About Authors:
*Ravi Patel, Dr Shori Thakur
Pharmacy and Pharmaceutical Science Department,
University of Hertfordshire, Hatfield
AL10 9AB, United Kingdom
Ravi_pharmacology@yahoo.com, Ravipatel12345@gmail.com
Abstract:
Oxidative stress play important role in the progression of many diseases. Superoxide anion is a key reactive oxygen species (ROS) produce from univalent reduction of oxygen shows cytotoxicity. Superoxide binds to Nitric oxide (NO) and produces peroxynitrite which is a deleterious ROS. Nitric oxide is produced with help of Nitric oxide synthase (NOS) which catalyse the conversion of L-arginine to citrulline. NO is small and produced in gaseous form which helps it in the diffuse from inside and outside of cells. NO has got many functions like vasodilation, nonspecific immunity, neurotransmission and also neurogenic vasodilation. NO produce from endothelial regulates vascular tone by various mechanisms like it produces vasodilation, inhibit platelet activation and leukocytes adhesion, and inhibit smooth muscle cell proliferation. Thereby NO plays important role in the progression of atherosclerosis so many researches are going on NO. NO also plays important role in the Inflammation and contribute in the organ failure. There are 3 types of NOS from which iNOS is non calcium dependant and can produces NO at very large level compare to other two types of NOS. DEM (Diethyl maleate) is a glutathione depleting agent which binds to glutathione through transferase reaction. It has been seen that DEM reduces the cytotoxicity induce by LPS, protect local skin inflammation, reduces lung injury and also increase survival rate in cerebral ischemia reperfusion. There by it get attraction of scientist for more research. The main aim of the study is to find out the effect of DEM on iNOS expression and cell viability. Results shows that DEM reduces the nitrite production and iNOS expression in LPS treated macrophages in dose dependant manner whereas it does not show any effect in macrophages not treated with LPS. It shows increase in the cell viability in LPS activated macrophages. These results indicate that a glutathione play regulatory role in iNOS expression but the mechanism underlining this is still unknown.
REFERENCE ID: PHARMATUTOR-ART-1797
1. Introduction
1.1 Oxidative stress
Oxidative stress is a condition in which overload of reactive oxygen species (ROS) overpower endogenous antioxidantsystems(Taniyama et al., 2003). When molecular oxygen reduced to water, production of superoxide anion radical, hydrogen peroxide and other hydrogen radical is taking place(Helmut, 1997). ROS has got different effects on each cell in the vasculature and play very important role both as physiological and pathological in various type cells. The main ROS in the vasculature is superoxide. Superoxide (O2·-) is formed from the univalent reduction of oxygen which is catalyse by the several enzymes like NADPH oxidase and xanthine oxidase (Taniyama et al., 2003). Even though superoxide directly acts on the different cells, it also produces some other ROS by reacting with the other substances e.g. superoxide reacts with Nitric oxide (NO)to produce peroxynitrite which is potentiallydeleterious ROS (Helmut, 1997). With the help of superoxide dismutase enzyme, Superoxide dismutation produces more stable ROS like hydrogen peroxide (H2O2) which is then converted to H2O. Hydrogen peroxide can also react with reduced metals and converted into the highly reactive hydroxyl free radicals (·OH). Nearly all types of vascular cells can produce O2·- and H2O2 (Taniyama et al., 2003).
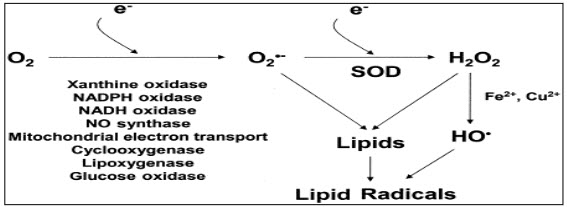
Figure 1: formation of different free radicals.
