 About Authors: RAJESH Z. MUJORIYA,
About Authors: RAJESH Z. MUJORIYA,
M.PHARM.(PHARMACEUTICS)
Assistant Professor,
sardar patel college of technology, balaghat M.P.
ABSTRACT
Pantoprazole sodium is proton pump inhibitor and used as an antiulcer agent. The study was undertaken with an aim to formulate pantoprazole sodium enteric coated pellets.
Before going to develop the formulation a detail product literature review was carried out to know about the MUPS and type of dosage form available in market. The present study was focused to formulate delayed release capsule by MUPS Technique.
Average pellets size was determined by sieve analysis and found to be 1680-1200 microns (ASTM sieve no. 12-16). Sieve analysis was the essential step before coating. Because uniform sized pellets undergo effective coating. The result indicates a effective enteric coating and delay the drug release, with 32% acryl ezee solution, is possible. The formulation developed can further be worked on. For identifying a best formulation for delayed release pellets of pantoprazole sodium.
[adsense:336x280:8701650588]
1. INTRODUCTION
A peptic ulcer is a hole in the gut lining of the stomach, duodenum, or esophagus. A peptic ulcer of the stomach is called a gastric ulcer; of the duodenum, a duodenal ulcer; and of the esophagus, an esophageal ulcer. An ulcer occurs when the lining of these organs is corroded by the acidic digestive juices which are secreted by the stomach cells. Peptic ulcer disease is common, affecting millions of Americans yearly
Proton pump inhibitors (or "PPI"s) are a group of drugs whose main action is a pronounced and long-lasting reduction of gastric acid production. These drugs are utilized in the treatment of many conditions such as Dyspepsia, Peptic ulcer disease (PUD), Gastroesophageal reflux disease Laryngopharyngeal Reflux Disease, Barrett's esophagus, prevention of stress gastritis. Gastrinomas and other conditions that cause hypersecretion of acid. Zollinger-Ellison syndrome.
Proton pump inhibitors act by irreversibly blocking the hydrogen/potassium adenosine triphosphatase enzyme system (the H+/K+ ATPase, or more commonly just gastric proton pump) of the gastric parietal cell. The proton pump is the terminal stage in gastric acid secretion, being directly responsible for secreting H+ ions into the gastric lumen, making it an ideal target for inhibiting acid secretion.
1.1 DELAYED RELEASE SYSTEMS1
The design of such system involves release of drugs only at a specific site in the gastrointestinal tract. The drugs contained in such a system are those that are:
i) Destroyed in the stomach or by intestinal enzymes
ii) Known to cause gastric distress
iii) Absorbed from a specific intestinal site or
iv) Meant to exert local effect at a specific gastrointestinal site
The two types of delayed release systemsare:
1. Intestinal release systems: A drug may be enteric coated for intestinal release for several known reasons such as to prevent gastric irritation, prevent destabilization in gastric pH etc.
2. Colonic release systems: Drugs are poorly absorbed through colon but may be delivered to such a site for two reasons-
a) Local action in the treatment of ulcerative colitis and
b) Systemic absorption of protein and peptide drugs
Advantage is taken of the fact that pHsensitive bioerodible polymers like polymethacrylates release the medicament only at the alkaline pH of colon or use of divinylbenzene cross-linked polymers that can be cleaved only by the azo reductase of colonic bacteria to release free drug for local effect or systemic absorption.
New drug delivery systems can be over looked as 2
Delayed release -use repetitive, intermittent dosing of a drug from one or more immediate release units, incorporated into a single dosage form.
e.g. - Repeat action tablets, enteric-coated tablets.
Sustained release –they show release over extended period of time.
Controlled release-provides constant drug levels, with zero order kinetics.
Prolonged release-provide extended release, but not necessarily constant drug levels. May not follow perfect zero order.
Site-specific and receptor release-targeting drug to the particular organ or tissue of the body. For receptor release, target is particular receptor for a drug within an organ or tissue.
1.2 CONTROLLED DRUG DELIVERY3
Controlled drug delivery is delivery of drug at a rate or at a location determined by needs of body or disease state over a specified period of time.1
advantages-1 improved patience compliance and convience due to less frequent drug administration
2 . Reduction in fictuation in steadyvstate and therefore better control of disease conditions and reduced intensityof local and systemic side effect
3.Increased safety margin of high potency drugs due to better control of plasma levels.
4 .Reduction in health care costs trough improved theary, shorter treatment period, less frequency of dosing and reduction in personnel time to dispence, administer and monitor patients.
Disadvantages- 1 .poor in vitro –invivo correlations.
2.possibility of dose dumping due to food, physiologic formulation variables or chewing or grinding of oral formulation by the patient and thus increased risk of toxicity
3 .Retrival of drug is difficuil in conc. Of toxicity,posining or hypersensitivity reaction
The oral controlled release systems are classified as follows:
A) Continuous release systems
B) Delayed transit and continuous release systems
C) Delayed release systems
SUSTAINED RELEASE DOSAGE FORMS
The aim of any drug delivery system is to provide therapeutic amount of drug to appropriate site in the body to achieve immediate therapeutic response and to maintain the desired drug concentration.
In the recent years sustained release (SR) dosage forms continue to draw attention in the research for improved patient compliance and decreased incidence of adverse drug rections.
Sustained release, sustained action, prolonged action, extended action are the terms used to identify drug delivery system that are designed to achieve a prolong therapeutic effect by continuously releasing medication over an extended period of time after administration of a single dose.4
Advantages
* Improvement of patient compliance because of decreased frequency of dosage.
* Reduction in adverse effects-this is because of the nature of its release kinetics, a sustained release formulation should be able to use less total drug over the time course of therapy, than conventional preparation.
* Avoidance of costly interventions such as laboratory services.
* Allowing patients to receive medications as outpatients.
* Reduction in the overall use of medicinal resources and overall reduction in health care costs
* Optimization of duration of action of drug.
* Controlling the site release.
Disadvantages
* Increased variability among dosage units.
* Stability problems.
* Increased cost per unit dose.
* More rapid development of tolerance.
* Need of additional patient education and counseling
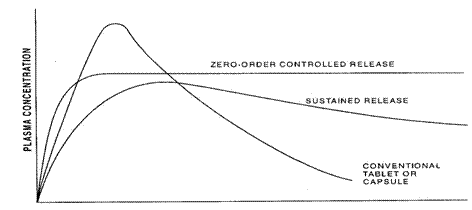
Figure 1: Plasma drug concentration profiles for conventional tablet or capsule formulation, a sustained release formulation and a zero order controlled release formulation.
1.3 PELLETIZATION
Pellets5: Pellets can be defined as small, free flowing ,spherical or semi-spherical solid units,typically from about 0.5 mm to 1.5 mm, and intended usually for oral
Administration, manufactured by the agglomerates of fine powders or granules of bulk drugs and Excipients using appropriate processing equipment. Pellets can be prepared by many methods, the compaction and drug-layering being the most widely used today.
1. Regardless of which manufacturing process is used, pellets have to meet the following requirements.They should be near spherical and have a smooth surface; both considered optimum characteristics for subsequent film coating.
2. The particle size range should be as narrow as possible. The optimum size of pellets for pharmaceutical use is considered to be between 600 and 1000mm.
3. The pellets should contain as much as possible of the active ingredient to keep the size of the final dosage form within reasonable limits.They should be near spherical and have a smooth surface; both considered optimum characteristics for subsequent film coating.
4. The particle size range should be as narrow as possible. The optimum size of pellets for pharmaceutical use is considered to be between 600 and 1000mm.
5. The pellets should contain as much as possible of the active ingredient to keep the size of the final dosage form within reasonable limits.
6. Regardless of which manufacturing process is used, pellets have to meet the following requirements . They should be near spherical and have a smooth surface; both considered optimum characteristics for subsequent film coating.
7 The particle size range should be as narrow as possible. The optimum size of pellets for pharmaceutical use is considered to be between 600 and 1000mm.
8 The pellets should contain as much as possible of the active ingredient to keep the size of the final dosage form within reasonable limits.They should be near spherical and have a smooth surface; both considered optimum characteristics for subsequent film coating.
SIGNIFICANCE OF PELLETS
Pellets may have varied applications in varied industries. It just requires an innovative bend to use it to derive maximum profitability. The smooth surface & the uniform size of the pellets allow uniform coating not only for each pellet but also from batch to batch.
Highlighted below are some of the few instances where smooth surfaced uniform pellets are being successfully used:
1. Improved appearance of the products. Coating of pellets can be done with different drugs to enable a controlled release rate.
2. In case of immediate Release Products larger surface area of pellets enables better distribution.
3. Chemically incompatible products can be formed into pellets & delivered in a single dose by encapsulating them.
4. In the chemical industries it is used to avoid powder dusting.
5. Varied applications are possible in the pellet form. Eg: sustained release.
6. Pellets ensure improved flow properties, and flexibility in formulation development and manufacture.
7. The coating material may be colored with a dye material so that the beads of different coating thickness will be darker in color and distinguishable from those having fewer coats.
