 About Author: Alka Lohani
About Author: Alka Lohani
M.Pharm (Pharmaceutics)
department of pharmaceutical sciences bhimtal campus
kumaun university nainital
Abstract:
Inconvenience of administration and patient compliance are gaining significant importance in the design of dosage forms. Difficulty in swallowing (dysphagia) is common among all age groups, especially in elderly and pediatrics. Ciprofloxacin hydrochloride is an orally administered antibacterial agent. The objective of this study was to develop ciprofloxacin hydrochloride soft gel using sodium alginate as a gelling agent and sodium citrate as a source of cation. Gels are formed by aggregation of polymers with minimum two components; the gelling agent and the fluid component. Different batches were prepared using three different concentrations of sodium alginate (0.1, 0.4, and 0.8%). The consistency of sodium alginate gel was dependent on the concentration of, sodium alginate, sodium citrate and co-solute. The results of dissolution study of soft gel F3 containing 0.4% sodium alginate and 0.3% sodium citrate revealed that Ciprofloxacin hydrochloride was 85% released in 45 min. and possessed acceptable sensory characteristics when evaluated by human volunteers. Short term stability study carried out for four weeks at different temperatures (0°C and room temperature) showed no considerable changes in performance characteristics of developed optimized formulation.
[adsense:336x280:8701650588]
Introduction:
Recently, more stress is laid down on the development of organoleptically elegant and patient friendly drug delivery system for pediatric and geriatric patients.1, 2 Many patients, elders and person with dysphagia find it difficult to swallow the tablets and hard gelatin capsules which results in high incidence of noncompliance and ineffective therapy. The patients with dysphagia can be choked by water while consuming liquid formulation which can be eliminated by administering liquid formulations with high viscosity.3, 4, The gel dosage form can be swallowed easily without water and are soft and smooth. The objective of this investigation was to develop hydrophilic gel dosage form for oral administration of Ciprofloxacin. Ciprofloxacin hydrochloride is a broad-spectrum fluoroquinolone antibacterial agent is more absorbed from the stomach and the proximal part of the small intestine.5
Materials and Methods-
Ciprofloxacin was obtained as gift sample from Finecure (P Ltd.)U. S. Nagar. Uttrakhand, Sodium alginate, Methyl paraben, propyl paraben, and all other chemicals like citric acid, sodium citrate, mannitol, purchased were of analytical grade.
Preparation of oral soft gel-
All the required ingredients of the formulation were weighed accurately. Sodium alginate powder was dispersed in 50 ml of distilled water maintained at 95°C. The dispersion was stirred at 95°C for 20 min using a magnetic stirrer. Ciprofloxacin was added with stirring. Then sucrose, citric acid, and preservatives (methylparaben, propylparaben) were added with stirring. Finally, required amount of sodium citrate was dissolved in 10 ml of distilled water and added to the mixture. The weight of the gel was monitored continuously during manufacturing and finally it was adjusted to the 100 gm with distilled water. The mixture was allowed to cool to room temperature to form gel. The mixture containing Sodium alginate, ciprofloxacin, and other additives was packed in polyethylene bag with airtight seal. The gels were prepared using three different concentrations of sodium alginate (0.1, 0.4, and 0.8%), each with two different sodium citrate concentrations (0.3 and 0.6%). The composition of Ciprofloxacin soft gel is shown in table-1.
|
INGREDIENTS |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Ciprofloxacin% |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
|
Sodium alginate% |
0.1 |
0.1 |
0.4 |
0.4 |
0.8 |
0.8 |
|
PEG 400% |
10 |
10 |
10 |
10 |
10 |
10 |
|
Citric acid % |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
|
Sucrose % |
66 |
66 |
66 |
66 |
66 |
66 |
|
Sucralose % |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|
Sodium citrate % |
0.3 |
0.6 |
0.3 |
0.6 |
0.3 |
0.6 |
|
Methylparaben (mg) |
0.18 |
0.18 |
0.18 |
0.18 |
0.18 |
0.18 |
|
Propylparaben (mg) |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Raspberry flavor % |
2 |
2 |
2 |
2 |
2 |
2 |
|
Water %, up to |
100 |
100 |
100 |
100 |
100 |
100 |
Evaluation of oral soft gel –
The ciprofloxacin soft gels were examined for appearance in terms of clarity, texture and consistency. Ciprofloxacin soft gels were also evaluated for viscosity, pH, and drug content and in vitrodrug release. Texture evaluationTexture of the soft gel was evaluated in terms of stickiness and grittiness by mildly rubbing the gel between two fingers.
