About Authors:
*1Ronak Patel, 2Mr. Ripal Mistry
1M.Pharm, Bhupal Nobels’ College Of Pharmacy, Udaipur 313001
2M.pharm, Indubhai Patel College Of Phaarmacy And Research Center, Dharmaj
*rk4989@gmail.com
{ DOWNLOAD AS PDF }
Abstract:
Ocular administration of the drug is primarily associated with the need to treat ophthalmic diseases and is not regarded as a means for gaining systemic drug action. Major classes of drugs used are miotics, mydriatics/cyclopegics, anti-inflammatory, anti-infectives surgical adjutants and diagnostics, which are all meant for local therapy. Systemic action by using eye as a portal is generally avoided in order to prevent the risk of eye damage from high blood concentrations of drug not intended for eye. The unique anatomy, physiology and biochemistry of eye renders this organ exquisitely impervious to for gain substances thus presenting a constant challenge to the formulator to circumvent the protective barriers of the eye without causing any permanent tissue damage. Currently, the knowledge in this field is rapidly expanding, and many concept and drug delivery strategies are emerging out.
REFERENCE ID: PHARMATUTOR-ART-2070
1. INTRODUCTION1 :
Human eye has a spherical shape with a diameter of 23mm. Amount of light-enter-pupil. Rapid removal of material instilled into the eye is occur unless - small in volume and chemically & physiologically compatible with surface tissue.
Eye Ball:
The wall of the human eye (globe) is composed of concentric layer;
- The outer fibrous layer.
- A middle vascular layer :
- Uvea tract - choroid,
- cilliary body, and iris.
- A nervous layer-the retina.
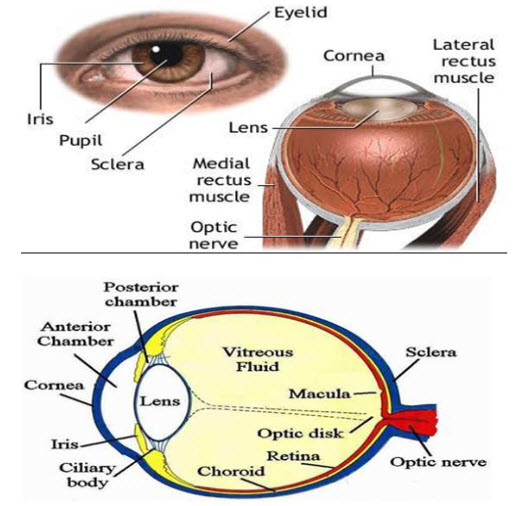
Anatomical view of Human eye
· Outer fibrous layer made up of 2 parts.
· Posterior 5/6 th is opaque and called the sclera
· Anterior 1/6 th is transparent and called the cornea.
Sclera contains the microcirculation, which nourishes the tissues of this anterior segment and is usually white.
Cornea is an optically transparent tissue that acts as the principal refractive element of eye. The corneal thickness is 0.5 to 0.7 mm. The cornea composed of epithelium, bowmen’s membrane struma descement membrane and endothelium.
Middle vascular area: The chorid remain just behind the retina forming the posterior 5/6 th of the middle coat, composed of numerous blood vessels and pigmented cells containing melanin.The cilliary body includes orbicularis ciliaris ciliar process and ciliary muscles. The iris nervous coat is called retina, which contain photosensitive receptors.
Conjunctiva
The conjunctival and corneal membrane covers the outer surface of the white portion of the eye, and inner aspects of the eyelids. It is attached loosely and then by permits free movements of the eyeball. Expect for the cornea the conjuctiva is the most exposed portion of the eye.
Lacrimal system The secretion of the lacrimal fluid the tear is delivered through a number of fine ducts into the conjunctival for nix.
COMPOSITION OF TEAR2:
The secretion is a clear, watery fluid containing numerous salts, glucose, other organic compounds, approximately 0.7% protein and the enzyme, lysosome.
Water-98.2%
Solids-1.8%
· Organic element:
- Protein0.67%
- Sugar-0.65%
- Urea-0.03%
- Nacl-0.66%
Other mineral elements sodium, potassium and ammonia-0.79%
The lacrimal fluid in humans has a normal volume of 7ml with fluid pH 7.4 and does occur, the volume can go up to 30ml without spillage.