8. The beads or granules of different thickness of coatings are blended in the desired proportions to give the desired effect.
9. The thickness of the coat on the pellets dictates the rate at which the drug/ contents are released from the coated particles. A smooth surface of the pellets & uniform coating thickness for each pellet.
10. By selecting the proper formulation, processing conditions and processing equipment it is possible to attain smooth surfaced & uniform pellets.
The most common advantages of pelletization are6
· Improved appearance of the product and the core is pharmaceutically elegant.
· Pelletization offers flexibility in dosage form design and development.
· Pellets are less susceptible to dose dumping.
· It reduces localized concentration of irritative drugs.
· It improves safety and efficacy of a drug.
· Pellets offer reduced variation in gastric emptying rate and transit time.
· Pellets disperse freely in G.I.T. and invariably maximize drug absorption and also reduce peak plasma fluctuation.
· Pellets ensure improved flow properties in formulation development
The most important reason for the wide acceptance of multiple unit products is the rapid increase in popularity of oral controlled release dosage forms, Controlled release oral solid dosage forms are usually intended either for delivery of the drug at a specific site within the gastrointestinal tract or to sustain the action of drugs over an extended period of time. With pellets, the above mentioned goals can be obtained through the application of coating materials (mainly different polymers), providing the desired function or through the formulation of matrix pellets to provide the desired effect. The advantage of multiple unit products as a controlled release dosage form is believed to be their behavior in vivo because of their advantageous dispersion pattern in the gastrointestinal tract and their special size characteristics.
1.4 Theory of pellet formation7
In order to judiciously select and optimize any pelletisation/granulation process, it is important to understand the fundamental mechanisms of granule formation and growth. Different theories have been postulated related to the mechanism of formation and growth of pellets. As the conventional granulation, the most thoroughly studied, most classified pelletisation process, which involves a rotating drum, a pan or a disc, has been divided into three consequtive regions: nucleation, transition and ball growth. However, based on the experiments on the mechanism of pellet formation and growth, the following steps were proposed: nucleation, coalescence, layering and abrasion transfer.
METHODS OF PREPARING PELLETS
Compaction and drug layering are the most widely used pelletization techniques in Pharmaceutical industry. Of the compaction techniques, extrusion and spheronization is the most popular method. Recently, however, melt pelletization has been used frequently in making compaction pellets using a different type of equipment, e.g. a high-shear mixer. Other pelletization methods such as globulation, balling and Compression are also used in development of pharmaceutical pellets although in a limited scale.
Powder layering:
Powder layering involves the deposition of successive layers of dry powders of drugs and excipients on preformed nuclei or cores with the help of binding liquids. As powder layering involves simultaneous application of binding agents and dry powders, hence it requires specialized equipments like spheronizer. The primary requirement in this process is that the product container should be solid walls with no perforation to avoid powder lose beneath the product chute before the powder is picked of by the wet mass of pellets that is being layered.
Solution / suspension layering:
Solution/suspension layering involves the deposition of successive layers of solution or suspensions of drug substances and binder over the starter/non-pareil seeds, which is an inert material or crystals/granules of the same drug. In fact the coating process involved in general is applicable to solution or suspension layering technology. Consequently conventional coating pans, fluidized beds, centrifugal granulators, wurster coaters have been used successively to manufacture pellets by this method. The efficiency of the process and the quality of the pellets produced are in part related to the type of equipment used.
PELLETIZATION BY EXTRUSION AND SPHERONIZATION8:
The process involves first making the extrudes from the powder material and then converting the extrudes into beads using the spheronizer. The powder material could be any kind of powder (drug powder, ayurvedic powder, food ingredient powder, detergent powder, nuclear powder etc). Beads as fine as 0.6mm can be made.
OTHER PELLETIZATION METHODS
Other pelletization methods such as globulation, cryopelletization, balling, compression are also used, although a limited scale in the preparation of pharmaceutical pellets.
Globulation or droplet formation consists two related processes, spray drying and spray congealing.
Spray drying:
It is the process in which drugs in the suspension or solution without excipients are sprayed in to a hot stream to produce dry and more spherical particles. This process is commonly used for improving the dissolution rates; hence bioavailability of poorly soluble drugs.
Spray congealing:
It is the process in which a drug is allowed to melt, disperse or dissolve in hot melts of gums, waxes or fatty acids, and is sprayed into an air chamber where the temperature is kept below the melting point of the formulation components, to produce spherical congealed pellets. Both immediate and controlled release pellets can be prepared in this process depending on the physiochemical properties of the ingredients and other formulation variables.
Cryopelletization:
It is a process in which the liquid formulation is converted in to solid spherical particles or pellets in the presence of liquid nitrogen as fixing medium. The shape depends up on the distance the droplet travel before contacting liquid nitrogen.
Compression:
It is one type of compaction technique for preparing pellets. Compacting mixtures or blends of active ingredients and excipients under pressure prepare pellets of definite sizes and shapes. The formulation and process variables controlling the quality of pellets prepared are similar to those used in tablets manufacturing.
Balling:
It is the pelletization process in which pellets are formed by a continuous rolling and tumbling motion in pans, discs, drums or mixtures. The process consists of conversion of finely divided particles in to spherical particles upon the addition of appropriate amounts of liquid.
Excipients for pellets:
Formulation aids or excipients are added to pharmaceutical dosage forms mainly to produce satisfactory delivery of the drug to the intended site, to impart favorable characteristics to the dosage form and to facilitate the manufacture of the product. Since pellets are intended to be administered orally, the excipients used in the pellet dosage forms are typically the same as those used in tablet or capsule formulations.
Excipients, disintegrant, surfactants, pH adjusters, Separating agents, Spheronization enhancers, glidants and release modifiers etc. some examples of such excipients are given in Table
Table:1 Examples of commonly used excipients
|
Filler |
MCC, starch, sucrose, lactose, mannitol |
|
Binder |
Gelatin, HPC, HPMC, MC, PVP, sucrose, starch |
|
Lubricant |
Calcium stearate, glycerin, PEG, Mg. stearate |
|
Separating agent |
Kaolin, talc, silicon dioxide |
|
Disintegrant |
Alginates, croscarmellose sodium |
|
pH adjuster |
Citrate, phosphate, meglumine. |
|
Surfactant |
Polysorbate, SLS |
|
Spheronization enhancer |
MCC , sodium CMC |
|
Glidant |
Talc, starch, Mg stearate. |
|
Release modifier |
Ethyl cellulose, carnauba wax, shellac. |
NON PAREIL SEEDS: (NEUTRAL PELLETS)
SUGAR SPHERES:
“Sugar spheres contain not more than 92% of sugar, calculated on dry basis. The remainder consists of maize starch.” defined according to European pharmacopoeia.
Possibility to analyze the sugar spheres according to the Ph. Eur., USP/NF and JP.
Produced accordance with the GMP.
Enteric coatings9
Enteric coatings are those which remain intact in the stomach, but will dissolve and release the contents once it reaches the small intestine. Their prime intension is to delay the release of drugs which are inactivated by the stomach contents or may cause nausea or bleeding by irritation of gastric mucosa.
Cracking of the film either during application or on storage will result in a loss of enteric properties. Therefore, consideration must be given to the mechanical properties of the applied film. Cracking problems can be effectively overcome by plasticization. Plasticizer can also be used to reduce the permeability of the polymer films to water vapor. The choice of suitable Plasticizer is restricted to non-water soluble materials because these are likely to be most effective.
An evaluation is made of the solubility parameters of species together with an assessment of the intrinsic viscosity of dilute solutions of the polymer on the plasticizers. This determines the maximum interaction between polymer and Plasticizer, and indicates which Plasticizer is likely to be most effective.
A general rule to follow is to use 1 part Plasticizer to 10 parts polymer.
One should also consider viscosity of the plasticizer, its influence on the final coating solution , its effect on film permeability , tackiness, flexibility, solubility and taste and its toxicity, compatibility with other coating solution components and stability of the film and the final coated product.
Important reasons for enteric coating are as follows 9:
* To protect acid-liable drugs from the gastric fluid
* To protect gastric distress or nausea due to irritation from drug
* To deliver drugs intended for local action in the intestines.
* To deliver drug that are optimally absorbed in the small intestine to their primary absorption site in their most concentrated form.
* To provide a delayed release component to repeat actions.
* Protect the drugs from harmful effect of the gastric contents; some of the drugs are prone to be hydrolyzed in acid media (E.g. Esomeprazole, omeprazole, pantaprazole)
Ideal enteric coating materials should have the following properties:
* Resistance to gastric fluids
* Ready susceptibility to or permeability to intestinal fluids
* Compatibility with most coating solution components and the drug substrates.
* The film should not change on aging
* Formation of continuous film.
* Non-toxicity
* Low cost
* Ease of application.
