About Author: Mr. Patil Kuldip, Tekade B. W., Thakare V. M., Dr. Patil V. R.
T. V. E. S’s College of Pharmacy,
Faizpur (M.S.), India
Abstract
The purpose of this research was to prepare a floating drug delivery system of atenolol. In the present study, preparation of atenololfloating microspheres, evaluation of Floating Drug Delivery System (FDDS) in vitro, prediction of the release, and optimization of stirring speed and polymers ratio to match target release profile was investigated. Floating microspheres were prepared by solvent evaporation (Oil-in-water emulsion) technique using hydroxylpropyl methylcellulose (HPMC), Ethyl cellulose (EC) and Eudrajit S100 as the rate controlling polymers. Particle size analysis, drug entrapment efficiency, surface topography, and release studies were performed. Results showed that the polymer ratio and stirring speed affected the size, incorporation efficiency and drug release of microspheres (> 12 h), and the best results were obtained at the ratio of EC (1:3). The mean particle size of prepared floating microspheres increased but the drug release rate from the microspheres decreased as the polymer concentration increased. The developed floating microspheres of atenolol may be used in clinic for prolonged drug release in stomach for at least 12 hrs, thereby improving the bioavailability and patient compliance.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1128
Introduction
The objective of the present investigation is to prepare floating microsphere of atenololto improve the bioavailability by increasing residence time in stomach. Cardiac dysrhythmia (also known as arrhythmia) is a term for any of a large and heterogeneous group of conditions in which there is abnormal electrical activity in the heart.1 the heart beat may be too fast or too slow, and may be regular or irregular. Some arrhythmias are life-threatening medical emergencies that can result in arrest and sudden death2,Atenolol is a non-selective beta-blocker mainly used in the treatment of hypertension and arrhythmia. The plasma half life of atenolol is short hence requiring taking thrice a day and it is decompose at alkaline pH3. The recommended adult oral dosage of atenolol is 10 mg 2 to 3 times in day4. Dosage forms that can be retained in stomach are called gastro retentive drug delivery systems (GRDDS). Gastro retentive systems can remain in the gastric region for several hours and hence significantly prolong the gastric residence time of drugs5. Prolonged gastric retention improves bioavailability, reduces drug waste and improves solubility for drugs that are less soluble in a high pH environment. It has applications also for local drug delivery to the stomach and proximal small intestines5. Gastric retention will provide advantages such as the delivery of drugs with narrow absorption windows in the small intestinal region6. Also, longer residence time in the stomach could be advantageous for local action in the upper part of the small intestine, for example treatment of hypertension and arrhythmia.
MATERIALS AND METHODS
Atenolol was obtained from market, EthylCellulose & hydroxylpropyl methylcellulose fromColorcon Pvt Ltd, Mumbai.Eudrajit S100 from Evonik Pvt Ltd, Mumbai.
Preparation of Microspheres: 7
Floating microspheres containing Atenolol were prepared using emulsion solvent evaporation technique. For the preparation of floating microspheres, the rate controlling polymers in varying concentration (Drug: polymer, 1:1, 1:2 and 1:3). , The drug and polymer mixture (1:1, 1:2 and 1:3) was dissolved in a Ethanol and dichloromethane (15ml) containing 0.01% of Tween 80, The resultant solution was stirred with a stirrer for 1 hour at 700 rpm. Various batches of floating microsphere was shown in table no. The parameter use in the formulation are given below .the formed floating microspheres were filtered and washed with water and dried at room temperature.
Evaluation of floating microspheres of Atenolol
Particle size analysis:8
The sizes of floating microspheres were measured by laser diffraction particle size analyzer. Firstly, 1gm offloating microspheres was floated in 200 ml of containing 0.02 % of Tween 20 in aqueous solution and stirred at 37 ± 0.5 °C. Second, particle size distribution was obtained when a laser light passed through the microspheres and then diffracted the intensity in an angular distribution. The data obtained were evaluated using volume distribution diameter (d) values of 10%, 50% and 90%.