Transporters in eye3:
* The traditional approach to improve ocular bioavailability is to modify the drug chemically to achieve the desired solubility and lipophilicity. However, a more rational approach would be a transporter-targeted modification of the drug. Transporters are membrane-bound proteins that play an important role in active transport of nutrients across biological membranes. The presence of transporters has been reported on various ocular tissues.
* Two types of transporter systems are of interest in ocular drug delivery: efflux transporters and influx transporters. Widely studied efflux transporters belong to the ATP binding cassette superfamily, whereas influx transporters belong to the solute carrier (SLC) superfamily. Efflux transporters lower bioavailability by effluxing the molecules out of the cell membrane and cytoplasm. Prominent efflux transporters identified on ocular tissues include P-gp, multidrug resistance protein (MRP), and BCRP. Various authors reviewed the emerging role of transporters in ocular drug delivery.
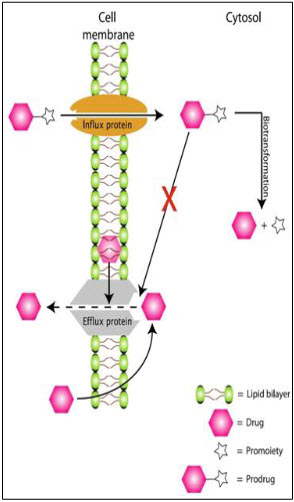
Circumvention of efflux proteins
Conventional Ophthalmic Dosage Forms4:
a) Eye Drops:It is defined as the liquid preparation in which all the ingredients are completely soluble in solution in a uniformly manner. Upon instillation most of the instilled volume is eliminated from the precorneal area.
b) Eye Gels: It is semisolid preparation comprising of small molecules interpenetrated by a liquid, which are applied to the eye. The residence time is more in the eye region by using gels due to which the absorption rate is increased and a prolonged therapeutic effect is produced.
c) Eye ointments: Eye ointments are sterile, semi solid preparation of homogenous appearance intended for application to conjunctiva or margin of the eyelids. Due to its hydrophobic nature it produces very low level of therapeutic effect. It produces blurring of vision due to its greasy nature.
d) Eye Suspension: Suspensions are the dispersion of finely divided relatively insoluble drug substances in an aqueous vehicle containing suitable suspending and dispersing agent. It must contain particle of such chemical characteristics and small dimensions that they are non-irritating to the eyes15. The particles are better retained in cul-de-sac thus bioavailability of the drug increases and give a slow release effect.
Novel Ophthalmic Dosage Forms1,5:
Delivery of drugs into eyes using conventional drug delivery systems, such as solutions, is a considerable challenge to the treatment of ocular diseases. Drug loss from the ocular surface by lachrymal fluid secretion, lachrymal fluid-eye barriers, and blood-ocular barriers are main obstacles. A number of ophthalmic drug delivery carriers have been made to improve the bioavailability and to prolong the residence time of drugs applied topically onto the eye.
* Controlled ocular delivery system1:
An inordinately high number of ocular conditions are by over treatment with topical ocular drugs.
Local installition of antibiotics, antiviral provides high drug and preservative concentration at epidermic surface.
Requisites of Controlled Ocular Delivery System:
1. To overcome the side effect of pulsed dosing
2. To provide sustained and controlled drug delivery
3. To increase ocular bioavailability
4. To provide targeting with in ocular globe
* Polymeric Solution:
The addition of polymer like methylcellulose, PVA, hydroxypropyl, PVP. This increase tear viscosity which decrease rapid initial drainage, increase contact time and thus remain sustain to some extend.
* Mucoadhesives:
Mucoadhesives are defined as they are retained in the eye by virtue of non-covalent bonds established with the corneal conjuctival mucin for extending the pre-ocular residence times.
Coating external surface of globe of eye is thin film glycoprotein is called as MUCIN. Natural and synthetic polymers (CMC, carbopol,sodium alginate etc.)that bind to mucin or epithelial surface has been used for the drug delivery via nose, buccal cavity and intestine. This bioadhesive polymers help in prolonging the release of drug from dosage form by localising it at a specific site where mucous is present. The remain in contact with precorneal tissue until mucin turnover causes elimination of polymers. Good mucoadhesion in the eye is achieved with the polymers possessing Correct charge density No. Of polar group for hydrogen bonding Balance of lipophilic to hydrophilic sections in the polymer chain. Hydrogen bonding plays an important role in bioadhesion since it is the protonated form of anionic mucoadhesive i.e. responsible for bioadhesion. Interaction between cationic polymer and mucin is due to the electrostatic attraction.