Enteric coating materials:
Enteric coatings work because they are selectively insoluble substances they won't dissolve in the acidic juices of the stomach, but they will when they reach the higher pH of the small intestine.
Most enteric coatings won't dissolve in solutions with a pH lower than 5.5. Commonly-used enteric coatings may be made from:
* Methacrylic acid copolymers
* Cellulose acetate (and its succinate and phthalate version)
* Polymethacrylic acid/acrylic acid copolymer
* Hydroxypropyl methyl cellulose phthalate
* Polyvinyl acetate phthalate
* Hydroxyethyl ethyl cellulose phthalate
* Cellulose acetate tetrahydrophtalate
* Acrylic resin
* Shellac
The earliest enteric coatings utilized formalized gelatin , this was unreliable because of the polymerization of gelatin could not be accurately controlled. Another was shellac , disadvantage was polymerization with time, resulting in poor dissolution of the coating.
The most extensively used polymers are CAP, PVAP. The most recently used polymers are HPMCP, Methacrylic acid copolymers.
Cellulose Acetate Phthalate (CAP):
Effective enteric coating, it only dissolves above pH 6 and may delay drug release longer than desired. it is permeable to moisture and simulated gastric fluid in comparison with other enteric polymers and it is susceptible to hydrolytic breakdown on storage.
Poly Vinyl Acetate Phthalate(PVAP):
Less permeable to moisture and simulated gastric juice, it is more stable to hydrolysis on storage. Enteric dosage forms coated with PVAP disintegrates at pH 5
Hydroxy Propyl Methyl Cellulose Pthalate (HPMCP):
It is available in two grades HP50 and HP55.
HP55 solutions are more viscous than HP50.
HP50 disintegrates at pH5 and HP55 disintegrates at pH5.5.
It has stability similar to that of PVAP and dissolves in the same pH range. The advantage is that it does not require Plasticizer.
Methacrylic acid copolymers:
Two grades are available A and B which differs in the ratio of free carboxyl to ester groups therefore:
Type A has a ratio of 1:1 and disintegrates at pH 6
Type B has a ratio of 1:2 and disintegrates at pH 7.
Available under the trade names Eudragit L and S correspond to NF types A & B.
1.5 COATING EQUIPMENTS:
Most of the coating processes use one of three general types of equipments.
1. The standard Coating pan
2. The Perforated Coating pan
3. The Fluidized bed coater
Conventional pan system:
The standard coating pan system consists of a circular metal pan mounted somewhat angularly on a stand, the pan is rotated on its horizontal axis by a motor, the hot air is directed into the pan and onto the bed surface, and is exhausted by means of ducts positioned through the front of the pan .Coating solutions are applied by spraying the material on the bed surface.
The Perforated Coating pan:
Neocota is an automatic coating system for tablets and pellets. Neocota is a completely updated automatic coating system having a batch capacity of 500 g to 1 kg. This model efficiently carries out the following operations: Aqueous film coating of tablets/pellets; Non-aqueous organic solvent based film coating of tablets/pellets; and enteric film coating of tablets/pellets.
The basic units of the system are: Coating pan has perforations along its cylindrical portion. It is driven by a variable speed drive with a flame-proof motor. Supply of hot air and exhaust of drying air are arranged to facilitate the coating system through stainless steel plenums positioned on both sides of the perforated coating pan. The pan is enclosed in a cylindrical airtight housing provided with a suitable door and front glass window.This housing of pan with drive is a stainless steel cabinet accommodating the gearbox, AC variable drive, power panel, hot air unit, ex-haust unit and an air fitter.
Liquid spray system is complete with stainless steel liquid storage vessel, variable flow-rate liquid dosing pump, automatic spray gun, and inter-connecting flexible hoses.
The Fluidized bed coater:
The Fluid Bed Technology offers a very efficient coating technique. The major advantage of the Fluid Bed Systems is that it is as per GMP standards it is a closed system.
The second advantage of the Fluid Bed Systems is that not only coating but granulation and pellet formation is also possible in the same machine.
Fluidized bed coating is a process that takes place inside a fluidized bed whereby a coat is introduced to cover the intended object in order to protect it or modify its behavior. Particulate coating is a form of fluidized bed coating involving the coating of solid
Particles inside the bed. In the process, a layer is deposited onto the surface of fluidized solid particles by spraying with a solution of the coating material. The fluidizing gas is also use to dry the deposited solution to form a coat on the surface of the particle.
There is considerable diversity in methods of using fluidized bed technology. For e.g. liquids can be applied to fluidized particles in a variety of ways, including top, bottom and tangential spraying. For a given product, each method can offer markedly different finished product characteristics.
Fluidized beds are used for coating because of their high energy- and mass transfer. Fluidized beds for film coating can be divided into three groups
¨ Top-spray,
¨ Tangential-spray
¨ Bottom-spray equipment.
Top spray:
The expansion chamber is lengthened to allow powder to remain fluidized longer and to move with a higher velocity, so that agglomeration is minimized The expansion chamber is conically shaped to allow uniform deceleration of air stream
The filter housing is larger and designed to shake the fines back into the bed interrupting fluidization ; this reduces agglomeration tendencies.
The nozzle is positioned low in the expansion chamber so that coating material impinge on the fluidized particle a short distance from the nozzle; this reduces droplet spray drying and provides for longer subsequent drying of the coated particles.
The top spray coater has been used to apply aqueous and organic solvent based film coatings, controlled release coatings.
Bottom spray coating10: (wurster process, Make-GLATT)
The wurster machine employs a cylindrical product container with a perforated plate. Inside the container is a second cylinder (coating partition) with is raised slightly above the perforated plate, centered in the plate below this partition is a spray nozzle used to dispense the coating solution. The perforated plated is designed with large holes in the area under the coating partition and smaller holes in the remainder of the plate, except for one ring of large holes at the perimeter. The design allows the substrate particles to be pneumatically transported upward through the coating partition, and downward outside this partition. Material passing through coating partition receives a layer of coating material, dries in the expansion chamber, and falls back in a semi fluidized state. Material circulates rapidly in this fashion and receives layer of coating material, dries in the expansion chamber, and falls back in a semi fluidized state material circulates rapidly in this fashion and receives a layer of coating on each pass through the coating partition. The ring of large holes on the periphery of perforated plate prevents the accumulation of material at the container wall. It has been used for coating small particles, pellets and tablets.
Table:2 Parameters Used in Bottom Spray Equipment
|
Inlet temperature |
38-42ºC |
|
Product temperature |
32-36ºC |
|
Exhaust temperature |
32-38ºC |
|
Spray rate |
8-12mg/min |
|
Peristaltic pump |
12-18 rpm |
1.6 FLUID BED COATING
Particles smaller than approx.2mm should be coated in fluid bed equipments, because with decreasing particle diameter the specific surface area of a substrate increase dramatically .thus ,the required coating weight gain is much higher than tablet coating processes. In order to achieve acceptable process times, the high efficiency of fluid bed compared to pan coating equipment shows clear advantages in particles coating processes.
SHAPE- in order to achieve good flow properties, spherical particles with smooth surfaces are preferred, while needle shaped particles show poor flow properties and tend to form lumps. Another advantage of the latter is the increased risk of breakage during the coating process, creating un coated spaces and leading to an increased coating weight gain .besides crystals and pellets, granules can be used as substrates as a disadvantages we may have uneven surfaces and often increased abrasion compared to the shapes mentioned first, which can also lead to increased surface areas which requires higher amounts of coating.
SIZE- usual particle sizes are in arrange of 0.2-1.2mm.smaller particles may have problematic flow properties in higher scale and may tend to break if the length /diameter-ratio is.2. Smaller particle size are required if particles are administered from sachets or incorporated into chewable tablets. in order to avoid damage by chewing, the coated particles should have a maximum size of o.4mm smaller end products may given a better mouth felling but increasing specific surface areas requires higher coating amounts.
TOP/ BOTTOM / TANGENTIAL SPRAY-The top spray method is known and used for particle coating and granulation processes. Compared to other fluid bed coating technologies, the top spray method is susceptible for porous film structure, especially if organic coating formulations are processed .bottom spraying (wurster process) is the usual method in particle coating .due to a more uniform particle movement, better film structures can be achieved compared to the top spray method, and the required polymer weight gain for a certain function is usually lower to some extent. A disadvantage is that in case of nozzle blockage during the coating process, the product must be discharged before the nozzles can be cleaned. Tangential spraying system, which is commonly fitted with a rotating bottom plate, can achieve film quantities nearly as good as bottom –spraying system. The rotation of the plate nicely supports product movement, so that the required air amount is mainly used for drying process and only to a smaller degree for the product movement.
NOZZLES FOR THE PARTICLE COATING –Common spray gun are air-borne with a round spray pattern. Some equipment is fitted with a double air supply which is used for common atomizing air and extra microclimate air, which surrounds the spray pattern, preventing over wetting of the product and reducing spray drying effects.