[adsense:468x15:2204050025]
Angle of repose:9
The angle of repose of floating microspheres was determined by fixed funnel method. The floating microspheres were allowed to fall freely through a funnel until apex of conical pile just touched the tip of the funnel. The angle of repose θ was determined according to the following formula
θ= tan-1 h/r
Where, h = height of pile, r = radius of the pile formed by the floating microspheres.
Bulk density and tapped density:9
The bulk density and tapped density of floating microspheres were determined by the tapping method.
Percentage compressibility index /Carr’s index:9
The percentage compressibility index was calculated according to following formula
Tapped density – Bulk density
% Compressibility Index = --------------------------------------- X 100
Tapped density
Hausner’s ratio:9
It is calculated by the following formula
Tapped density
Hausner’s Ratio = ---------------------
Bulk density
Percentage yield:10
The percentage yield of different formulations was determined by weighing the floating microspheres after drying. The percentage yield was calculated as follows.
Total weight of floating microspheres
% Yield = ---------------------------------------------------- X 100
Total weight of drug and polymer
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Determination of entrapment efficiency:11
The drug content of atenolol loaded microspheres was determined by dispersing 50 mg Microspheres in 10 ml of ethanol, which was stirred for 8 h to extract the drug. The samples were diluted and analyzed spectrophotometrically at 275 nm and the percentage drug entrapment was calculated.
SEM study:12
The surface topography of the microspheres was observed by scanning electron microscopy.
In-vitro release studies:11
In-vitrorelease of Atenolol from floating microspheres was carried out using the USP dissolution test apparatus (Type-I). A weighed amount of floating microspheres equivalent to 25 mg of drug were filled into a capsule and placed in the basket. Dissolution media used was 900 ml of 0.1 N HCI (pH 1.2) maintained at 37 ± 0.5°C and stirred at 50 rpm. At predetermined time intervals, 10 ml of sample was withdrawn and replaced with equal amount of 0.1 N HCI (pH 1.2). The collected samples were filtered and suitably diluted with 0.1 N HCI and analyzed spectrophotometrically at 275 nm to determine the amount of drug released in the dissolution medium. Then drug content in dissolution sample was determined by software (PCP disso v2.08) version.
Stability study13
The floating microspheres (A3) were placed in borosilicate screw capped glass containers and stored at temperature (40 ± 2°C) with relative humidity (75±5 RH) for a period of 90 days. The samples were assayed for drug content at regular intervals of 30 days.
Differential scanning calorimetry (DSC): 14
The DSC thermo gram of microsphere was recorded using Differential scanning calorimeter (Extar DSC 6220, Japan).
Kinetic modeling:15
The result of in-vitro drug release study of floating microspheres were fitted with various kinetic equation
RESULT AND DISCUSSION:
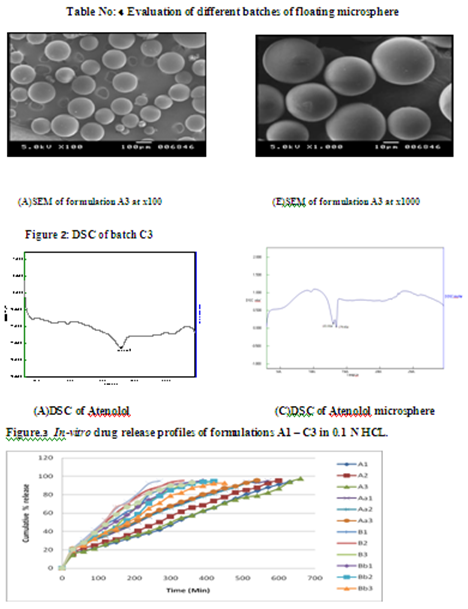