* Phase transition system: These are liquid dosage forms which shift to the gel or solid phase when instilled in the cul-de-sac due to physiological condition of eye.After converting into gel it remain in contact with the cornea of the eye for prolonged period of time due to which drug elimination is also slow down.Thus increasing the precorneal residence time and enhancing the ocular bioavailability of the drug.
Possible mechanisms for phase – transition system:-
A) Temperature Dependent System:-
In situ gelling by a temperature change is produced when the temperature of polymeric dispersion is raised from 25 to 37°C.It can be prepared by using poloxamer 407 and Lutrol FC 127.
B) pH dependent system:-
At formulation pH (4 – 6 ) ; it forms clear free flowing system but at physiological pH ( 7.4) of the eye, it forms firm gel which has capacity to release the drug for long time. The polumers used for this purpose are Carbopol, Cellulose acetate phthalate and chitosan.generally, this system is used along with the viscosity imparting agents like MC,HPMC etc.
C) Ion Induced System:-
This system is converted into gel when introduced in the ion concentration at the physiological condition of eye.
Examples :
1.Gelrite : which is the low acetylated gellan gum.
2. Sodium alginate:
D) Enzyme dependent system:
Xanthan gum has capacity to form gel with the enzyme LYSOZYME which is present in the tear fluid. No. of patented autoclavable in –situ gelling eye preparations are made by using vehicle containing the xanthan gum.
Vesicular System for Ocular Drug Delivery:
1. Niosomes:1,2
· Niosomes are the vesicles containing non-ionic surfactant(dialkylpolyoxyethylene ether) can entrap both hydrophillic and lipophillic drugs either in aqueous layer or in vesicular membrane made of lipid materials. It helps in preventing the metabolism of the drug by enzymes present at the tear/corneal surface.
· In order to circumvent the limitations of liposomes such as chemical instability, oxidative degradation of phospholipids, cost, and variable purity of natural phospholipids,neosomes were developed.
· It is reported that a vesicular system is formed when a mixture of cholesterol and a single-alkyl chain, non-ionic surfactant is hydrated.
· The resultant vesicles, termed ‘‘niosomes’’, can entrap solutes, are osmotically active, and relatively stable.
· Disc shaped neosomes are known as discosomes.
· Discomes, in addition to their many advantages, seem to have a special advantage towards the ocular route, wherein their large size may prevent their drainage into the systemic pool.
· Furthermore, their ‘‘disc’’ shape provides for a better fit in the cul-de-sac of the eye.
· Non-ionic surfactant-based discoidalniosomes (discomes) of timolol maleate have been reported to be promising systems for the controlled ocular administration of water-soluble drugs, releasing the drug in a biphasic profile.
2. Liposomes:1,6
· Liposomes are microscopic vesicles composed ofone or more concentric spheres of lipid bilayers separated by water or aqueous buffer compartments with a diameter ranging from 80nm to 100 mm.
· The lipid layers are comprised mainly of phospholipid. They have the ability to entrap hydrophillic compound in the aqueous compartment and to incorporate hydrophobic molecule in the lipid bilayers.
· Drug molecules depending upon their solubility are encapsulated in either the aqueous phase or the lipid bilayer . Thus, liposomes can accommodate both hydrophilic(Dihydro streptomycin sulphate) and lipophilic compounds(Triaminoloneacetonide),and it is possible to apply water-insoluble drugs in a liquid dosage form.
· Lipophilic drug are mostly used due to 2 fold increase in lipophilic drug concentration and aq. Humor level of hydrophilic drug decreases.
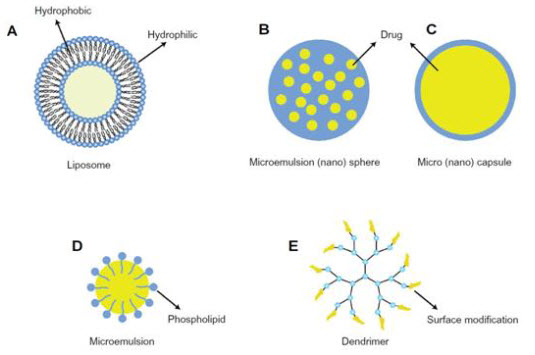
vesicular system
· Liposomes can enhance corneal drug absorption, achieved through their ability to come into intimate contact with the corneal and conjunctival surfaces.