Rheological measurement-Viscosity of the all the batches of soft gels was measured using Brookfield DV-II+Pro viscometer. The Ciprofloxacin soft gel was squeezed out from the polyethylene plastic bag by making a cut of uniform size on the bag and viscosity was measured using spindle number LV4 at the rotation of 50 rpm at room temperature. The viscosity measurements were made in triplicate using fresh samples each time. Results are shown in table 2.
Table-2, showing viscosity and pH
|
Parameters |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
Viscosity(cPs) |
1862±35 |
2552±50 |
7150±80 |
7562±90 |
10152±105 |
12182±130 |
|
pH |
6.93 |
6.08 |
6.99 |
6.12 |
6.05 |
6.10 |
pH of the soft gel – The pH of the final gel has got influence on stability, and on the taste. The pH of ciprofloxacin soft gel was measured by using Digital pH meter at room temperature. Results are shown in table 2.
Drug content –Drug content of the ciprofloxacin soft gel was estimated by eluting the drug from 10g of gel in phosphate buffer pH 6.8. The drug content was estimated by UV- spectrophotometer at 278 nm after filtering the sample through whattman filter paper.
Taste evaluation- Ten healthy, adult human volunteers participated in taste evaluation of ciprofloxacin soft gel (F3). One dose of the ciprofloxacin soft gel (10 g) containing 250 mg of ciprofloxacin was given to every volunteer and they were told to keep the gel in mouth for 5 sec. The volunteers were instructed not to swallow the gel. The volunteers were asked to comment on the bitterness, aftertaste, sweetness and flavor of the gel. Mouth feel in terms of grittiness was also checked. The results of taste evaluation of ciprofloxacin soft gel are shown in table-3
Table -3, Taste evaluation (formulation F3)
|
Parameters |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
Bitterness |
NB |
NB |
NB |
NB |
NB |
NB |
NB |
NB |
NB |
NB |
|
Aftertaste |
NB |
NB |
NB |
NB |
BT |
NB |
NB |
NB |
NB |
NB |
|
Sweetness |
VS |
SW |
VS |
VS |
VS |
VS |
VS |
VS |
VS |
SW |
|
Flavor |
GD |
GD |
GD |
GD |
GD |
GD |
GD |
GD |
GD |
GD |
|
Mouth feel |
GD |
GD |
GD |
GD |
MD |
GD |
GD |
MD |
GD |
MD |
NB- non-bitter, BT-bitter, SW-sweet, VS- very sweet, GD-good, MD-moderate
In vitro drug release6- In vitro drug release studies was carried out using USP dissolution apparatus 2 using paddle at a speed of 100 rpm using 900 ml of pH 6.8 phosphate buffer as dissolution media at 37±2°C. Soft gel (5 gm) containing 250 mg of ciprofloxacin was used in the dissolution test. Sample was withdrawn at the interval of every five minutes and the drug solution was replaced with the same volume of phosphate buffer (pH 6.8) maintained at 37±2°C. Absorbance was measured at 278 nm using UV- Spectrophotometer after suitable dilution of the samples.
Stability studies of soft gel- Thesamples were kept at different temperatures (0-8°, 25±5°, 45±2°) for four weeks. The samples were observed for pH, viscosity and appearance at the interval of 2 week. The results of the stability studies are shown in table-4
Table-4 stability studies of ciprofloxacin soft gel (Formulation F3)
|
Temperature |
0°C |
Room Temperature* |
||
|
weeks |
2 |
4 |
2 |
4 |
|
pH |
6.91 |
6.89 |
6.99 |
6.92 |
|
Viscosity(cPs) |
7150 |
7168 |
7162 |
7169 |
*about 28°C
Results and Discussion-
All the batches of soft gels were transparent in appearance. The gel of formulation F1, F2, and F3 were non-sticky and non-gritty while the gel of formulation F4 was slightly sticky but non gritty. The non-gritty nature of the formulation F5 to may be due to the suitable concentration of sodium alginate and sodium citrate but F6 was gritty due to higher concentration of both sodium alginate and sodium citrate.
The gel of formulation F1 and F2 exhibited fluid like consistency while the gel of formulation F5 and F6 were very thick in consistency.
Viscosity is the one important parameter which provides vital information during the optimization of the soft gel. The viscosity of the formulation F1 and F2 were low because of its fluid like consistency while the viscosity of the formulation F5 and F6 were high because they were very thick in consistency. As formulation F5 and F6 were thick in consistency, sticky and gritty, they failed to give good mouth feel. The viscosity of the formulation F3 and F4 were acceptable. The consistency and viscosity of the soft gels are related to each other because both are dependent on concentration of sodium alginate, sodium citrate, and co-solute. Effect of concentration of co-solutes (sucrose and sucralose) on the viscosity and consistency of all the batches of the soft gel was same because the co-solutes were used at same level in all the batches. formulation F3 consisting of 0.4% sodium alginate and 0.3% sodium citrate was considered as an optimum batch considering viscosity and appearance. The pH of the maximum stability of ciprofloxacin in aqueous phase is in between 1.5 to 7. Therefore, the pH of the formulated gels was adjusted and maintained in between 5 to 7 with help of buffering agents such as citric acid and sodium citrate. Sucrose may crystallize in presence of citric acid on standing.[8] Therefore, the amount of citric acid was kept minimum, just to adjust to the required pH. Sodium citrate was selected as a salt to contribute cation because it also act as sequestrant, buffering agent and helps in maintaining mechanical property of the gel.7 The drug content of the formulations F3 and F4 were 99.6±1.56% and 99.1±1.48%, respectively which is well within the acceptable limit.