Floating microspheres containing Atenolol were prepared using emulsion solvent evaporation techniqueusing EC, HPMC, and Eudrajit S100 polymer in ratio 1:1, 1:2, 1:3. The parameters which were evaluated for microspheres are given in the Table below .The mean particle size of floating microspheres formulation which shows high percentage of entrapment was in the range of 172-275 mm this show that more particle size more entrapment of drug as shown in Table, The bulk density value of different microspheres ranged from 0.416 - 0.569 gm/cm3 as shown in Table. Angle repose, percentage compressibility index, Hausner’s of floating microspheres was observed in range of 22°.02’- 25°.84’, 11.37% - 19.89%,1.128-1.248 respectively as shown in Table which shows good flow properties The percentage yields of different formulation were in range of 33.60% - 41.02% in which A3 batch shows highest % yield as shown in Table The drug entrapment efficiency of different batches of floating microspheres was found in the range of 46.11 % - 89.72 % w/w in which A3 batch shows highest entrapment efficiency as shown in tableMicrospheres were subjected to in vitro release using USP dissolution apparatus Type I in 900 ml of simulated gastric pH medium (0.1M HCL ).But the release seems to be somewhat sustained with increased in the amount of polymer. Drug release profiles of different batches of formulations are shown in the Figure . The release rate was found to be decreased in accordance with the increase in ratio of polymer used. The best release was found to be with lower drug polymer ratio (1:3) of batch A3.DSC result suggested that drug and polymer were compatible with each other.SEM show microsphere are spherical with no visible major surface irregularity. Few wrinkles and inward dents were appeared may due to collapse of floating microspheres during the in- situ drying process. Mechanism of releasedata obtained for in-vitro release were fitted into equations for the zero-order, first-orderKorsmeyer Peppas, and Hixson crowel and matrix release models. The interpretation of data was based on the value of the resulting regression coefficients. The in-vitro drug release showed the highest regression coefficient values for matrix model
CONCLUSION
In-vitro data obtained for floating microspheres of atenolol showed good incorporation efficiency, and prolonged drug release. Microspheres of different size and drug content could be obtained by varying the formulation variables. From the results it can be concluded that the drug release from the floating microspheres matrix was controlled by the polymer proportion. Prepared formulation showed best drug release rate for longer period in stomach.
REFERENCES:
1. Rang HP, Dale MM, Pharmacology, 5th Edn, Elsevier Science publisher,2005, 312
2. en.wikipedia.org/wiki/Cardiac_dysrhythmia , (1-9-2010, 3.15 PM).
3. Indian Pharmacopoeia, 2007, Government of India, ministry of health and family welfare, Ghaziabad, The India Pharmacopoeia commission publisher , New Delhi, 749.
4. drugs.com/atenolol.html,(1-9-2010,12.30 PM).
5. Garg R, Gupta GD, Progress in controlled gastro retentive drug delivery system, Trop J Pharm Res,7,2008,1055-1066.
6. Shah SH, Patel JK, Patel NV, Stomach specific floating drug delivery system:A Overview., Int J Pharm Tech, 1, 2009, 623-633.
7. SemaltyM,YadavS, Semalty A, Preparation and characterization of gastroretentive floating microspheres of ofloxacin hydrochloride Int J Pharm Sci Nano ,3, 2010,819-823 .
8. PatilHS, Patil MP, Tekade BW, Thakare, VM, Patil VR, Formulation and in-vitro evaluation of floating microspheres of acyclovir ., Arch Pharm Sci Res ,1, 2009, 194 -198 .
9. Banker SG, Anderson RN, Tablets. In, Lachman L, Liberman AH, Kanig LJ. Text book of the theory and practice of industrial pharmacy, 3rd Edn. Mumbai, Varghese Publication House, 1991,317-324.
10. Ramachandran S, Shaheedha SM, Thirumurugan G, Dhanaraju MD, Floating controlled drug delivery system of famotidine loaded hollow microspheres (microballoons) in the stomach.,Current Drug Del , 7, 2010 ,93-97.