· Depending on the composition, liposomes can have a positive, negative, or neutral surface charge.
· In vitro liposome–corneal interaction studies showed that liposomes were taken up by the corneain the order
· MLV+> SUV+>> MLV->SUV-> MLV=SUV
· The reason for this apparent difference is not clear, but it is known that the corneal epithelium is thinly coated with negatively-charged mucin to which the positive surface charge of the liposomes may absorb more strongly.
ADVANTAGES:
- Control the rate of encapsulated drug
- Protection of drug from metabolic enzymes present at the tear corneal epithelium interface.
- Biodegradable
- Non toxic
DISADVANTAGE:
- instability (due to the hydrolysis of phospholipids normally used in their preparation)
- limited drug-loading capacity
- technical difficulties in obtaining a sterile liposomalpreparation.
PREPARATION:
- Matrix of phospholipid (phosphotidylcholine, phosphotidyl serine, cardiolipins) is agitated in aq. medium
- This may produce multilamellar vesicles(by sonication) or unilamellar (by reverse phase evaporation or solvent injection or detergent removal)
- Drug used owing to their amphiphilic in nature.
3. Nanoparticles:
· Nanoparticles are solid particles of polymeric nature ranging in size from 10-1000nmin which the drug is dissolved, entrapped, encapsulated, or adsorbed.The drugs are bound to small particles, which are than dispersed aqueous vehicle.Due to very small in size these are not washed away with tears quickly.
· They are further classified into nanospheres (small capsules with a central cavity surrounded by a polymeric membrane) or nanocapsules (solid matricial spheres).
· The nanocapsules show a better effect than the nanospheres, probably because the drug (betaxolol, carteolol) is in an unionized form in the oily core and can diffuse at a greater rate into the cornea. Diffusion of the drug from the oily phase to the corneal epithelium seems to be more effective than diffusion from the internal matrix of the nanospheres.
· The better efficiency of nanocapsules is due to their bioadhesive properties, resulting in an increase in the residence time and biological response.
· However,it was observed that nanoparticles consisting of poly(alkylcyanoacrylate) damaged the corneal epithelium by disrupting the cell membranes.
* PREPARATION:
· It is prepared by emulsion polymerisation.
· Poorly soluble monomer is dissolved in continuous phase (aq. Or organic) and give treatment with irradiation (with gamma rays,UV,visible rays) or by chemical initiation polymerisation takes place(pH <3).By adding emulsifier (polyalkyl cyanoacrylates) stabilize polymer particle formed. Then adding drug and adjust pH to desired value.
· Ex:1) Pilocarpine nanoparticle-----enhance miotic response by 22-23%
------miosis time --> 180 to 240min
2) In inflammation concentration of pilocarpine nanoparticle with polyhexylcyanoacrylate 3-times higher than in normal eye.
3) Eudragitself assembling nanoparticle of diclofenacàimproved efficacy.
Ophthalmic inserts:
• Inserts are defined as a thin disks or small cylinders made with appropriate polymeric material and fitting into the lower or upper conjunctival sac. Their long persistence in preocular area can result in greater drug availability with respect to liquid and semisolid formulation.
• Polymer used: collagen and fibrin.
• Criteria for controlled release inserts:-
* Comfort
* Lack of explosion
* Ease of handling and insertion
* Non interference with vision and oxygen permeability
* Reproducibility of release kinetics
* Ease of manufacture
* Sterility and stability
Classification of ophthalmic insert;
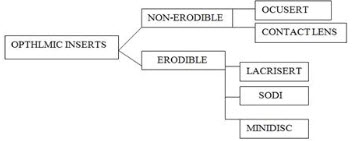
1. Erodible insert :
a) Lacrisert
b) SODI
c) Ocular therapeutic system or minidisc
2. Non erodible insert:
a) Ocusert
b) Contact lenses
1. ERODIBLE INSERT:- 1,4
A) Lacrisert:
· A rod shaped,sterile pellet of Hydroxypropyl Cellulose without preservative is commercially available (Lacrisert). This device is designed as a sustained release.