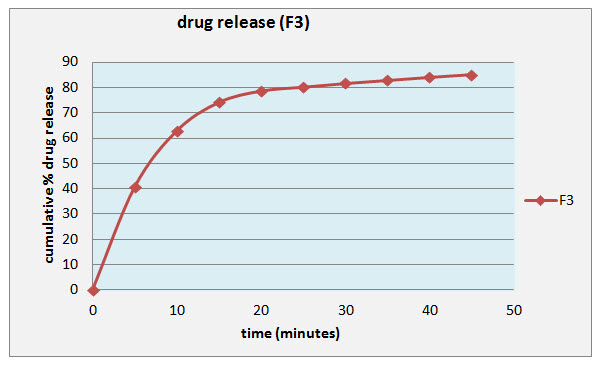
The results shown reveal that gels of the formulation F3 exhibited acceptable consistency and viscosity. Thus, it was subjected to dissolution study. Dissolution studies of the ciprofloxacin soft gel containing 0.3% of sodium alginate and 0.3% of sodium citrate showed 85% release of the drug within 45 min.
The results of taste evaluation of the formulation F3 ciprofloxacin gel are shown in Table 3. All the ten volunteers perceived the soft gel as non-bitter. Addition of flavors and sweeteners is the foremost and simplest approach for taste masking especially in the case of pediatric formulations. Sucrose (66%) was not able to mask the bitter taste completely because sugar molecules might have been trapped into the gel network. Sucralose was selected as an auxiliary sweetener because it is non-carcinogenic and 300-1000 times sweeter than the sucrose.8 Raspberry flavor was selected because to certain extent it helps in masking the bitter taste of drug and also improves patient acceptance.
The results of stability studies, shown in Table 4 indicate no considerable changes in pH, viscosity and appearance of the formulations. Precipitation of ciprofloxacin in the soft gels was not observed.
Figure-1 showing drug release (F3)
References:
1. Wadhwani AR, Prabhu NB, Nandkarni MA, Amin PD. Consumer friendly mucolytic formulations. Indian J Pharma Sci 2004;7:506-7.
2. Bhusan SY, Sambhaji SP, Anant RP, Kakasaheb RM. New drug delivery system for elderly. Indian Drugs 2000;37:312-8.
3. Yokoyama H, Hirata A, Hamamoto H, Ishibashi M, Yamasaki K, Fujii T. Biguanide drug containing jelly preparation, 2007, U.S Patent app,0053939
4. Ninomiya H, Shimizu T, Dairaku M, Komagata T. Jellied medicinal composition for oral administration, 1999, U.S Patent No,5,932,235.
5. Rajaonarivony M, Vauthier C, Couvrraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm. Sci 1993; 82(9): 912.
6. Indian Pharmacopoeia. Vol. 2. New Delhi: Controller of Publication; 1996. p. 555-6.
7. Rowe R, Sheskey P, Weller P, editors. Handbook of Pharmaceutical Excipients. 4th ed. Chicago: The Pharmaceutical Press; 2003.
8. Itoh A, Niwas T. PCT Int. Appl WO 9830, 209 , through CA., 129 (10),1998
9. Singh B, Kim K. Effects of divalent cations on drug encapsulation efficiency of deacylated gellan gum. J Microencapsul. 2005;22:761–71.
10. Miyazaki S, Nakayama A, Oda M, Takada M, Attwood D. Chitosan and sodium alginate based bioadhesive tablets for intraoral drug delivery. Biol Pharm Bull. 1994;17:745-747.
11. Deasy P, Quigley J. Rheological evaluation of deacylated gellan gum (Gelrite) for pharmaceutical use. Int J Pharm. 1991;73:117–23.
12. Rytting E, Lentz K, Chen X, Qian F, Venkatesh S. Aqueous and co-solvent solubility data for drug-like organic compounds. AAPS PharmSciTech. 2005;7(10).
Reference ID: PHARMATUTOR-ART-1027
FIND OUT MORE ARTICLES AT OUR DATABASE