11. Indian Pharmacopoeia, Government of India, ministry of health and family welfare, Ghaziabad, The India Pharmacopoeia commission publisher , New Delhi, 2007,749.
12. MallikarjunaKR, Gnanaprakash K, ChandraSekhar KB, Madhusudhana CC, Formulation and in-vitro characterization of floating microspheres of amoxycillin trihydrate agaist H.pylori ., J Pharm Res, 4, 2011,836-840
13. Qin, W., Zhang, G. G. Z., Stability and excipient compatibility study, Novartis, 2006, 30-35.
14. Aulton ME, The design of dosage form. The sciences of dosage form design, 2nd Edn. UK Harcourt, 2002, 1-5.
15. Barhate SD, Rupnar YS, Sonvane RM, Pawar KR, Rahane RD, Formulation and evaluation of floating microspheres of ketorolac trometamol., Int J Pharm Res Dev ,1, 2009, 1-8.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TABLES AND FIGURES:
Table No.1 Parameter used in Batches of floating microspheres of Atenolol
|
Batch |
Temperature |
Speed (rpm) |
|
A |
27oc |
700 |
|
Aa |
27oc |
700 |
|
B |
27oc |
700 |
|
Bb |
27oc |
700 |
|
C |
27oc |
700 |
Table No.2Solvent used in Batches of floating microspheres of Atenolol
|
Batch |
Solvent used |
|
A |
Ethanol and Dichoromethane |
|
Aa |
Methanol |
|
B |
Ethanol and Dichoromethane |
|
Bb |
Methanol |
|
C |
Ethanol and Dichoromethane |
Table 3: Composition of batches of floating microspheres of atenolol
|
Sr no. |
Formulation code |
Atenolol(mg) |
Ethyl cellulose(mg) |
HPMC(mg) |
Eudrajit L100(mg) |
|
1 |
A1 |
500 |
500 |
|
|
|
2 |
A2 |
500 |
1000 |
|
|
|
3 |
A3 |
500 |
1500 |
|
|
|
4 |
Aa1 |
500 |
500 |
|
|
|
5 |
Aa2 |
500 |
1000 |
|
|
|
6 |
Aa3 |
500 |
1500 |
|
|
|
7 |
B1 |
500 |
250 |
250 |
|
|
8 |
B2 |
500 |
500 |
500 |
|
|
9 |
B3 |
500 |
750 |
750 |
|
|
10 |
Bb1 |
500 |
250 |
250 |
|
|
11 |
Bb2 |
500 |
500 |
500 |
|
|
12 |
Bb3 |
500 |
750 |
750 |
|
|
13 |
C1 |
500 |
|
|
500 |
|
14 |
C2 |
500 |
|
|
1000 |
|
15 |
C3 |
500 |
|
|
1500 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table No: 4Evaluation of different batches of floating microsphere
