 About Author: Saurabh Jain.*1, M.D.Kharya and Jain Aakansha2
About Author: Saurabh Jain.*1, M.D.Kharya and Jain Aakansha2
1 Department of Pharmaceutical Sciences
2 Department of Microbiology
Dr. H. S. Gour Central University Sagar (M.P.), India.470003
Abstract
Due to increasing resistance to existing synthetic antimicrobial agents, herbal drugs are being looked as importance source for treating various ailments related to skin infections and also for the discovery of new antimicrobial molecules for their synthesis. Cassia tora is a well known plant possessing wide range of pharmacological activities. It has been used in India as folk remedy in the form of decoctions and infusions to treat bacterial and fungal infections and also claimed to be effective against variety of skin conditions like psoriasis, acne, wounds etc. Although this plant, particularly its seeds have been reported to have antimicrobial activities, but no targeted and specific study has been reported. The present investigation was carried out to study this unexplored area of this drug. As the seeds of C. tora possess good antimicrobial activity, it was thought worked to scientifically explore its potential on microorganisms effecting human beings especially on skin, so that safe and effective herbal treatment can be developed for the same.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1108
Introduction
Traditional medicines hold a great promise as source of easily available effective therapy for skin diseases. Cassia tora Linn.(Family: Leguminosae), is a common Indian herb having various medicinal properties for the treatment of many disease. According to Ayurveda the leaves and seeds of C.tora are acrid, laxative, antiperiodic, antihelmintic, ophthalmic, liver and cardiotonic and expectorant and useful in leprosy, ringworm, flatulence, colic, dyspepsia, constipation, cough, bronchitis and cardiac disorders. It has been reported for their usefulness in traditional system of medicines for treating skin diseases like psoriasis, leprosy etc (Zahra et al, 2000; Harrison and Dorothy, 2003). C.tora is used as natural pesticides. Antimicrobial activity of chrysophanic acid-9-anthrone from C. tora has been reported. (Kim et al, 2004). Humans are natural hosts for many bacterial species that colonize the skin as normal flora. Staphylococcus aureus and Streptococcus pyogenes are infrequent resident flora, accounting for a wide variety of bacterial pyrodermas, conditions like impetigo, ecthyma, erysipelas, cellulitis, psoriasis, atopic dermatitis, Erythroderma and other skin infections (Tomi et al, 2005). As C.tora has been reported to possess antimicrobial activity causing skin infections, the present investigation was carried out to evaluate the antimicrobial activity of Cassia tora, against Staphylococcus aureus, Escherichia coli, Aspergilus niger and Microsporum gypseum.
Materials and Methods:
Seeds of C. tora collected from local market of Sagar, were dried and authenticated in Department of Botany, Dr. H.S.Gour Central University, Sagar (M.P.), India.
Chemicals and Reagents: All the solvents used were of AR grade from Rankem.
Apparatus: Kitchen blender, Soxhlet apparatus, sieve size #10.
Extraction of Cassia tora: Seeds were washed in tap water; shades dried, powdered in a kitchen blender and were stored in airtight plastic bags. Then powdered seeds (250g.) passed through sieve # 10 was defatted by the Whatman filter paper and introduced in to the Soxhlet apparatus using petroleum ether (60-80) as a solvent. After complete defatting drug was air dried for removing trace of petroleum ether. The defatted drug was then packed in Whatman filter paper and introduced in to the Soxhlet apparatus and extracted with benzene as a solvent for complete extraction. The extract was filtered, concentrated and dried in a water bath. Dried extract was transferred to air tight bottles and the percentage yields was calculated and stored at cool place. (Table-1).
Isolation of Chrysophanic acid: Isolation of chrysophanic acid was done by column chromatography of benzene extract. The column was washed three times with acetone and packed with activated silica gel (100-120 mesh). The elution was started with the chloroform solvent system. Since most of the components were yellowish- brown coloured, it was easy to govern the elution pattern. Each fraction collected separately and concentrated by evaporation. There were around twelve fractions and each fraction was compared with its adjacent fractions by TLC. Those fractions given similar pattern combined together and each fraction qualitatively analyzed for the particular class of compound. Then after, obtained isolated compound was identified by various methods as apperance, melting point, solibilty profile, Modified Borntrager’s test and UV-visible spectra range etc.