1.1a Role of ROS
Impaired endothelium dependant vasorelaxation: Some study suggests that ROS plays very important role in the endothelium dysfunction and treatment with the lipid lowering drug with the antioxidant reduces the endothelium dysfunction. Glutathione peroxidase (GPx) is very important for the removal of the ROS (peroxides) and deletion of GPx produces impaired endothelium dependant vasorelaxation (Taniyama et al., 2003).Apoptosis: when the endothelium is expose to the various stimuli its produces ROS which induces the apoptosis and ultimately leads to loss of endothelium cells. Activation of adhesion molecules: In normal condition endothelium present as an inert inflammatory surface but on exposure to the various pro-inflammatory stimuli, induces the activation of adhesion molecules. This leads to adhesion of monocytes on the endothelium cells and produces the atherosclerotic lesion formation. However some other ROS dependant adhesion molecules are also express which are adhesion molecule-1(VCAM-1) and intracellular adhesion molecule-1 (ICAM-1). NO· acts as a antioxidant as it has a antioxidant properties called as scavenging properties and thereby it reduces the VCAM-1 expression. Angiogenesis: angiogenesis is a process of formation of new blood vessel which is very important in the embryonic development, would repair and also in pathological processes like cancer. ROS is known to be involved in the proliferation and migration of endothelial cells and in the intervene lymphocyte-activated tubulogenesis. ROS is also act as intermediary of the angiogenic growth factors e.g. vascular endothelial growth factor (VEGF). Growth of vascular smooth muscle cells: ROS also involve in the regulation of the VSMCs functions. In arthrosclerosis it is found that VSMCs growth is up regulated and ROS is involved in the hypertrophic and proliferative of VSMC growth. Regulation of matrix: Matrix metalloproteinase (MMPs) is involved degradation and reorganisation of extracellular matrix for the physiological and pathological vascular remodelling. It is known that ROS plays important role in the modulation of MMPs activity by the gene expression mechanism. When VSMCs is exposed to the mechanical stretch leads to increase in MMP-2 mRNA. Inflammatory gene expression: Arthrosclerosis is an inflammatory disease where various cytokines are known to involve in the progression of the atherosclerotic lesion. Nuclear factor-?B (NF-?B) is the ROS sensitive transcription factor and plays important role in the inflammatory gene expression and pro-inflammatory gene expression like monocyte chemotactic protein-1 (MCP-1) andinterleukin-6. Angiotensin (Ang II) and TNF-α are also ROS dependant gene inducers which are (Taniyama et al., 2003).
1.2 Role of endothelial
Endothelium is continues layer between epithelial cells and blood. It has important functions like involved in capillary transport, regulation of plasma lipids, control of hemostasis and muscular smooth muscle cells proliferation (Vanhoutee et al., 1986).
This all process is achieved by several mechanisms like,
1) Endothelium acts as a physical barrier between vascular smooth muscle cells, hormones and other vasoactive substances in blood.
2) It metabolise the vasoactive substances like norepinephrine, serotonin and kinins and by doing so it prevents these substrates activity.
3) It plays important role in conversion of precursor like angiotensin to vasoactive products.
4) It secretes vasoactive substances like Prostacyclin.
5) It also secretes some unknown inhibiters in response to vasoactive stimuli (Vanhoutee et al., 1986).
Some study suggests that endothelial dysfunction involved in the development of arthrosclerosis. The underlining mechanism associated with impaired endothelial function and the progression of atherosclerotic disease is unknown(Thomas et al., 2001). Endothelium is having active role in the regulation of vascular homeostasis i.e. endothelium is very important for the acetylcholine –induced vasodilation. Endothelium-derived relaxing factor (EDRF) as nitric oxide regulate the platelet function, vascular growth, remodelling, and adhesion of inflammatory cells to endothelial surface. Endothelium also releases some other factors which influence vasomotor tone, vascular growth, vascular inflammation, thrombosis, and fibrinolysis. So alteration in release of this factor leads to endothelium dysfunction but loss of EDNO (endothelial derived nitric oxide) action play major in endothelium dysfunction. There is an evidence of endothelium dysfunction in the arthrosclerosis progress (joseph and joseph, 2000).
Apart from NO, endothelium regulates vasomotor tone by release of some other vasodilators like prostacyclin; endothelium derived hyperpolarizing factor (EDHF), and adenosine. EDHF is found to play important role when EDNO release become impaired in some diseases. Endothelium also releases some vasoconstrictors substances like endothelin, angiotensin II, thromboxane A2, and some other prostanoid vasoconstrictor. So by balancing release of vasoconstrictor and vasodilator, endothelium regulates vasomotor tone. Endothelium also plays important role in the control of fibrinolysis by producing tissue-type plasminogen activator (t-PA) and plasminogen activator-1 (PAI-1). Heparin surface proteoglycans is also release by endothelium which inhibits thrombosis as like NO and Prostacyclin (joseph and joseph, 2000).