· Weight= 5mg
· Diameter = 12.7mm
· Length = 3.5mm
· It should be inserted in to inferior fornix, where it imbibes water and form hydrophilic film which stabilize tear film. This will hydrate and lubricate cornea
lacrisert
• Indication and usage:
* LACRISERT is indicated in patients with moderate to severe dry eye syndromes, including keratoconjunctivitissicca.
* LACRISERT is also indicated for patients with:
* Exposure keratitissicca.
* treatment of dry eye syndromes
* Decreased corneal sensitivity
* Recurrent corneal erosions
• Contraindications:
* LACRISERT is contraindicated in patients who are hypersensitive to hydroxypropyl cellulose.
· Contraindications:
- LACRISERT is contraindicated in patients who are hypersensitive to hydroxypropyl cellulose.
B) Soluble ocular drug insert (SODI):
· A SODI is a soluble copolymer of acrylamide, N-vinyl pyrrolidone, and ethyl acrylate.
· It is in the form of sterile thin films or wafers of oval shape, weighing 15 to 16 mg.
· After introduction into the upper conjunctival sac, the SODI softens in 10 to 15 sec, conforming to the shape of the eyeball; in the next 10 to 15 min the film turns into a polymeric clot, which gradually dissolves within 1 hr, while releasing the drug.
· Advantage:
Reduced role of the clinician, since the form is dissolved by total or partial solubilization and there is no need to surgically remove the insert once the drug has been released.
· Drawback:
They blur vision while the polymer is dissolving.
· Release of the drug from the SODI is proposed to occur in two stages:
1) hydration of the matrix by penetration of dissolution medium; and
2) diffusion of the medium deep into the matrix and back-diffusion of the dissolved active principle.
· A single SODI application has been reported to replace 4 to 12 drops instillations or 3-6 application of ointment and constitute the valid once a day therapy for treatment of glaucoma and trachoma.
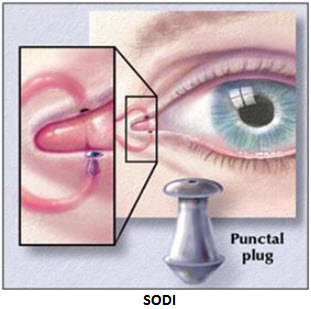
C) Ocular therapeutic system or minidisc (OTS):
- It consists of contoured disc with convex front and concave back surface in contact with eyeball.
- Is like a miniature contact lenses.
- Diameter-4-5mm.
- The symmetric circular design of the minidisc in contrast with the elliptical or rod shape elimination the need to align a particular geometric axis of device with eyelid margin.
- It is a silicon based prepolymer-a-w-bis (4-methyloxy)butylpolydimethylsiloxane.
- Can be hydrophilic or hydrophobic to permit extended release of both water soluble and insoluble drugs.
- Ex. Sulfisoxazole (poorly water soluble) incur-orrate in hydrophilic matrix and drug was release for 170 hrs.
- Gamma irradiation and heat exposure,slow down drug release because cross linking of polymer matrix.
Advantages:
- Sophisticated and effective delivery system.
- Flexibility in drug type and dissolution rate.
- Needs only insertion into eye and not to remove.
Disadvantages:
- Patient discomfort.
- Requires assistance for insertion.
- Movement of system around the eye can cause abrasion.
- Occasional product loss during sleep or while rubbing eyes.
- Interference with vision and difficulty in placement.
2) NON-ERODIBLE:-
A) Ocusert:-
· It is flat, flexible, elliptical device.
· A truly continuous controlled release and Zero order kinetic fashion was achieved using ocusert.For hydrophilic Drugs.
· Pilocarpineocuserts( byAlza corporation of California.)
· The system consists of a Pilocarpine – alginate core (drug) in gel form sandwiched between two transparent, rate controlling ethylene-vinyl-acetate copolymer membranes. Titanium dioxide encloses the drug reservoir circumferentially.
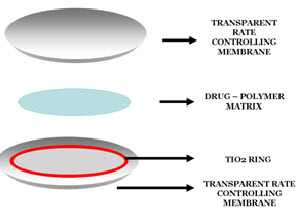
· The microporousmembrane permit the tear fluid to penetrate into the drug reservoir compartment to dissolve drug from the complex.
· Higher release rate achieved (for chronic glaucoma ) by:
- Making its rate controlling membrane thinner
- Use of flux enhancer di(2 ethyl-hexyl)phthalate
· Two types of Ocuserts® are available:
1. Ocusert®pilo- 20
2 .Ocusert®pilo- 40
· This device is more popular among younger patients as compared to elder population who have difficulties in insertion, do not retain device well and often do not notice if it falls out.