PUMP SYSTEM –peristaltic pumps fitted with silicon tubing are standard. Tubing can be selected in a wide range of internal diameters in order to keep the flow speed high and hence to prevent sedimentation .therefore the use of tubing with small internal diameters recommended. Alternative pump systems include gear pumps and piston pumps 9,10
ROATING DISK GRANULATION-Granulation techniques utilizing centrifugal fluidizing drive have been studied only recently. These techniques have been extended to coating operations and combined with an expansion chamber to form the rotating disk granulator and coater fluid bed device. The basic design employs a rotating disk in the product container.
The disk can be moved up or down to create a variable slit opening between the outer perimeter of the disk and the sidewall of the container. Air is drawn into the product container through the slit under negative pressure. This fluidizes the material along the circumferential surface of the product container. At the same time the disk rotates at varying speeds and moves the product by the centrifugal force to the outer portions where it is lifted by the fluidizing air stream into the expansion chamber. As the material decelerates, it descends to the center of the disk and repeats the same sequence.
The fluidization pattern is often described as a spiraling helix or rope-like pattern around the inside of the rotor chamber.
Spray nozzles can be immersed in the bed of fluidized material and spray applied in tangential fashion with respect to the particle flow.
2. LITERATURE REVIEW
Bergstrand et al 11 ( 2004) The active compound was susceptible to degradation / transformation in acidic and neutral media .the degradation was catalyzed by acidic compounds and was stabilized in mixture with alkaline compounds. The stability of the active substances was also affected by moisture, heat, organic solvent and some degree by light. The proton pump inhibitors were best protected from contact with acidic gastric juice by an enteric coating layer. before applying enteric coating the core covered by one or more separating layers of alkaline compounds with using coating pan / coating granulator / fluidized bed coater .The material for separating layer are Sugar, Polyethylene Glycol, PVP, PVA, HPC, Methyl Cellulose , Ethyl Cellulose, HPMC .
Rahman et al 12 (August 08) The multiparticulate formulation of Sodium Paraminosalicylate for oral administration was developed by extrusion and spheronization technique microcrystalline cellulose was used as filler in conc. of 14.4% w/w. Pellets were coated by Eudragit L30D55 using fluidized bed processor. Different weight gain of acrylic polymer were applied on the pellets and evaluated for invitro dissolution behavior in 0.1N Hcl for 2 hours. And then media was charged to pH6.8 phosphate buffer. A 60% w/w coating level of Eudragit L30D55 has Produce the most acceptable result.3% seal coat of HPMC E5 was also applied in order to protect the drug from migration into the Eudragit coat and film coat was applied in order to prevent aggregation of pellets in dissolution media. Morphological characteristics of developed pellets were also investigated by scanning electron microscopy and found to be smooth and spherical. Developed system was found to be suitable for the delivery of Sodium Paraminosalicylate)
Tanberk et al 13(feb.1997)In the production of omeprazole pellets the inert core based on sacharose, starch, glucose. The inert core covered with the micronized and sieved active substance which is in a buffered dispersion being added within anionic surface active agent. In order to finally receive an enteric covering in a fluidized bed with HPMC Phthalate, Diethyl Phthalate, Acetone, Ethyl Alcohol, being afterwards dried to obtain water content of less than 1%. Then sieved, weighted and encapsulated in gelatin capsule. Inert core prepared by 65-85% of Sacharose, 2-6% of Glucose being sieved through 150 mesh & dispersing in a buffered aqueous dispersion at pH 7.1 with addition of anionic surface active agent.
Claudio et al 14 (Januuary 2000)The goal of the present study was to evaluate the influence of the formulation and operating conditions on pellets preparation by pan technique application of powdered drug on sugar based cores .inert cores were intermittently treated with micronized drug powder and adhesive solution .drug layering by GS automated pan coating system. Core resulting in the production of pellets that can further coated by different polymers to obtain modified release formulations different procedures have been used to evaluate a series of important parameters such as initial cores weight. Speed of powder application, speed type and position of the atomizers, atomization degree, temperature and air spray. At first covered with seal coating then followed by enteric coating.
Heinamalk et al 15 (Feb. 2002)The effects of filler used in the cores (i.e. waxy, cornstarch, or lactose) and the enteric film coat thickness on the diffusion and dissolution of freely soluble drug were studied.
Two kinds of pellets core containing riboflavin sodium phosphate as model drug , microcrystalline cellulose as basic filler ,and waxy cornstarch or lactose as a co filler were film coated (theoretically weight increases 20% or 30%) with an aqueous enteric coated pellets was investigated by CLSM. In vitro release test was performed using a USP apparatus I (basket method)
Comoglu et al 16 (June 2008) Pantoprazole is proton pump inhibitor prodrug used in the treatment of gastric ulcers and gastroesophageal disease.pantoprazole must be absorbed in the gastrointestinal tract and because it is unstable under acidic conditions, enteric delivery system are required . the purpose of this study was prepare pantoprazole loaded microspheres by emulsion solvent evaporation technique using two different types of enteric coating polymers Eudragit S100 and hydroxypropyl methylcellulose phthalate. The microspheres have been characterized in terms of their morphology, encapsulation efficiency, and ability of stabilizing pantoprazole in acidic media pantoprazole determinations were carried out using a validated spetrophotometric method for analysis of drug dissolution media.
Rekkas et al17(July 2008) This study was examined the effect of rotor speed, amount of water sprayed, and atomizing air pressure on the geometric mean diameter and geometric standard deviation of pellets produced in a fluid-bed rotor granulator using a 23 factorial design and an optimization technique. Pellets were prepared by wet granulation. Equal amounts of microcrystalline cellulose, a-lactose monohydrate, and distilled water were used as the granulation liquid. The size and the size distribution of the pellets were determined by sieve analysis.
The size of the pellets was found to be dependent on the amount of water added, while an increase in rotor speed decreased their size. Both factors were found to be statistically significant (P < .05). The effect of atomizing air pressure on pellet size was not statistically significant. None of the 3 factors significantly affected the geometric standard deviation of the pellets. The rotor speed and the amount of water sprayed were further selected in order to construct a mathematical model that correlates these factors with the geometric mean diameter of the pellets. For this purpose, the optimization technique 32 was used. The derived equation described the relationship between the selected factors and the size of the pellets.
As a result, the experimental design techniques applied were found to be suitable in optimizing the pelletization process carried out in a fluid-bed rotor granulator.
Jakob Kristensen et al18 (March 2005) The aim of the present study was to investigate the use of different grades of microcrystalline cellulose (MCC) and lactose in a direct pelletization process in a rotary processor. For this purpose, a mixed 2- and 3-level factorial study was performed to determine the influence of the particle size of microcrystalline cellulose (MCC) (~60 and 105 μ m) and lactose (~30, 40, and 55 μ m), as well as MCC type (Avicel and Emcocel) on the pelletization process and the physical properties of the prepared pellets. A 1:4 mixture of MCC and lactose was applied, and granulation liquid was added until a 0.45 Nm increase in the torque of the friction plate was reached. All combinations of the 3 factors resulted in spherical pellets of a high physical strength. The particle size of MCC was found to have no marked effect on the amount of water required for agglomerate growth or on the size of the resulting pellets. An increasing particle size of lactose gave rise to more spherical pellets of a more narrow size distribution as well as higher yields.
The MCC type was found to affect both the release of the model drug from the prepared pellets and the size distribution. Generally, the determined influence of the investigated factors was small, and direct pelletization in a rotary processor was found to be a robust process, insensitive to variations in the particle size and type of MCC and the particle size of lactose.
US PATENT 19 Proton pump inhibitor and antibiotic clarithromycin were contained in separate Layer of single pellets .upon administration of the drug was released in different
Sites of the living body in a more stable form .double pellet formulation mainly used for the treatment of peptic ulcers, Helicobacter pylori infection, gastritis, stomach duodenal ulcer. Combinations of more than two drugs were follows one was proton pump inhibitor and another Amoxicillin, Metronidazole, Furazolidine, Bismuth Subsalicylate, Clarithromycin, Roxithromycin, Metroidazole, Ranitidine. Inventive double pellets prepared by dissolving a proton pump inhibitor and an alkalizing agent together with a coating base in a solvent and then coating the solution on an inert sugar particles. Then sugar particle coated with enteric coating material methacrylic acid copolymer, cellulose acetate phthalate, cellulose acetate butylate, hydroxyl propyl methyl cellulose acetate succinate, and other suitable enteric coating bases used.
WIPO PATENT 20 Lansoprazole is acid labile benzimidazole derivative very effective for GERD, peptic ulcer, lansoprazole having poor stability in the solid state it degrade in the presence of heat, moisture, light and having solution or suspension. Its stability deceases with decreasing pH. Sub coatthe prepared pellets in order to avoid a reaction between the API and the outer acidic enteric coating. It shows degradation and prone towards discoloration of API. Avoid antitacking agent in the nucleus form. Salt form was used in the pellets of lansoprazole e.g. Calcium, Magnesium, Hydroxide, Sodium, Potassium, Citrate. In above preparation SCMC, Povidone K30, used as binder and Polymethacrylic acid used for gastric resistance, Triethyl Citrate used as plasticizer,
3. AIM AND OBJECTIVE
The main objective of the work is to prepare enteric coated pellets of pantoprazole sodium by using methacrylic acid copolymer with drug release above pH 5.5, by using extrusion and spheronization method. Proton pump inhibitors are widely used to treat peptic ulcer, gstroesophageal reflux disease, zollinger-ellison syndrome, also in eradication of H.pyroli infection.