Endothelium is also having important role in the regulation of the inflammatory response which is atherogenesis. In normal condition endothelium provides a barrier for infiltration and attachment of leukocytes. When endothelium cells are exposed to cytokines such as interleukin 1-β (IL-1 β) or tumour necrosis factor alpha (TNF-α), endothelium get activated and release endothelial-leukocyte adhesion molecules including VCAM-1, intercellular adhesion molecule (ICAM-1) and E-selectin, which help in leukocyte binding (joseph and joseph, 2000).
1.3 Oxidative stress and atherosclerosis:
1.3a Atherosclerosis: Arthrosclerosis is a very complex disease and associated with the death of the many people in the develop world and because of these, many research and the study are going on the arthrosclerosis. Arthrosclerosis can be seen in the younger but it gets more complex in the middle aged and elderly (Andrew, 2003). Atherosclerosis is a Greek word, athero means gruel or paste and sclerosis means hardness. Atherosclerosis is a process in which fatty substances, cholesterol, cellular waste products, and calcium which is called as plague, deposits in the inner lining of an artery. Plague formation significantly reduces the blood flow through the arteries and most of the damage occurs when this plague become fragile and rupture. Rupturing of this plague leads to blood clots which obstruct the blood flow. When these plagues get break down, it travel to the other part of the body and this again take part in the blocking another part of arteries and this blocking causes the heart attack. If the plague blocks the blood vessel that provides nutrient to the brain it causes stroke and if the plague block the blood arteries which supply blood to arms/legs leads to gangrene (American heart association, 2009). Formation of atherosclerosis lesions in large and medium-sized arteries lead to ischemia of brain and heart which results in infarction(Russell, 1999).
Arthrosclerosis is associated with the multifactor like hypertension, diabetes mellitus, obesity, smoking, and hypercholestramia but in some cases it also seen to occur with the one of this factor also (Andrew, 2003).
Risk factors associated with the atherosclerosis are,
- High blood cholesterol (especially LDL or "bad" cholesterol over 100 mg/dL)
- Cigarette smoking and exposure to tobacco smoke (free radicals formation)
- High blood pressure
- Diabetes mellitus
- Obesity
- Physical inactivity
- Infection-herpes viruses, Chlamydia pneumonia (American heart association, 2009).
1.3b Pathophysiology of arthrosclerosis: Arthrosclerosis known to affect arteries like aorta, the carotid arteries, arteries of the lower extremities and the coronary arteries. One of the main characteristic of the arthrosclerosis is the presence of the macrophages and foam cells in the arteries. There is a clear evidence of the role of the endothelial smooth muscle proliferation in the development of the arthrosclerosis(Arnold, 2004). All factors which described above affects on the normal endothelium which plays an important role in the disturbing of normal homeostatic and causes endothelium dysfunction leads to progression of atherosclerotic plague formation (Russell, 1999). Exposure of these risk factors to the endothelium leads to increase adhesiveness of the endothelium with reverence to platelets and leukocytes. This injury induce endothelium to produces pro-coagulant instead of anticoagulant properties which cause formation of vasoactive molecules like cytokines and growth factors and if this response is not stop then it will continue indefinitely. The various inflammatory response lead to migration and proliferation of smooth muscle cells which intermixed with the other inflammatory area to form inflammatory lesion. These further thicken the artery wall that is compensates by slow dilation and lumen remain unaltered. This phenomenon is called as “remodelling”. Monocyte-derived macrophages and specific subtypes of T lymphocytes are mainly associated with the inflammatory response. On continuous inflammation, macrophages and lymphocytes are emigrate and multiply at the place of lesion and then become active and release hydrolytic enzymes, cytokines, chemokines and growth factors. These further produces damage and focal necrosis. These cycles of accumulation of mononuclear cells, migration and proliferation of smooth muscle cells and formation of fibrous tissue continues and leads to enlargement and formation of atherosclerotic lesion which is contain fibrous cap which is called as complicated lesion (Russell, 1999).