· Clinical studies with the pilocarpineOcusert® demonstrated that slow release of the drug can effectively control the increased intraocular pressure in glaucoma, with a minor incidence of side-effects, such as miosis, myopia, browache, etc.
Advantages of occuserts:
· Increased ocular residence time hence a prolonged drug activity and a higher bioavailability with respect to standard vehicle.
· Possibility of releasing drug at a slow constant rate.
· Accurate dosing (contrary to eye drop that can be improperly instilled by the patient and are partially lost after administration each insert can be made to contain a precise dose which is fully retained at the administration site).
· Reduction of systemic absorption (which occurs freely with eye drops via the nasolacrimal duct and nasal mucosa).
· Better patient compliance, resulting from a reduced frequency of administration and a lower
· incidence of visual and systemic side effects.
· Possibility of targeting internal ocular tissues through non corneal(conjunctival sectional) routes
· Increased shelf life with respect to aqueous solutions.
· Exclusion of preservative, thus reducing the risk of sensitivity reactions.
· Possibility of incorporating various level novel chemical/technological approaches, such as prodrugs, mucoadhesives, permeation enhancers, microparticulates, salts acting as buffers etc.
Disadvantages of occusert:
· Initial discomfort, their movement around the eye.
· Occasional inadvertent loss during sleeps or while rubbing the eye.
· Interference with vision and a difficult placement.
Classification of occusert:
They are classified on the basis of their solubility. a) Insoluble ophthalmic inserts. b) Soluble ophthalmic inserts. c) Bioerodible ophthalmic inserts. a) Insoluble ophthalmic inserts: Sub classified into The Pharma Research Year: 2009, Vol: 01 78
* Diffusional Inserts.
* Osmotic Inserts.
* Contact Lenses.
Diffusional Inserts: The diffusional inserts are composed of a central reservoir of drug enclosed in specially designed semi permeable, which allow the drug to diffuse from the reservoir at precisely determined rate. The lacrimal fluid permeating through the membrane until the sufficient internal pressure is reached to drive the drug out of reservoir controls the drug release from such a system.
The principle for its operation can be operated by the Fick’s diffusion equation. J=-DAdc/dx J=solute flux D=Difference co-efficient for the drug within the polymer membrane A= Area of membrane dc/dx= Drug concentration gradient within the membrane along the direction of drug flow.
Osmotic Inserts: The osmotic inserts are composed of two distinct compartments. One compartment contains drug and other contains osmotic solute, which is sandwiched between the rate controlling membrane. The tears diffuse into osmotic compartment inducing an osmotic pressure due to which drug diffuses.
Contact Lenses:
· Contact lenses are covalently cross-linked hydrophilic or hydrophobic polymer that forms a three-dimensional network capable of retaining water aqueous drug solution or solid components.
· Currently, approximately 100 million people are estimated to be wearing contact lenses, and the number is increasing exponentially. Although the main use of contact lenses is for correcting ametropia problems.
· Ocular drug administration is particularly challenging and recent research has been directed towards the design of novel drug delivery systems capable of prolonging the permanence of the drug in the precorneal area and, thus, potentially increasing bioavailability and minimizing adverse effects.
· Conventional hydrogel soft contact lenses have the ability to absorb some drugs and release them into the post-lens lacrimal fluid, minimizing clearance and sorption through the conjunctiva.
· Their ability to be a drug reservoir strongly depends on the water content and thickness of the lens, the molecular weight of the drug, the concentration of the drug loading solution and the time the lens remains in it.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
· However, the ability of contact lenses to load drugs and to control their release is in general inadequate and the following approaches,
(i) covalent binding of the drug to the lens network via labile bonds;
(ii) Inclusion of the drug in colloidal structures that are dispersed in the lens and are responsible for controlling drug release;
(iii) Functionalization of the network with chemical groups that work as ion-exchange resins; and
(iv) Creation in the lens structure of imprinted pockets that memorize the spatial features and bonding preferences of the drug and provide the lens with a high affinity and selectivity for a given drug. In this review, the possibilities and the advantages/drawbacks of these new types of contact lenses as drug delivery systems are critically analyzed.