Phytochemical evaluation: Phytochemical examinations were carried out for the extract as per the standard methods. (Trease and Evans, 1987). (Table-2)
|
S.No. |
Phytochemicals |
Test Procedure |
|
1. |
Alkaloids |
Extract was dissolved in dilute Hydrochloric acid and filtered. The filtrate was used to test for the presence of alkaloids. |
|
Mayer’s Test |
Filtrate was treated with Mayer’s reagent (Potassium Mercuric iodide). Formation of a yellow cream precipitate indicates the presence of Alkaloids. |
|
|
Dragendroff’s Test |
Filtrate was treated with Dragendroff’s reagent (solution of potassium bismuth iodide). Formation of red precipitate indicates the presence of alkaloids. |
|
|
2. |
Carbohydrates |
Extract was dissolved in 5 ml distilled water and filtered. The filtrate was used to test for the presence of carbohydrates. |
|
Molisch’s Test |
Filtrate was treated with 2 drops of alcoholic α-naphthol solution in a test tube and 2 ml of Conc. Sulphuric acid was added carefully along the sides of the test tube. Formation of violet ring at the junction indicates the presence of Carbohydrates. |
|
|
3. |
Glycosides |
Extract was hydrolysed with dil. HCl, and then subjected to test for glycosides. |
|
Modified Borntrager’s Test |
Extract was treated with Ferric Chloride solution and immersed in boiling water for about 5 minutes. The mixture was cooled and shaken with an equal volume of benzene. The benzene layer was separated and treated with ammonia solution. Formation of rose-pink colour in the ammoniacal layer indicates the presence of anthraquinone glycosides. |
|
|
Legal’s Test |
Extract was treated with sodium nitroprusside in pyridine and methanolic alkali. Formation of pink to blood red colour indicates the presence of cardiac glycosides. |
|
|
4. |
Saponins Froth Test |
Extract was diluted with distilled water to 20ml and this was shaken in a graduated cylinder for 15 minutes. Formation of 1 cm layer of foam indicates the presence of saponins. |
|
5. |
Steroids Libermann Burchard’s Test |
Extract was treated with chloroform and filtered. The filtrate was treated with few drops of acetic anhydride, boiled and cooled. Conc. Sulphuric acid was added carefully along the sides of the test tube. Formation of brown ring at the junction indicates the presence of steroids |
|
6.
|
Fixed oils & fats Stain Test |
Small quantity of extract was pressed between two filter papers. An oily stain on filter paper indicates the presence of fixed oil.
|
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Chrysophanic acid as a remedy for skin infections:
Formulation of the ointment (Lachman et al., 1987)
Each 50 g. of ointment contains:
Chrysophanic acid……………………………1 g.
Benzoic acid (as preservative)……………….250 mg
Menthol (as cooling agent)…….…………….0.1 mL
Ointment base………………………………..q.s. to 50 g.
In-vitro Antimicrobial activity:
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bacterial Concentration (MBC):
Since agar disc-diffusion assay is a qualitative method used in antimicrobial testing, MIC can be defined as the minimum concentration of the extract, which can inhibit the growth/proliferation of microorganism and MBC is lowest extract concentration that completely kills at microbes. It indicates the potency of the extract as an antimicrobial agent. Those extracts which showed antimicrobial property were further used for the MIC and MBC studies at various concentrations with respective microorganisms (Aboaba et al, 2006).
The benzene extract and test ointment that exhibited considerable activity (>6 mm) were separately dispensed separately in a series of five test tubes containing sterile nutrient broth to get a final concentration (25, 50, 75, 100 and 125 mg/mL) of extracts and a final tube with the solvent control. An aliquot of 0.5 mL of the respective bacterial suspension was inoculated into each tube and mixed well. All the tubes were incubated at 370C for 24 hour. The lowest concentration that did not permit any visible growth when compared with the control was considered as the minimum inhibitory concentration (MIC). Aliquots of culture media from all the test tubes that did not show any visible growth were inoculated on nutrient agar, incubated at 370C for 24 hour. The minimum bacteriocidal concentration was considered as the lowest concentration that could not produce a single bacterial colony.
Micro-organisms used: Two bacterial species Staphyllococcus aureus (Gram-Positive) and Escherichia coli (Gram-Negative) and two fungal species, Aspergilus niger and Microsporum gypseum were procured from Department of Microbiology, Dr.H.S.Gour Central University, Sagar (M.P.).
Determination of zone of inhibition: Preparation of the samples: 200 mgs of benzene extract of Cassia tora, prepared test ointment and standard ointment (Fusidic acid ointment) screened for their antimicrobial activity. All the samples were dissolved in 10 ml of DMSO to get the concentration of 20 mg/ml. Evaluation of the activity was carried out by cup-plate technique using nutrient agar medium and the antibacterial and antifungal activity was measured in terms of zone of inhibition.