|
Sr No |
Batch code |
Mean particle size (mm) |
Angle of Repose (°) |
Bulk Density gm/cm3 |
Tapped Density gm/cm3 |
Compressibility index |
Hausner’s Ratio |
% Yield
|
Entrapment Efficiency (%) |
|
1 |
A1 |
230.6±0.06 |
22.94 ± 0.15 |
0.441 ±0.02 |
0.548±0.05 |
19.21±0.00 |
1.237±0.00 |
39.86±0.41 |
62.94 ± 0.09 |
|
2 |
A2 |
243.3±0.62 |
23.42 ±0.12 |
0.434 ±0.03 |
0.532±0.05 |
17.53±0.02 |
1.213±0.03 |
40.62±0.35 |
69.43 ± 0.92 |
|
3 |
A3 |
236 ±0.85 |
24.38 ± 0.24 |
0.416 ±0.05 |
0.481±0.09 |
13.42±0.01 |
1.155±0.02 |
35.95±0.88 |
82.21 ± 0.90 |
|
4 |
Aa1 |
188.6±0.01 |
22.64 ± 0.19 |
0.445 ±0.06 |
0.523±0.05 |
14.84±0.01 |
1.174±0.01 |
39.80±0.20 |
48.04 ± 0.56 |
|
5 |
Aa2 |
178.6±0.05 |
22.20 ± 0.02 |
0.426 ±0.04 |
0.494±0.02 |
13.72±0.02 |
1.159±0.03 |
40.97±0.60 |
51.66 ±0.66 |
|
6 |
Aa3 |
172 ±0.60 |
24.31 ± 0.26 |
0.450 ±0.05 |
0.535±0.09 |
15.98±0.02 |
1.190±0.02 |
36.83±0.23 |
57.5 ± 0.66 |
|
7 |
B1 |
226.6±0.74 |
23.66 ± 0.10 |
0.471 ±0.01 |
0.580±0.02 |
18.84±0.01 |
1.232±0.02 |
36.36±0.01 |
65.61 ± 0.27 |
|
8 |
B2 |
227 ±0.76 |
22.81 ± 0.07 |
0.459 ±0.05 |
0.573±0.06 |
19.89±0.00 |
1.248±0.00 |
41.02±0.96 |
63.05 ± 0.09 |
|
9 |
B3 |
227.3±0.16 |
24.28 ± 0.11 |
0.486 ±0.01 |
0.577±0.01 |
15.65±0.02 |
1.186±0.03 |
38.30±0.90 |
65.72 ± 0.93 |
|
10 |
Bb1 |
260 ±0.15 |
22.75 ± 0.19 |
0.494±0.06 |
0.596±0.06 |
17.00±0.01 |
1.205±0.02 |
33.60±0.59 |
46.11 ± 0.46 |
|
11 |
Bb2 |
275 ±0.11 |
25.2 ± 0.14 |
0.489 ±0.02 |
0.569±0.06 |
14.12±0.00 |
1.164±0.01 |
37.64±0.46 |
63.83 ± 0.27 |
|
12 |
Bb3 |
252.3±0.25 |
22.02 ± 0.17 |
0.495 ±0.02 |
0.559±0.06 |
11.43±0.03 |
1.130±0.04 |
37.61±0.42 |
65.77 ± 0.09 |
|
13 |
C1 |
264 ±0.22 |
25.84 ± 0.05 |
0.556 ±0.01 |
0.634±0.00 |
12.28±0.02 |
1.140±0.03 |
35.60±0.41 |
56.11±0.54 |
|
14 |
C2 |
274.6±0.09 |
24.44 ± 0.10 |
0.569 ±0.04 |
0.643±0.05 |
11.37±0.01 |
1.128±0.02 |
37.02±0.50 |
64.16±0.20 |
|
15 |
C3 |
268.6±0.03 |
22.12 ± 0.11 |
