran and L. James, 2006. Quick dissolving films: A novel approach to drug delivery Drug. Development Technologies, pp: 1-7. Drugdelierytech.Com.
4. (gas-x.com/.)
5. Wolany, G.J.M., J. Munzer, A. Rummeltand H.P. Merkle, 1990. Buccal absorption of Sandostatin (octreotide) in conscious beagle dogs. Proceed.Intern. Symp.Control. Rel. Bioact. Mater., 17: 224-225
6. Shojaei, A.H., 1998. Buccal Mucosa as A Route for Systemic Drug Delivery: A Review. J. Pharmacy and Pharmaceutical Sci., 1(1): 15-30.
7. Harris, D. and J.R. Robinson, 1992. Drug delivery via the mucous membranes of the oral cavity. J. Pharmaceutical Sci., 81: 1-10.
8. Galey, W.R., H.K. Lonsdale and S. Nacht, 1976 The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water, J. Investigative Dermatol., 67: 713-717.
9. Aungst, B.J. and N.J. of absorption-promoting actions of Laureth-9, Na salicylate, Na2EDTA and Aprotinig on rectal, nasal, and buccal insulin delivery. Pharmaceutical Res., 5(5): 305-308.
10. Oh, C.K. and W.A. Ritschel, 1990.Biopharmaceutic aspects of buccal absorption of insulin. Methods and Finding in Experimental and Clinical Pharmacol, 12: 205-212.
11. Wolany, G.J.M., J. Munzer, A. Rummelt and H.P. Merkle, 1990. Buccal absorption of Sandostatin (octreotide) in conscious beagle dogs Proceed. Intern. Symp. Control. Rel. Bioact. Mater17: 224-225.
12. Kurosaki, Y.S.Hisaichi, L.Hong, T. Nakayama and T. Kimura, 1989. Enhanced permeability of keratinized oral-mucosa to salicylic acid with 1- dodecylacycloheptan-2-one (Azone). In vitro studies in hamster cheek pouch. International J. Pharmaceutics, 49(1): 47-55.
13. Kurosaki, Y., S. Hisaichi, T. Nakayama and T. Kimura, 1989. Enhancing effect of 1-dodecylazacycloheptan-2-one (Azone) on the absorption of salicyclic acid from keratinized oral mucosa and the duration of enhancement in vivo. International J. Pharmaceutics, 51(1): 47-54.
14. Siegel, I.A.and H.P. Gordon, 1985. Effects of surfactants on the permeability of canine oral mucosa.
15. Siegel, I.A. and H.P. Gordon, 1985. Surfactant- induced increase of permeability of rat oral mucosa to non-electrolytes in vivo. Archives of Oral Biol., 30: 43-47.
16. Siegel, I.A. and H.P. Gordon, 1985. Effects surfactants on the permeability of canine oral mucosa molecular basis of barrier function in oral epithelium. In- vitro. Toxicology Letters, 26(2-3): 153-157.
17. Kurosaki, Y., S. Hisaichi, C. Hamada, T. and T. Kimura, 1988. Effect of surfactants on the Lipid content and w