Endothelial dysfunction: Changes in the endothelial permeability to lipoprotein and other plasma constituents. The normal endothelium permeability is mediated by nitric oxide, prostacyclin, platelet-derived growth factor, angiotensin II and endothelin. So unbalance in these constituents leads to increase endothelial permeability and this leads to formation of atherosclerosis lesions. In the starting stage fatty steaks, it is made of lipid-laden monocytes and macrophages along with T lymphocytes and then in the later stages they combine with the various other smooth muscle cells. Smooth muscle cells migration is stimulated by the various factors like fibroblast growth factor 2, transforming growth factor ß, and platelet-derived growth factor. T-cell stimulation is achieved by the tumour necrosis factorα, granulocyte–macrophage colony-stimulating factor, and interleukin-2(Russell, 1999). Foam cell formation is stimulated by macrophage colony-stimulating factor, tumour necrosis factorα, and interleukin-1 and oxidized low-density lipoprotein, macrophage colony-stimulating factor (Russell, 1999). Platelet adherence and aggregation enthused with the help of integrins, fibrin, P- selectin, thromboxane A2 (Russell, 1999). In the later stage of endothelial dysfunction, formation of fatty steak cap can be seen which is occurs with the help of platelet-derived growth factor, transforming growth factor ß, tumour necrosis factor α, osteopontin, interleukin-1 (Russell, 1999). After the fibrous cap formation, rupturing of the fibrous cap can be seen at the site of thinning of the fibrous cap which occurs due to continuing influx and activation of macrophages that release metalloproteinase and other proteolytic enzymes which leads to thinning of fibrous cap. Release of this enzymes leads to degradation of matrix which causes thrombus formation and occlusion of the artery (Russell, 1999).
1.3c Role of oxidative stress and atherosclerosis:It has been known that reactive oxygen species(ROS) plays very important role in the progression of the atherosclerosis including smooth muscle cell proliferation and activation, apoptosis of endothelial cells, oxidation of lipids, stimulation of adhesion molecules. Oscillatory shear which is present at the site of the atherosclerotic lesion is known to stimulate the superoxide production. NADPH oxidase activation by angiotensin IIis also found to play important role in the long time production of ROS. Decreased concentration of the vascular extracellular superoxide dismutase enzyme is also seen in the coronary artery disease (David et al., 2003).
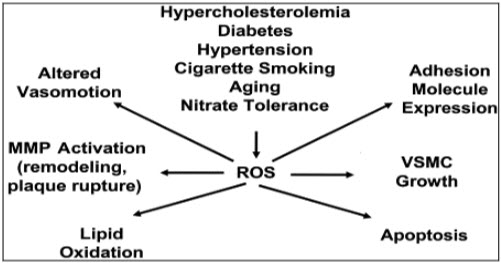
Figure 2: role of ROS
ROS start the disease progress with the help of the enzymes like xanthine oxidase, nicotinamide adenine dinucleotide phosphate oxidase and NOS (David et al., 2003).
These enzymes utilize different substrates as a source of electron which reduces molecular oxygen to produce a different ROS. If one electron reduction of oxygen occurs, leads to generation of superoxide and if two electron oxidation of oxygen occur leads to formation of hydrogen peroxide. Other different ROS (lipid radicals, thiyl radicals, hydroxyl radical, hypochlorous acid and carbon-centred radicals) are produces from superoxide and hydrogen peroxide (David et al., 2003). The increase oxidative stress plays very important role in the endothelial dysfunction(Thomas et al., 2001). Some hypothesis suggests that imbalance between increased oxidative stress and impaired antioxidant defence that not only affect the endothelial function but also contribute to atherosclerotic disease progression (Heitzer, Schlinzing, Krohn, Meinertz, & Munzel, 2001). When the endothelium (ECs) and vascular smooth muscle cells (VSMCs) exposed to extracellular stimuli leads to production of the ROS which ultimately leads to various alteration in the cellular function. This alteration contributes in the vascular dysfunction (Taniyama et al., 2003).