Acid labile and moisture sensitive pantoprazole difficult to stabilize in various dosage form. Moisture penetration prevented by seal coating. Pellets are of great interest to the pharmaceutical industry for Varity of reasons .pelletized product not only offer flexibility in dosage form design and development, but also utilized to improve safety and efficacy of bioactive agent.
A multiunit pellet system (MUPS) is an approach to develop capsule formulation capsule dosage form containing MUPS, when administered drug dispersed it. Protected from acid and dissolved in duodenum, each pellets acts as a single subunit. Consequently as a separate drug delivery system. The MUPS have good desirable distribution characteristic, reproducibility, transit time and reduce chance of localization of drug delivery .it having less prone to adherence to the intestinal walls, nasogastric and gastromy tubes and giving predictable delivery of the drug product to the site of drug release.
APPLICATION2
1. To protect drugs that are unstable in acid from disintegrating in the gastric juice e.g. antibiotics enzymes, peptides proton pump inhibitors.
2. pH Dependent controlled release of drugs for optimal absorption
3. GI targeting of different sections of small intestine or of the colon (absorption window, targeting localized effects)
4. Colon targeting for local treatment and systemic therapies. The key to controlling the release of the drug is the pHdependent dissolution of the film coating, which takes advantage of the different pH values that exist along the gastrointestinal tract. Since the coatings dissolution is controlled by pH, or by gradually permeability, the drug is release in a precise manner in specific sections of the digestive tract, or at specific times after intake.
Table No 3: Relationship between pHprofile and transit times in human GIT
|
Region |
pH (Fasted) |
pH(Fed) |
Transit time |
|
Stomach |
1.7 (1.4 – 2.1) |
5 |
1-5 hrs |
|
Duodenum |
4.6 (2.4 –6.8) |
4.5 – 5.5 |
>5 hrs |
|
Jejunum |
6.1 ( 6.0 – 7.0) |
4.5 – 5.5 |
1 -2 hrs |
|
Ileum |
6.5 |
6.5 |
2 -3 hrs |
|
Colon |
8 |
8 |
15 -48 hrs |
4. PLAN OF WORK
• Literature collection
• Selection of drug and excipients
• Preformulation studies
• Formulation of pellets
• Seal coating of pellets
• Enteric coating of pellets
• Evaluation of pellets
• Analysis of result & Identify the best formulation
INTRODUCTION 23, 24, 25
Drug name- Pantoprazole sodium sesquihydrate.
Mechanism of action- Drug inhibits H /K ATPase pump function. Thereby reducing gastric acid secretions
Dosage- Tablets, delayed-release 20 mg;
Tablets, delayed-release 40 mg
Suspension, delayed-release, oral 40 mg
40 mg injection for IV use
Use- In peptic ulcer, in gastro esophageal reflux Disease, Zollinger -Ellison syndrome
PHYSIOCHEMICAL PROPERTIES-
Description- White fine powder
Chemical name- 5(-difluromethoxy)-2 [3, 4-Dimethoxy- 2-pyridyl) methylsulphinyl]-1H- benzimidazole
Molecular formula- C 16 H15 F 2 N 3 O 4 S 1.5H20
Molecular weight- 383.371g/mol
Solubility- freely soluble in ethanol, soluble in water, and slightly Soluble in hexane
Category- Proton pump inhibitor
PHARMACOKINETIC PROPERTIES-
Time of peak serum conc. (Tmax) - 2.5h (0ral)
Bioavailability- 77% (oral)
Plasma protein binding- 98%
Volume of distribution- 11 to 23.6 L
Route of metabolism-Hepatic metabolism by Demethylation Through CYP2C19
Route of Elimination- Urine (71%), Feces (18%)
Biological half life - 1h
DRUG INTERACTION-
Diazepam: Inhibit Oxidation
Phenytoin: Inhibit Oxidation
Ketoconazole: Decrease absorption
Itraconazole: Decrease absorption
[adsense:468x15:2204050025]
6. EXCIPIENT PROFILE26
MICROCRYSTALINE CELLULOSE
Nonproprietary names- BP - Microcrystalline cellulose; USPNF- Microcrystalline cellulose
Synonym- Avocet PH, Cellulose gel, crystalline cellulose.
Functional category - Tablet disintigrant, Tablet and Capsule diluents.
Application- Used as binder, diluents in oral solid dosage form. It is used in Wet granulation and direct compression also.
Table No. 4: Use and conc. of MCC
|
Use |
Concentration ( % ) |
|
Adsorbent |
20-90 |
|
Antiadherant |
5-20 |
|
Capsule binder/diluents |
20-90 |
|
Tablet disintigrant |
5-15 |
|
Tablet binder/ diluents |
20-90 |
Stability & storage - microcrystalline cellulose is stable through hygroscopic material. The bulk material should be stored in a well closed container in a cool, dry place.
Incompatibilities - Microcrystalline cellulose is incompatible with strong Oxidizing agent.
POLYSORBATE 80
Chemical Name- Polyoxyethylene 20 sorbitan monooleate
Synonym- Tween 80, Polyoxyethylene 20 oleate
Functional category- Nonionic surfactant, solubiling agent, in various pharmaceutical Preparation
Table No.5: Use and conc. of polysorbate 80
|
Use |
Conc. ( % ) |
|
Emulsifying agent |
1-15 |
|
Solubilizing agent |
1-10 |
|
Wetting agent |
0.1-3 |
Application- Used as wetting agent, dispersing agent, solublizing agent in various pharmaceutical preparation.
Stability and storage- Stable with electrolytes& weak bases. Unstable with strong bases, polyoxyetylene surfactant. Store in well closed container ,protected from light , keep at dry &cool place
Incompatibilities- Discoloration, precipitation occurs with phenols, tannins, tars.
METHACRYLIC ACID COPOLYMER DISPERSION
Methacrylic acid copolymer of grades L, S, FS contains carboxylic acid groups and is thus anionic character. enteric coating with above material grades release the active ingredient between pH 5.5 and > pH7, allowing GI targeting from the small intestine to the colon .the release of active ingredient also depends on the thickness of the film coatings and the solubility characteristics of the active ingredient under physiological conditions. The different methacrylic acid copolymers are available as aqueous dispersions, powders or organic solutions used for enteric coating. It can be applied to conventional solid dosage forms such as tablets, capsule, and small particles
Application -Effective and stable enteric coating with rapid dissolution. Drug release in the colon, duodenum, jejum, ileum
TRIETHYL CITRATE
Nonproprietary names- BP-Triethyl citrate; USPNF- Triethyl citrate
Synonym- Citric acid, Ethyl ester, Citrofol
Functional category- Plasticizer
Application- It provide flexibility to coated film, generally used in capsule, Tablets, beads, and granules for immediate release sustained And enteric coating .sometimes used for taste masking.
Stability & storage- Triethyl citrate should be stored in a closed container in a cool & dry place. When stored in a accordance with these Conditions, Triethyl citrate are a stable product.
Incompatibilities- Triethyl citrate is Incompatible with strong alkali and Oxidizing agent.
HPMC
Nonproprietary name- BP- Hypomellose.; USP- Hypomellose; JP- Hydroxy propyl methyl cellulose
Synonym- Hypomellose, Methocel, Hydroxypropyl Methylcellulose
Functional Category- Coating agent, Film former Rate controlling polymer Stabilizing agent, binder, viscosity enhancer
Application- Use as tablet binder used in film coating & as and extended Release tablet matrix.
Table No.6: Use and conc. of HPMC
|
Use |
Conc.% |
|
Binder ( wet /dry granulation) |
2-5 |
|
Rate controlling polymer |
10-80 |
|
Film former |
2-20 |
|
Thickening agent (Eye drop) |
0.45-1 |
Stability & storage- HPMC powder is stable material, although it is Hygroscopic after drying. Powder should be stored in a Well closed container
Incompatibilities- HPMC is ISncompatible with some oxidizing agent.
CROSPOVIDONE
Nonproprietary names- BP - Crosspovidone; USNF-Crosspovidone
Synonym- Cross linked povidone, kollidon CL, Polyvinylpyrrolidine
Functional category - Tablet Disintegrant.
Application – Crosspovidone is a water Insoluble tablet disintegrant And dissolution agent.
Use - 2-5% conc. used in tablet prepared by direct Compression, wet, dry granulation method.
Stability & storage- Since crosspovidone is hygroscopic, it should be stored. In airtight container and cool and dry place.