0.569 ±0.05 |
0.661±0.08 |
13.92±0.01 |
1.161±0.01 |
39.58±0.33 |
59.77±0.73 |
|
Sr. No. |
Time (min) |
Cumulative % release ±S.D |
|||||
|
A1 |
A2 |
A3 |
Aa1 |
Aa2 |
Aa3 |
||
|
1 |
0 |
0.00 |
0.000 |
0.00 |
0.000 |
0.00 |
0.000 |
|
2 |
30 |
14.9±1.09 |
15.8±1.3 |
14.5±1.7 |
20.2±0.8 |
19.6±0.6 |
21.4±0.0 |
|
3 |
60 |
18.7±1.12 |
21.0±0.9 |
18.3±0.6 |
26.5±1.2 |
25.9±1.2 |
25.9±0.3 |
|
4 |
90 |
21.9±1.72 |
24.7±0.5 |
21.5±1.6 |
30.8±0.7 |
31.3±1.7 |
32.1±1.2 |
|
5 |
120 |
25.0±1.83 |
28.9±0.7 |
26.0±1.5 |
34.7±1.3 |
35.2±0.1 |
36.6±1.4 |
|
6 |
150 |
28.2±0.20 |
31.3±0.4 |
30.5±1.8 |
39.7±0.1 |
41.5±0.0 |
41.6±1.6 |
|
7 |
180 |
31.8±0.44 |
35.8±1.0 |
33.4±1.1 |
44.4±1.6 |
45.3±0.2 |
46.9±1.3 |
|
8 |
210 |
35.0±0.82 |
40.5±0.6 |
36.7±1.0 |
49.9±1.1 |
50.7±0.5 |
51.9±0.0 |
|
9 |
240 |
38.2±1.72 |
45.6±0.8 |
40.0±0.6 |
56.0±0.7 |
56.2±0.5 |
57.7±0.5 |
|
10 |
270 |
41.9±0.49 |
50.2±1.1 |
44.5±0.6 |
62.4±0.5 |
60.9±0.7 |
63.4±1.3 |
|
11 |
300 |
47.1±1.03 |
56.3±0.4 |
49.5±0.5 |
66.7±1.2 |
65.6±1.0 |
67.7±1.2 |
|
12 |
330 |
52.9±1.0 |
61.5±0.9 |
54.3±1.2 |
70.6±1.3 |
69.1±1.9 |
72.2±0.4 |
|
13 |
360 |
57.4±1.1 |
66.0±1.0 |
57.5±1.4 |
75.3±0.4 |
73.7±1.3 |
75.7±0.5 |
|
14 |
390 |
62.0±0.5 |
68.2±1.2 |
62.7±1.8 |
79.6±0.5 |
77.7±0.6 |
80.4±0.8 |
|
15 |
420 |
65.8±0.4 |
73.6±0.4 |
66.4±1.3 |
82.7±1.0 |
80.8±0.5 |
83.3±1.0 |
|
16 |
450 |
71.4±1.3 |
77.9±0.6 |
70.9±1.2 |
85.1±0.6 |
85.0±1.0 |
87.3±0.9 |
|
17 |
480 |
75.9±1.7 |
82.3±1.2 |
74.6±1.2 |
88.3±0.7 |
88.0±0.5 |
89.3±0.2 |
|
18 |
510 |
80.5±0.2 |
85.2±0.4 |
77.5±1.1 |
93.9±0.2 |
92.4±0.4 |
92.9±1.6 |
|
19 |
540 |
84.2±0.9 |
87.7±1.3 |
79.0±0.6 |
92.8±0.9 |
94.9±1.0 |
95.8±1.0 |
|
20 |
570 |
88.1±0.9 |
92.7±1.3 |
82.4±0.9 |
95.3±1.9 |
|
|
|
21 |
600 |
91.2±1.2 |
95.5±0.3 |
86.9±0.2 |
|
|
|
|
22 |
630 |
94.1±1.31 |
|
93.9±1.1 |
|
|
|
|
23 |
660 |
|
|
97.6±1.3 |
|
|
|
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table No: 6In-vitro dissolution profile of formulation B1-B3 in 0.1 N HCL.