Preparation of Inoculums: Suspension of organism was prepared as per McFarland nephelometer standard (Ellen and Sydney 1990). A 24 hour old culture was used for the preparation of bacterial and fungal suspension. Suspension of organism was made in a sterile isotonic solution of sodium chloride (0.9% w/v) and the turbidity was adjusted such that it contained approximately 1.5X 10 8 cells/ml. It was obtained by adjusting the optical density of the bacterial and fungal suspension to that of a solution of 0.05ml of 1.175% of barium chloride and 9.95 ml of 1% sulphuric acid.
Antibacterial Assay: Antibacterial assay was carried out on Mueller Hinton Agar. The composition of the medium is given below.
Mueller Hinton Agar (g/L):-
Beef infusion : 4.0
Starch : 1.5
Casein hydrolysate : 17.5
Agar : 15.0
Final pH : 7.4 at 370C
Antifungal Assay: Antifungal studies were performed on Sabouraud Dextrose Agar (SDA) media.
Sabouraud Dextrose Agar (g/L):-
Meat Peptone : 5
Casein Peptone : 5
Dextrose : 40
Agar : 15
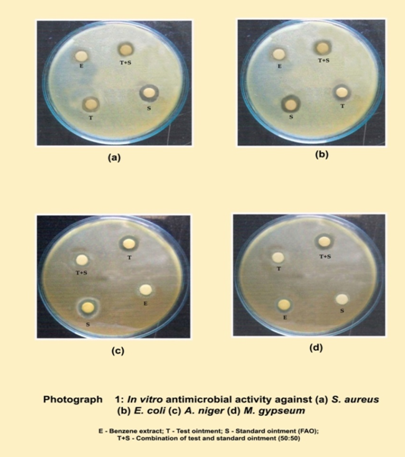
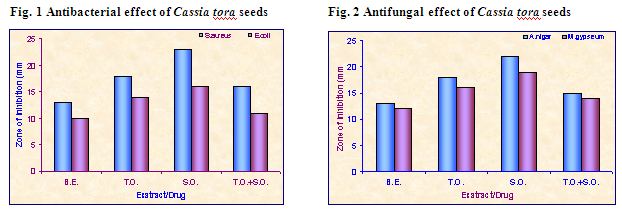
Procedure: The medium was prepared by dissolving the ingredients in distilled water and subjected to sterilization in an autoclave at1210C for 15 minutes. The Petri plates were washed thoroughly and sterilized in hot air oven at 1600C for 1 ½ hours. 30 ml of sterile molten agar medium seeded by organisms, in semi hot conditions (400C) was poured aseptically in sterile petri plate and allowed to solidify at room temperature. Bores were made on the medium using sterile stainless steel borer and 0.1 ml of the samples were added to respective bore of Fusidic acid ointment at a concentration of 100 μg / ml was taken as standard. The petri plates seeded with organisms, containing extracts and the standard were kept in refrigerator at 40C for 1 hour to facilitate the diffusion of the extracts and the standard in to the media. After diffusion the petri plates were incubated at 37 ± 10C for 24 hours in a incubator and zone of inhibition was observed and measured using a scale. The results of the antibacterial and antifungal activity of samples are tabulated. (Table 3 and 4; Fig. 1 and 2) (Photograph 1)
Result and discussion:
In the Ayurvedic system of medicine, C.tora has great reputation in all kinds of skin infections. Seeds of C.tora constitute a valuable remedy in skin diseases, chiefly for ringworm and itch. The benzene extraction yield 6.5% extract.
Table 1: Percentage yield of extract
|
Plant & Plant part |
Wt. of part taken (g) |
Solvent |
Solvent used (mL) |
Crude extract (g) |
Appearance |
% yield |
|
Cassia toraseeds |
228 |
Benzene |
1500 |
14.8 |
Dark yellowish-brown semisolid |
6.5 |
Cassia tora is well known traditional medicine for its effectiveness against wide range of diseases including skin infections due to the advantage of the diversity of secondary metabolites responsible for their antimicrobial activity.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 2: Phytochemicals Studies of C.tora seeds in benzene extract
|
S.No. |
Tests/Reagents |
Result |
|
1. |
Alkaloids (Dragendorff’s reagent and Mayer’s reagent) |
Absent |
|
2. |
Resins (Acetone-water test) |
Absent |
|
3. |
Steroids (Liebermann’s test) |
Absent |
|
4. |
Terpenoids (Copper acetate test) |
Absent |
|
5. |
Flavonoids (Shibata’s reaction) |
Absent |
|
6. |
Saponins (Froth test) |
Absent |
|
7. |
Tannins (Gelatin Test) |
Absent |
|
8. |
Phenols (FeCl3 test) |
Absent |
|
9. |
Cardiac Glycosides (Legal’s test) |
Absent |
|
10. |
Anthraquinone Glycosides (Modified Borntrager’s test) |
Present |
|
11. |
Carbohydrates/Sugars (Molisch’s test) |
Present |
|
12. |
Proteins and Amino acids (Ninhydrin test) |
Absent |
|
13. |
Wax/gums (Water+ethanol) |
Absent |
Qualitative phytochemical analysis of C.tora seeds has mainly shown the presence of anthraquinones.