Incompatibilities- Povidone is compatible with most organic and inorganic Pharmaceutical ingredients.when exposed to high water Level, crosspovidone may form molecular adduct with Some materials
7. MATERIAL AND INSTRUMENT
Table No.7 The following list includes the raw materials and chemicals used in this works
|
No. |
Material |
Source |
|
1 |
Microcrystalline cellulose |
FMC biopolymer |
|
2 |
Polysorbate 80 / SLS |
Palmo Industries |
|
3 |
Crosspovidone / Crosscarmalose Sodium |
Anshul agencies |
|
4 |
HPMC / PVPK30 |
Dow chemicals |
|
5 |
Sodium Carbonate |
S D fine chemical |
|
6 |
Magnesium Stearate |
Kant Healthcare |
|
7 |
Talc |
Vijay minerals |
|
8 |
Triethyl Citrate |
Lancaster |
|
9 |
Magnesium Oxide |
Macco organics |
|
10
|
Methacrylic acid copolymer dispersion. |
Degussa |
|
11
|
Titanium Dioxide |
Kivnos |
|
12
|
Brilliant blue lake |
Colocorn Asia ltd |
|
13 |
Sodium Lauryl Sulphate
|
Stephan co. ltd |
|
14
|
Purified Water |
LIST OF EQUIPMENT
Table No. 8 The following table includes the list of equipment used in this work
|
No. |
Equipment |
Manufacturer |
|
1 |
Electronic balance |
Shimadzu-AUW20D |
|
2 |
Mesh |
12,16,18,30,60,8 |
|
3 |
Tap density tester |
Electolab ETD-1020 |
|
4 |
Conventional coating pan /spray gun
|
Rinak , kalweka HD410AC |
|
5
|
Fluidized bed coater |
Umang ,Thane |
|
6 |
Hardness Tester |
Dr.schleuniger, pharmatron,USA |
|
7 |
Dissolution test apparatus |
Electrolab USP XXII |
|
8 |
Homogenizer |
Remi motors ,Mumbai |
|
9 |
Fluidized bed dryer |
S.P.Panchal& company |
|
10 |
Extruder with 1mm screen |
R.R.Enterprises,vasai |
|
11 |
Speronizer |
R.R.Enterprises,vasai |
|
13 |
Overhead stirrer |
R.R.Enterprises,vasai |
|
14 |
Moisture balance / LOD Apparatus
|
Sartorious |
|
15 |
Vernier caliper |
Mituloya absolute |
|
16 |
Capsule hand filling machine |
Rinak , kalweka |
8 EXPERIMENTAL DETAILS
8.1 PREFORMULATION
PHYSICAL CHARACTERISTIC
1) Determination of bulk density and tap density –
An accurately weighted quantity of the powder (W) was carefully poured into the granulated cylinder and volume (Vo) was measured. then the graduated cylinder was closed with lid .set into the density determination apparatus (bulk density apparatus)the density apparatus was set for 500 taps,750 taps , and 1250 taps .after that the volume(Vf) was measured and continued the operation till the two consecutive reading were equal. The bulk density and the tapped density were calculated using the formulas.
Bulk Density – W/Vo
Tapped Density- W/Vf
Where W- Weight of the powder.
Vo- Initial volume.s
Vf- Final volume.
Bulk density of pantoprazole powder was found to be0.39g/ml
Tap density of pantoprazole powder was found to be 0.62g/ml
2) Hausner ratio– It indicates the flow properties of the powder and measured by the ratio of
Tapped density to bulk density
Hauser ratio- Tapped density /Bulk density
Table No.10: Range of hausner ratio and its properties
|
No. |
Hausner ratio |
Properties |
|
1 |
0-1.2 |
Free flowing |
|
2 |
1.2-1.6 |
Cohesive powder |
Pantoprazole is cohesive powder .And hausner ratio was found to be 0.63g/ml
3) Sieve Analysis-
The main aim of sieve analysis was to determine the different size of drug Particles present. series of standard sieve were stacked one above the other so that sieves with larger pore size (less sieve number)occupy top position followed by sieve of decreasing pore size (large sieve number) towards the bottom.
PROCEDURE - A series of sieves were arranged in the order of their deceasing pore diameter (increasing sieve number) i.e. sieve no. ASTM 40, 60, 80 ,100 With 40grams of drug were weighed accurately and transferred to sieve 40 which were kept on top. The sieves were shaken for about 5-10 minutes .Then the drug retained on each sieves were taken, Weighted separately and expressed in terms of percentage.
69.4% pantoprazole powder pass through sieve 100 (NLT 65% Should pass through 100 mesh)
8.2 FORMULATION
Table No.11 Composition of the core pellets in the formulation trials (batch for 4000capsule)
|
Ingredient used |
F1 |
F2 |
F3 |
F4 |
F5 |
|
Pantoprazole sodiumSesquihydrate |
194.24 |
194.24 |
194.24 |
194.24 |
194.24 |
|
MCC (Avicel pH101) |
208.96 |
323.76 |
183.76 |
156.76 |
156.76 |
|
Lactose monohydrate |
---- |
--- |
--- |
133.2 |
133.2 |
|
Polysorbate 80 |
24 |
---- |
24 |
---- |
--- |
|
SLS |
---- |
10 |
--- |
3.72 |
3.72 |
|
Crosspovidone |
40 |
30 |
40 |
--- |
--- |
|
CCS |
---- |
--- |
--- |
28.08 |
28.08 |
|
HPMC |
4.8 |
6 |
6 |
--- |
--- |
|
Sodium carbonate |
28 |
28 |
28 |
32 |
32 |
|
Magnesium oxide |
---- |
---- |
190 |
--- |
---- |
|
Purified water |
300 ml |
340 ml |
400 ml |
270 ml |
268 ml |
|
Total |
508 |
600 |
674 |
558 |
558 |
· Magnesium stearate and talc used to prevent agglomeration during spheronization process .
in first 3 batches 4 g. and remaining two batches 5 g of magnesium stearate and talc
used .
· All quantity used in g only.
· The no. of pellets in each capsule contains 45.1mg of pantoprazole sodium sesquihydrate.
Table No.12 Composition of ingredient for seal coating (Trial for 3030 capsule batch)
|
Ingredient used |
F1 |
F2 |
F3 |
F4 |
F5 |
|
Pantoprazole core pellets |
384 |
454 |
393 |
412 |
430 |
|
HPMC |
12.12 |
12.12 |
12.12 |
16.96 |
16.96 |
|
PVPK30 |
--- |
--- |
--- |
0.60 |
0.60 |
|
Triethyl citrate |
1.515 |
1.515 |
1.515 |
---- |
---- |
|
Propylene glycol |
---- |
--- |
--- |
3.96 |
3.96 |
|
Magnesium oxide |
6.66 |
6.66 |
6.66 |
--- |
--- |
|
Titanium Dioxide |
--- |
--- |
|
8.01 |
8.01 |
|
Color |
0.80 |
0.80 |
0.80 |
1.09 |
1.09 |
|
Isopropyl alcohol |
---- |
---- |
--- |
153.55 ml |
153.55 ml |
|
Purified water |
219.5 ml |
219.5 ml |
219.5 ml |
153.55 ml |
153.55 ml |
|
Total |
405 |
475 |
414 |
442.6 |
460.6 |
· 10% seal coating suspension was prepared. And 5 % weight was built up.
· All quantity used in g only.
Table No.13Composition of ingredient for enteric coating (Trial for 3000 capsule batch)
|
Ingredient used |
F1 |
F2 |
F3 |
F4 |
F5 |
|
Pantoprazole sodium seal Coated pellets |
405 |
475 |
414 |
442.6 |
460.6
|
|
Methacrylic acid copolymer dispersion |
54.54 (16.36) |
54.54 (16.36) |
54.54 (16.36) |
--- |
--- |
|
Acryl ezee ready made enteric coating bend |
--- |
--- |
--- |
43.5 |
43.5 |
|
Triethyl citrate |
1.515 |
1.515 |
2.5 |
--- |
--- |
|
Titanium Dioxide |
2.424 |
2.424 |
2.424 |
--- |
--- |
|
Purified talc |
4.242 |
4.242 |
4.242 |
--- |
--- |
|
Color |
0.9 |
0.9 |
0.9 |
0.9 |
0.9 |
|
Purified water |
127.2 ml |
127.2 ml |
132.17 ml |
217.5 ml |
217.5ml |
|
Total |
430.4 |
500.4 |
440.4 |
487 |
505 |
· 20% enteric coating dispersion / suspension was prepared. And 18, 20, 30, 30 and 32 % weight was built up for respective batches.
· Methacrylic acid copolymer dispersion is in liquid form but value in bracket indicates solid content.
· All quantity used in g only.