EXAMPLE:-Bionite lens (which was made from hydrophilic polymer:2-hydroxy ethyl methacrylate )
* It is a type of potential drug delivery device in which they are inserted by presoaking them in drug solution.
* It has been seen that most of the drug from contact lens is released within the first 30min.
* The use of preservative benzalkonium chloride has come in question due to its toxic effect on the corneal epithelium.
* So, an alternative approach to presoaking soft contact lenses in drug solutions is to incorporate the drug either as a solution or suspension of solid particles in the monomer mix.
* The polymerization is then carried out to fabricate the contact lenses.
* This technique has demonstrated the promise of longer times of release up to 180h as compared to presoaked contact lenses.
* Here drug is added without any preservative.
· Therapeutic soft lenses are often used to aid corneal wound healing in patients with infection and corneal ulcers.
· Advantages:
- Prolonged delivery
- Controlled rate of release
- Flexibility for type of drug selected
- Sustained release
· Disadvantages:
- Patient discomfort
- Irritation to eye
- Patient placement and removal
- Tissue fibrosis
4. CORNEAL COLLAGEN SHIELDS:
• Collagen is a structural protein, found in bones, tendones, ligaments & skin and applied to body.
• It comprises more than 25% of the total body protein in mammals.
• To prepare collagen shields, collagen is extracted and moulded into a contact lens configuration.
• The shields are 14.5mm in diameter with 9mm base curve and thickness of 0.15-0.19mm.
• The shields are sterilized by gamma radiation then dehydrated and individually packed for storage and shipping.
• Drugs can be incorporated in the collagen matrix during manufacture absorbed into the shields in the eye.
• As the shield dissolves the drug is released gradually in the tear film, maintaining high concentration in the corneal surface and increasing drug permeation through cornea.
• The simplicity of use and the convenience afforded by shields make them an attractive delivery device.
• The study showed that wafer shaped collagen inserts impregnated with gentamicin produced the highest levels of drug in tear film, and tissue, in the rabbit eye compared to drops, ointments and conjuctival injection.
• ADVANTAGE
* Need not to be removed.
* Biological inertness
* Structural stability
* Good biocompatibility
* Low cost of production
• DISADVANTAGE
* It may cause inflammatory response in ocular tissues. If collagen shields are not used along with antibacterial secondary infection may occur.
* Insertion technique is difficult
* Expulsion of the shield may occur
* Not fully transparent and thus reduce visual acuity
VARIOUS CHEMICAL APPROACHES FOR OCULAR DRUG DELIVERY
A) CHEMICAL DRUG DELIVERY SYSTEM (Prodrug):-
· A chemical delivery system (CDS), is an inactive species obtained by chemical modifications of the active agent based on metabolic considerations.
· Conceptually, a CDS upon its administration will undergo several predictable enzymatic transformations via inactive intermediates and finally deliver the active species to the target site.
· In order to enhance the partitioning and corneal bioavailability of topically applied drugs, intensive research is being done on the prodrug approach, which is also a type of CDS.
· This approach to enhance corneal permeability of drug.
· The method includes modification of the chemical structure of the drug molecule, thus making it selective, site-specific, and a safe ocular drug delivery system.
· Ex:- dipivalyl epinephrine,
· o-butyltimolol ester (prodrug)àincrease ocular absorption of timolol 4-6 times.
· Site specific and stereo specific beta blockers(Alprenolol and Betaxolol are also the examples of cdds.
· Prostaglandin F2a ester prodrugs have been reported to have better aqueous stability than prostaglandins.
B) ENHANCEMENT OF PERMEABILITY BY ION PAIR EFFECT
· Most of the beta blockers like TImolol and Carteolol are highly hydrophilic in nature.therefore they suffer permeability problems.
· Preservative serves as penetration enhancer. ex: 0.01% of benzalkonium chloride.
· Addition of sorbitol into the preparation produce ion-pair complex with hydrophilic drug and this ion pair complex has property similar to the lipophilic drug soit can penetrate easily through the corneal epithelium and problem of permeability can be solved.
· Ex:- sodium cromoglycate ion pair with dodecylbenzylmethylbethyl ammonium chloride.
C) SOFT DRUG APPROACH(Targeting of ocular drug):
· It allow localized treatment of the retina without exposing whole body to toxic effect of drug.