It is clearly evident that prepared test ointment showed no irritancy, good extrudability, homogeneity and spreadability. The pH of ointment was suited to skin secretions. The physico-chemical characteristics and percent assay of drug in the formulation was found to be satisfactory. Ointment formulation was stable and had good physical properties.
in vitro study, the extract and prepared test ointment was analyzed for antimicrobial property against two bacterial species, Staphylococcus aureus and Escherichia coli and two fungal species, Aspergilus niger and Microsporum gypseum. The observations were compared with that of standard antibiotic by measuring the diameter of clearance zone of inhibition. The benzene extract was found most potent (13 mm) against Staphylococcus aureus, drug in ointment (test) showed 18 mm, while Fucidin ointment (standard drug) showed a clearance zone of 23 mm. The minimum inhibitory concentration and bacteriocidal concentration of the extract was 75 mg/mL and 125 mg/mL respectively, while for drug in ointment the minimum inhibitory concentration and bacteriocidal concentration 50 mg/mL and 100 mg/mL respectively. In case of antifungal study benzene extract was found most effective against A. niger (13 mm), drug in ointment showed 18 mm, while the activity of Fucidin ointment against these fungi was 22 mm.
Table 3: Comperitive antibacterial study of Cassia tora seed extract
|
Test samples |
Diameter (mm) of zone of inhibition |
|
|
S.aureus |
E.coli |
|
|
Benzene extract |
13 |
10 |
|
$Test ointment |
18 |
14 |
|
$$Standard ointment |
23 |
16 |
|
Combination of Test and Standard ointment (50:50) |
16 |
1 |
Table 4: Comperitive antifungal study of Cassia tora seed extract
Test samples |
Diameter (mm) of zone of inhibition |
|
|
A.niger |
M.gypseum |
|
|
Benzene extract |
13 |
12 |
|
$ Test ointment |
18 |
16 |
|
$$ Standard ointment |
22 |
19 |
|
Combination of Test and Standard ointment (50:50) |
15 |
14 |
$ Test ointment is prepared in laboratory.
$$ Fusidic acid ointment (FAO) used as Standard ointment.
Conclusions
Phytochemical analysis of Cassia tora seeds shows that it mainly contains anthraquinones. In vitro studies proved that Cassia tora seeds possess antimicrobial property. The order of in-vitro activity in present study; Standard drug ointment > Test drug ointment> Combination of test+ standard drug ointment (in 50:50 ratio)> extracted drug. The present phytochemical and pharmacological findings provide scientific evidence to the traditional use of Cassia tora seeds in skin infections.
References
Aboaba OO, Smith SI and Olude FO. 2006. Antibacterial Effect of Edible Plant Extract on Escherichia coli. Pakistan Journal of Nutrition 5(4): 325-27.
Ellen JB and Sydney MF. 1990. Diagnostic microbiology. 8th ed., USA, Missouri. p.453.
Harrison and Dorothy. 2003. Natural therapeutic composition for the treatment of wounds and sores. CIPO Patent 2392544.
Kim YM, Lee CH, Kim HG and Lee HS. 2004. Anthraquinones isolated from Cassia tora (Leguminosae) seed show an antifungal property against phytopathogenic fungi. J Agric Food Chem. 52(20): 6096-100.
Lachman Leon, Lieberman A. Herbert and Kanig L. Joseph. 1987. The Theory and Practice of Industrial Pharmacy, 3rd Edition, Varghese Publishing House, Bombay: 534-58.
Tomi NS, Kränke B and Aberer E. 2005. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. Journal of the American Academy of Dermatology 53(1): 67-72.
Trease E and Evans WC. 1987. Pharmacognosy, Billiare Tindall.London 13 th edition: 61-62.
Zahra A, Mohammed A, Mohamlkmed HK.2000. Evaluation of immunomodulatory effects of five herbal plants. J.Ethanopharmacol 72:167-72.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