8.3 METHOD OF PREPARATION.
METHOD OF PREPARATION OF CORE PELLETS Core pellets was prepared by extrusion and spheronization method.The required quantity of pantoprazole sodium was weighted and sifted through sieve 30,
Microcrystalline cellulose, disintigrant (crosspovidone or crosscarmelose sodium), sodium carbonate, sodium lauryl sulphate, hydroxyl propyl methyl cellulose were weighted accurately and sifted through sieve, and blend was prepared. Antitacking agents talc and magnesium steareate were passed through sieve 60
Hydroxyl propyl methyl cellulose was dissolved in purified water. And It is used as granulating fluid for granulation. All ingredients mixed properly and HPMC solution was added the prepared wet mass was passed through extruder; cylindrical shaped extrudates were formed and then keep in to spheronizer for rounding the extrudates. Extrudate rotate in spheronizer at different RPM 75, 100, and 125 for 10 min. checked the LOD of wet pellets. Fluid bed dryer was used for drying the pellets at a temperature below 40 0C
PARAMATERS-
· LOD of wet mass- 30 %
· LOD of wet pellets- 27 %
· LOD of dried pellets- 1.5 %
SEAL COATING ON CORE PELLETS
The required quantity of Hydroxyl propyl methyl cellulose, PVPK30, and plasticizer were weighed and dispersed in purified water with the help of overhead stirrer for over a period of 10 min.
Pigment suspension was prepared by dissolving magnesium oxide \ titanium dioxide, color, in a sufficient volume of purified water by using homogenizer. The pigment suspension was added in HPMC solution then specified volume of Isopropyl alcohol was added and continuous stirring for another 5 minutes.
Prepared coating suspension was sprayed on core pellets in fluid bed processor. The coating was carried out with the help of bottom spray. The coating was continuous until 5% build up was achieved.
FLUDIZED BED PROCESSOR WAS OPERATED WITH FOLLOWING CONDITIONS
· Inlet temperature - 40-420 C
· Bed temperature – 35-400 C
· Exhaust (blower) - 3000 RPM
· Spray rate- 7 RPM
· Air pressure- 15 kg\cm2
ENTARIC COATING ON SEAL COATED PELLETS The required quantity of methacrylic acid polymer dispersion and triethyl citrate were weighed and dispersed in specified volume of purified water.
In another S.S vessel titanium dioxide, purified talc,color were added in purified water undergo homogenization (pigment suspension) The methacrylic acid polymer solution added in pigment suspension and continuous stirring for another 5 min.
Enteric coating suspension was sprayed on seal coated pellets by using fluid bed processor. The coating was carried out until 20% build up was achieved. Bottom spray was used for enteric coating.
FLUDIZED BED PROCESSOR WAS OPERATED WITH FOLLOWING CONDITIONS
· Inlet temperature - 35-420 C
· Bed temperature – 30-400 C
· Exhaust (blower)- 3OOO RPM
· Spray rate- 1RPM
· Air pressure- 15 kg / cm2
9. EVALUTION OF ENTERIC COATED PELLETS
1) DISSOLUTIO PROFILE-
Buffer preparation-
· 6.8 pH phosphate buffer- Dissolve 6.8 g of potassium Dihydrogen phosphate in Purified water. Make up the volume upto1000 ml by purified water and adjust the PH by using sodium hydroxide solution
· 0.1N HCl- 8.5 ml HCl added in purified water and make up volume up to 1000ml by purified water.
Standard preparation- 45.1 mg of pantoprazole sodium sesquihydrate dissolved in (6.8 pH phosphate buffer) dissolution media, from above solution 5ml taken and volume makeup up to 100 ml by 6.8 pH phosphate buffer, Pellets dissolution test was conducted in a USP II dissolution Test apparatus into two different dissolution media. Initially in 0.1 N HCl for 2 hr. after that in 6.8 pH Phosphate buffer for 45 min.
Dissolution at acid stage medium
· Media- 0.1 N HCl
· SApparatus- USP II apparatus (paddle)
· RPM- 100 RPM
· Amount of media- 1000 ml
· Temperature- 37 0C (with + or – O.50C)
· Time- Up to 2hr
Acid stage working standard solution- Dilute an appropriate volume of the standard stock solution to 1 L with acid stage medium in such a way to obtain a final conc. Of about 10% of the label claim per L
Test solution - pass a portion of solution under test through a suitable 10 µm filter
Procedure- Determine the amount of pantoprazole dissolved by employing UV absorption at the wavelength of maximum absorbance 305 nm of portions of the test solutions in comparison to acid stage working standard solution using a 4 cm path length cell and acid stage medium as blank .drain the acid stage medium from each vessel and replace with buffer stage medium . Calculate the amount of pantoprazole dissolved by the formula
= Au X Cs X 1 X 100 / As X L
In which Au and C are the absorbance obtained from the test solution and acid stage working standard solution respectively .Cs is the conc. In mg per L of pantoprazole in the acid stage medium, 100 is conversion factor to percentage and l is label claim in mg.
|
Time in hr |
Amount dissolved |
|
2 hr. |
Not more than 10% |
Dissolution at buffer stage medium
· Media- pH 6.8 phosphate buffer
· Apparatus USP II apparatus (paddle)
· RPM- 100 RPM
· Amount of media- 1000ml
· Temperature- 37 0 C (with + or – O.50C)
· Time- Up to 45 min.
Buffer stage working standard solution- Dilute an appropriate volume of the standard stock solution to 250ml with buffer buffer stage medium in such a way to obtain a final conc. Of about 100% of the label claim per L
Test solution - pass a portion of solution under test through a suitable 10 µm filter.
Procedure- Determine the amount of pantoprazole dissolved by employing UV absorption at the wavelength of maximum absorbance 288 nm on portions of the test solutions in comparison to buffer stage working standard solution using a 0.5 cm path length cell and buffer stage medium as blank. Calculate the amount of pantoprazole dissolved by the formula
= Au X Cs X 1 X 100 / As X L
In which Au and C are the absorbance obtained from the test solution and acid stage working standard solution respectively .Cs is the conc. In mg per L of pantoprazole in the buffer stage medium, 100 is conversion factor to percentage and l is label claim in mg.
|
Time in min. |
Amount dissolved |
|
45 min. |
Not less than 75% |
2 ASSAY BY HPLC
CHROMATOGRAPHIC CONDITION
Stationary phase- (4.6mm X 25CM) 5H C12, (Hypersil BDS)
Mobile phase- buffer: acetonilrile (40:60), SpH 7.4 with KOH solution.
Flow rate- 1ml/min
Control temp. - Ambient
Detector- 280 nm
Injection volume- 20 micro liters
Preparation of buffer- Dissolve 2.72 g of KH2PO4 & 0.525 g of K2HPO4 and volume makeup up to 10000 ml by purified water
Preparation of standard-
40.4 mg of pantoprazole sodium sesquihydrate dissolved in 20 ml of methanol from this 2ml of solution dilute. And volume makes up upto 50 ml by using mobile phase
Preparation of sample – weight equivalent to 40 mg pantoprazole sodium into 20 ml volumetric flask then add 15 ml methanol and sonicate it. Passed through 0.45 micrometer filter take 2 ml of filtrate add volume make up upto 50mL
3) DESCIPTION – For checking the appearance of pellets 20 gm of pellets taken From respective batch and observed for the color, shape of pellets
4) DIMENSION – The size of pellets were determined by using mitutoyo absolute Verniar caliper from the respective sample.
5) HARDNESS – Hardness tester was used to determine the hardness of pellets. The test pellet was held between the edge of the fixed and movable part of the instrument and holds the pellets between the edges and the nozzle in edgewise position .the scale was adjusted by sliding so that the zero on the scale coincides with the pointer. The adjustable knob slowly moved till the pellet breaks. The pressure indicated on the dial was in N. Also hardness test was performed by Dr. Schieuniger hardness tester. Keep the pellets in between the Two edges. After breaking of pellets reading display.
10. RESULTS AND DISCUSSION
1) STANDARD CURVE FOR PANTOPRAZOLE SODIUM SESQUIHYDRATE
Table No.13 Conc. and absorbance for standard curve
|
Concentration |
Absorbance |
|
0 |
0 |
|
10 |
0.131 |
|
20 |
0.272 |
|
30 |
0.414 |
|
40 |
0.552 |
|
50 |
0.692 |
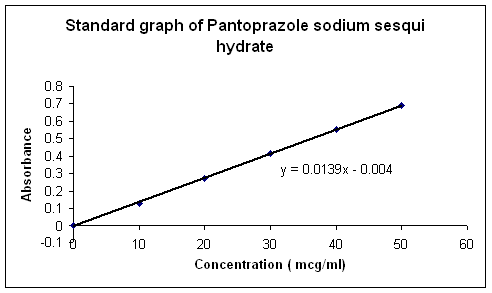
Graph1 .Std curve for pantoprazole sodium sesquihydrate enteric coated pellets.