|
Sr. No. |
Time (min) |
Cumulative % release ±S.D |
||||||||
|
B1 |
B2 |
B3 |
Bb1 |
Bb2 |
Bb3 |
C1 |
C2 |
C3 |
||
|
1 |
0 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
2 |
30 |
20.0±1.3 |
20.7±0.8 |
19.6±1.7 |
19.3±0.1 |
19.4±1.7 |
19.4±1.9 |
20.7±1.1 |
19.3±1.0 |
20.7±0.8 |
|
3 |
60 |
25.7±1.1 |
28.1±0.8 |
25.9±0.7 |
26.6±1.4 |
27.9±1.1 |
24.8±1.6 |
31.2±1.7 |
27.4±0.0 |
27.7±1.0 |
|
4 |
90 |
39.6±1.0 |
39.4±1.3 |
33.3±1.9 |
36.7±1.7 |
33.7±1.1 |
32.1±1.8 |
41.8±1.3 |
38.9±1.1 |
38.3±1.8 |
|
5 |
120 |
47.4±0.9 |
50.4±0.6 |
39.5±1.1 |
44.1±1.0 |
37.5±1.3 |
38.8±1.0 |
52.3±0.1 |
52.0±1.4 |
47.4±2.1 |
|
6 |
150 |
53.7±0.3 |
60.5±1.2 |
46.0±1.0 |
50.7±1.6 |
43.6±1.5 |
43.4±1.6 |
65.1±1.4 |
66.6±0.7 |
58.6±1.9 |
|
7 |
180 |
61.5±1.0 |
69.3±1.0 |
57.6±0.3 |
57.9±1.9 |
51.9±1.3 |
49.5±1.3 |
72.3±1.1 |
70.0±0.6 |
66.6±0.1 |
|
8 |
210 |
66.3±1.9 |
79.1±0.5 |
67.7±1.8 |
66.6±1.2 |
58.8±0.7 |
56.0±1.0 |
81.5±0.2 |
77.2±1.4 |
71.4±1.5 |
|
9 |
240 |
79.5±1.8 |
85.6±0.0 |
72.7±1.0 |
74.3±1.7 |
70.4±1.5 |
65.0±1.0 |
92.1±1.8 |
83.2±1.6 |
81.4±1.2 |
|
10 |
270 |
84.5±1.0 |
89.0±1.8 |
80.2±1.0 |
80.0±1.3 |
78.4±1.0 |
71.0±0.5 |
95.0±0.7 |
88.4±1.2 |
85.2±0.5 |
|
11 |
300 |
89.3±1.0 |
93.7±0.7 |
85.2±0.2 |
88.8±1.2 |
84.7±0.7 |
78.9±1.5 |
|
91.9±0.4 |
89.8±1.5 |
|
12 |
330 |
92.2±1.2 |
95.3±0.7 |
90.1±0.7 |
91.4±1.2 |
88.2±1.0 |
82.2±1.2 |
|
93.1±0.9 |
90.2±0.4 |
|
13 |
360 |
93.8±0.7 |
|
92.2±1.0 |
94.0±0.4 |
92.0±0.4 |
87.4±1.0 |
|
|
93.4±0.4 |
|
14 |
390 |
95.2±0.4 |
|
93.3±0.4 |
95.0±0.7 |
93.9±0.7 |
89.6±0.7 |
|
|
|
|
15 |
420 |
|
|
93.9±0.4 |
|
94.6±0.3 |
92.2±0.7 |
|
|
|
|
Batch Code |
A1 |
A2 |
A3 |
Aa1 |
Aa2 |
Aa3 |
B1 |
B2 |
B3 |
Bb1 |
Bb2 |
Bb3 |
C1 |
C2 |
C3 |
|
|
Matrix
|
(R2)
|
0.97 |
0.94 |
0.99 |
0.98 |
0.98 |
0.98 |
0.98 |
0.98 |
0.97 |
0.97 |
0.97 |
0.97 |
0.97 |
0.97 |
0.98 |
|
(K) |
3.28 |
3.57 |
5.91 |
3.59 |
3.81 |
3.99 |
4.81 |
4.19 |
4.22 |
4.19 |
4.28 |
4.25 |
5.16 |
5.03 |
4.94 |
|
|
Sr. No |
Days |
Colour changes |
% Drug Content 40±2 °C |
% Max Drug Release |
|
1 |
0 |
white |
99.6 ±0.18 |
93.22±0.47 |
|
2 |
30 |
No change |
99.1 ±0.11 |
93.19±0.58 |
|
3 |
60 |
No change |
99.0 ±0.16 |
93.14±0.26 |
|
4 |
90 |
No change |
98.9 ±0.22 |
93.11±0.32 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