· Goal:-maximize ocular drug absorption & Minimize systemic drug absorption
· Systemic toxicity reduced by ‘SOFT DRUG’- defined as biologically active drugs which are metabolized to non-toxic moieties after therapeutic role.
· Optimize time of drug administration. So, increase ratio of ocular to systemic absorption.
Advantages:-
- Good corneal permeability
- Better stability
- Less side effects (bronco-constriction) because site specific activity
D) COLLOIDAL DOSAGE FORMS FOR OCULAR DRUG DELIVERY :3
Colloidal dosage forms have been widely studied and employed in the field of ocular drug delivery These dosage forms include liposomes, nanoparticles, microemulsions, and nanoemulsions etc. Barriers to ocular drug delivery have already been described earlier in the context of structure and function of various ocular tissues and how each tissue can act as a barrier. The chronic nature of many ocular diseases necessitates frequent drug administration. Advantages of colloidal dosage forms include sustained and controlled release of the drug at the targeted site, reduced frequency of administration, ability to overcome blood–ocular barriers, and efflux-related issues associated with the parent drug.
E) MICRONEEDLE-, ULTRASOUND-, AND IONTOPHORESIS-BASED OCULAR DRUG DELIVERY SYSTEMS :3
All these delivery systems are noninvasive methods designed to deliver drugs to intraocular regions, mainly for the treatment of posterior segment diseases. Researchers have developed drug-coated microneedles with a length of 500 to 750 μm. The drug to be delivered can be coated on thesolid metal. Following administration, coated moleculesdissolve rapidly, and subsequently, microneedles are removed from the tissue. This delivery system generates a much higher concentration compared to a free drug solution . Sodium fluorescein and pilocarpine were coated and delivered using a similar technique. In the case of sodium fluorescein, permeation was found to be 60 fold higher, and in the case of pilocarpine, rapid constriction of pupil was observed. Similarly, intrascleral hollow microneedles have also been developed. This delivery system is able to deliver microparticles, nanoparticles, and drugs in a solution with minimal invasion. To deliver microparticles, concomitant administration of spreading enzymes such as hyaluronidase and collagenase is also necessary. These enzymes rapidly hydrolyze the collagenous and extracellular matrix structure of the sclera making the delivery of microparticles feasible.
CONCLUSION:
Ocular drug delivery has to overcome unique barriers. However, several approaches have been shown experimentally to improve ocular drug absorption and the constantly increasing understanding of the absorption processes offers new possibilities in the future.To improve ocular drug bioavailability, there is a significant efforts have been directed towards development of new drug delivery systems for ophthalmic administration. The development of ophthalmic drug delivery systems in the present includes polymeric gels, colloidal systems, cyclodextrins and collagen shields. Hydrogels generally offer a moderate improvement of ocular drug bioavailability with the disadvantage of blurring of vision. In-situ activated gel-forming systems are preferred as they can
REFERENCES:
1. Vyas SP., Khar KR. Controlled Drug Delivery Concept & Advances.1st ed. Vallabh Prakashan; 2002.
2. Dr. ramesh k Goyel, Human Anatomy Physiology and Health Education,7 th edi,B.S. shahPrakashan; 2007-2008.
3. Ripal Gaudana, Ocular Drug Delivery, The AAPS Journal, Vol. 12, No. 3, September 2010.
4. Jain NK, Advances In Controlled and Novel Drug Delivery, CBS Publication,Ed.2011 Page no 85
5. E Lavik, Novel drug delivery systems for glaucoma, Eye : Macmillan Publishers Limited All rights reserved, 2011,25, 578–586.
6. Miki Honda Tomohiro Asai, Liposomes and nanotechnology in drug development: focus on ocular targets, International Journal of Nanomedicine,2013:8 495–504
7. Harish Ravivarapu,Ravichandran Mahalingam and Bhaskara R. Jasti, Biodegradable Polymeric Delivery Systems, McGraw-Hill Companies,U.S.A.,2006. Chapter
9.page no 271 : Design of Controlled Release Drug Delivery Systems.
8. https://www.pharmatutor.org/articles/colon-drug-delivery-system-recent-conventional-and-novel-approach
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 02/012/2014; Accepted On: 13/12/2014; Published On: 15/01/2014 How to cite this article: R Patel, R Mistry, Drug Design Concept in Ocular Drug Delivery, PharmaTutor, 2014, 2(1), 49-61 |