2) INVITRO DISSOLUTION TEST -
Table No. 14 The percentage of drug release of the no. of pellets in each capsule in Different Trials
|
Dissolution Media |
Sampling time
|
Percentage of drug release in different trials
|
USP Specification
|
||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
|||
|
0.1 N HCl |
2 hr |
--------------- |
2.5 % |
1 % |
NMT 10% drug release |
||
|
6.8 pH phosphate buffer
|
10 min |
F1,F2,F3 trial batches |
29.1 %
|
30.7%
|
|
||
|
20 min |
were partially or fully
|
58.5 %
|
61.5% |
||||
|
30 min |
Dispersed in 0.1 N HCl |
64%
|
66.2%
|
||||
|
45 min |
------------- |
77.9% |
78.2 % |
NLT 75% drug release |
|||
Table No. 15 Comparative study of percentage drug release of the Innovator product with F4 and F5 formulation''
|
Time (min ) |
F4 (%) |
F5 (%) |
Innovator Tablet sample (%) |
|
120 |
2.5 |
1 |
0 |
|
130 |
29.1 |
30.7 |
34.75 |
|
140
|
58.5 |
61.5 |
68.39 |
|
150 |
64 |
66.2 |
74.03 |
|
160 |
77.9 |
78.2 |
83.73 |
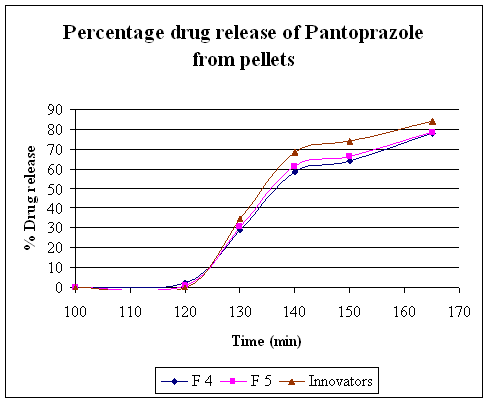
Graph 2. Invitro Release Of Pantoprazole Sodium Sesquihydrate Enteric Coat Pellets
3) DESCRIPTION– Blue coloured, spherical shape having enteric coated pellets.
4 )DIMENSIONS– Size of the pellets in the range of 1.28 -1.40 mm determine by Using vernier caliper
5) HARDNESS– Hardness of pellets in the range of 9 -17 N determined by Dr.Schieuniger hardness tester.
Table No.16 Hardness of pellets of different formulation
|
Formulation |
F1 |
F2 |
F3 |
F4 |
F5 |
|
Hardness (N) |
9 N |
10 N |
14 N |
17 N |
17 N |
11. CONCLUSION
Pantoprazole sodium is proton pump inhibitor and used as an antiulcer agent. The study was undertaken with an aim to formulate pantoprazole sodium enteric coated pellets.
Before going to develop the formulation a detail product literature review was carried out to know about the MUPS and type of dosage form available in market. The present study was focused to formulate delayed release capsule by MUPS Technique.
In preliminary trials various formulation combinations and parameters such as spheronization speed, time of spheronization, amount of granulating fluid, quantity of MCC, were responsible to produce spherical and smooth surface pellets. Shape, surface area, uniform sized pellets undergo efficient coating. Microcrystalline cellulose has been found to be effective diluent in the extrusion and spheronization method. With increase in quantity of MCC the surface of pellets more plastic and easily adopt spheroid shape and also needed more amount of granulating fluid. 28% w/w content MCC was found to be optimum to produce the pellets of required characteristics.
Similarly pellets were prepared with varying amount of granulating liquid. Higer amount of granulating liqid result in harder pellets that could not obey the dissolution criteria specified in USP. 300 ml of granulating fluid was found to sufficient for pellets preparation
Average pellets size was determined by sieve analysis and found to be 1680-1200 microns (ASTM sieve no. 12-16). Sieve analysis was the essential step before coating. Because uniform sized pellets undergo effective coating.
Drug turns brown when come in contact with moisture therefore pellets were dried and undergo seal coating as early as possible. To avoid barrier diffusion and moisture penetration seal coating was applied 5 % by using HPMC. FBD was used to dry the pellets.
To avoid degradation of drug in upper part of GIT, above developed pellets are further coated with enteric coating polymer. Coating was performed in Fluid bed coater. Methacrylic acid copolymer dispersion was coated 18, 20, and 30 % for first three trials. But still pellets were fully and partially dispersed in 0.1N HCl. Then acryl ezee blend was used as coating suspension (20 % coating suspension) weight gain 30, 32 % for remaining two trials. 32 % Increasing coat thickness by using acryl ezee suspension was satisfied the USP dissolution criteria. Above developed formulation were evaluated for invitro dissolution .Dissolution was carried out in USP 32 apparatus II (paddle) using 1000 ml of 0.1 N HCl for 2 hrs, coated pellets slowly release (not more than 10%) and after that in 1000 ml of pH 6.8 phosphate buffer rapid dissolution of pellets were observed. drug show 78.2 % release after 45 min. and hence F5 trial was satisfy the dissolution criteria.
The result indicates a effective enteric coating and delay the drug release, with 32% acryl ezee solution, is possible. The formulation developed can further be worked on. For identifying a best formulation for delayed release pellets of pantoprazole sodium.
12. BIBLIOGRAPHY
1. Bramankar D M and Jaiswal S B , ‘Biopharmaceutics and Pharmacokinetics A Treatise’, 1ed,Vallabh prakashan, Delhi , p.335,337 (1995)
2. Vyas S P and Khar R K , ‘Controlled drug delivery system : Concepts andadvances’,1ed, Vallabh Prakashan, New Delhi , p.167(2002)
3. Bramankar D M and Jaiswal S B , ‘Biopharmaceutics and Pharmacokinetics A Treatise’, 1ed,Vallabh prakashan, Delhi , p.335,337 (1995)
4. Lachman L and Lieberman H A , Kaing J L, ‘The Theory and Practice of Industrial Pharmacy’,3rded, Bombay ,Varghese publishing house p. (1987)
5. Gennrao R A , ‘Controlled release drug delivery system ,The science and Practice of pharmacy, remingtan 20th ed, vol 1, and p.903-930
6. Vyas S. P. And Khar R K , ‘Controlled drug delivery: Concepts and Advances’,1 ed ,Vallabh Prakashan, New Delhi , p.15 (2002)
7. Chien Y.W, ‘Rate controlled drug delivery system’: controlled release Vs Sustained Release, med.prog.tech, 1989 (15) P.21-46
8. Gibson ,pharmaceutical preformulation & formulation , p. 450,429-431
9. Degussa creating essential , Guideline for formulation development & process Technology for Enteric coatings, P. 29
10. D. Wurster Method for Applying Coating to Tablets, US Patent 2,648,609, (1953)
11. Bergstrand , ‘ Multiple unit Pharmaceuical preparation containing Proton pump Inhibitor’, Patent WO9601625,(2004)
12. Rahman A, ‘Development and in vitro Evaluations of enteric coated multiparticulate system for resistant tuberculosis’, Indian journal of pharmaceutical sciences, P. 477,481(July-august 2008)
13. Tanberk , ‘New galenic process for the omeprazole pellets’, EP0519144,(Feb.1977)
14. Claudio N, ‘ Influence of formulation and process parameter on pellets production by powder layering technique using aqueous solution’,AAPSPharmScitech1(2)Article1, (Januvary 2000)
15. Heinamalk J , ‘Diffusion of freely water soluble drug in aqueous enteric coated pellets’, AAPSPharmScitech 3(2)Article16,(May 2002)
16. Comoglu T, ‘Development and in vitro Evaluation of pantoprazole loaded Microsphrers’, www.freshpatentsonline.com/y2004/0028737,P. 295,302 (2004)
17. Rekkas , ‘Optimization of the Pelletization Process in a Fluid-Bed Rotor Granulator Using Experimental Design, (July 2008)
18. Kristensen J, ‘Direct pelletization in a Rotary Processor Controlled by Torque Measurements. III. Investigation of Microcrystalline Cellulose and Lactose Grade’, AAPSPharmScitech6(3),P495-503, (Octomber 2005)
19. Wipo patent, ‘Double Pellet formulation of Proton Pump Inhibitors & clarithromyin for the treatment of gastrointestinal ulcer’.
20. wipo patent, ‘New stabilized galenic formulation comprising lansoprazole and their preparation.’
21. Degussa creating essential , Guideline for formulation development & process Technology for Enteric coatings, P. 43,44
22. Lian D H, ‘Preparation and invitro / invivo evalution of sustained release metformin hydrochloride pellets,Europian Journal of pharmaceuticas and biopharmaceutics (64) P.185,182 (2OO6)
23. wikipedia.org
24. www.druginformation.com
25. Medline Plus, The National Library Of Medicines Consumer Health Website, www.nim.nih.gov
26. Rowe R C, Handbook of pharmaceutical excipient 4th edition p.-184, 287 ,354 , 421, 665
27. United State Pharmacopoeia, official monograph Pantoprazole Sodium Delayed Release Tablet, P. 3202,3203
Reference ID: PHARMATUTOR-ART-1009