1.4 Nitric oxide: NO is a key molecule in the cell signalling for the regulation of various pathological and physiological process. Endothelium cells produce NO from L-arginine and oxygen by either Ca2+ independent or dependant endothelial isoform of nitric oxide synthase by the nitric oxide pathways(Joseph and Joseph, 2000). NO production is taking place by two mechanism, flow dependant NO production and receptor-mediated NO production. When there is an increased in the blood flow or shear stress that active calcium channel this leads to activation of cNOS and leads to production of NO. Where as in other mechanism due to sensitivity of the different ligands to receptor it leads to increased in calcium production and subsequently leads to iNOS expression and NO production(cardiovascular physiology concepts, 2008). NO is produced in the gaseous form has very small molecules size and thereby it can diffuse easily into and outside the cell. NO reacts with haem groups, cysteine residues, iron and zinc clusters thereby it shows its effect in the cell. (Reproductive and cardiovascular disease research group, 2009)
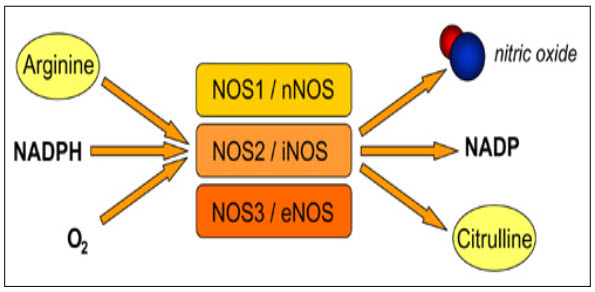
Figure: 3 NO production (Reproductive and cardiovascular disease research group, 2009)
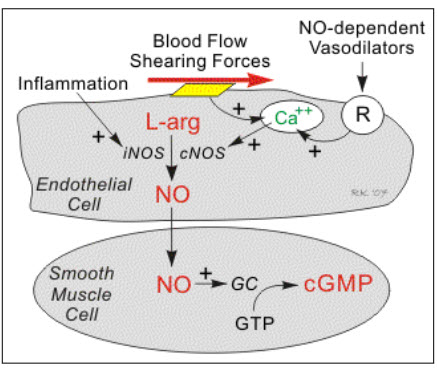
Figure 4: Mechanism of NO production (cardiovascular physiology concepts, 2008).
NO produced by the calcium dependant NOS, maintain the gastrointestinal mucosal integrity where as NO produced by the calcium independent NOS was found to be involved in both cell protection against the cytotoxins and also for acts as a cytotoxic to cells. NO binds to the reactive oxygen metabolites and generate peroxynitrite and leads to instigate lipid peroxidation which acts as a cytotoxic (damage cell membrane). This cytotoxicity can be reverse by the treatment of the NO synthase inhibitors (Candice & Barry, 1997). Study suggests that concentration of production of NO reflect the degree of inflammation(Hwa et al., 2008).
Role of NO:
Vasodilation: No produces in the endothelial plays very important role in the vasodilation which is very important in the control of blood pressure. NO react with the ferrous iron and increases the concentration of cyclic GMP that leads to relaxation of vascular muscles. NO produced from the platelet and endothelial are involved in the control of platelet aggregation through cyclic GMP mechanism by binding with prostacyclin. NO also play role in the inhibition of leukocytes activation and inhibition of smooth muscle cells proliferation (Moncada & Higgs, 1993).
Neurotransmitter: NO acts as a neurotransmitter in the central nervous system and involved in the development of memory.
Neurogenic vasodilation: NO control nonadrenergic and noncholinergic nerve system for the neurogenic vasodilatation and to control different gastrointestinal, respiratory tract functions.
Nonspecific immunity: No produce from macrophages acts as a cytotoxic and plays important role in the host defence in the inflammation reaction(Salvador& Annie, 1993).
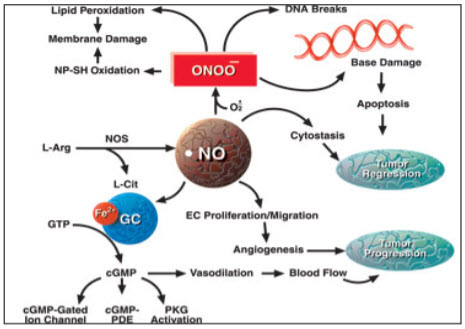
Figure 5: Nitric oxide pathway(Daily strength, 2009).
1.4a NOS: There are mainly 3 different types of NOS has been identified and they are named depending on their activity or first place where they identified- Neuronal NOS (nNOS), Inducible NOS (iNOS) and Endothelial NOS (eNOS). Most tissues have more than one isoform of NOS. All of these three types of NOS are involved in the NO production but in the different or some part of body. nNOS is present in the brain is involved in production of NO in neurons , vascular endothelium, glial cells, astrocytes, vascular smooth muscle, and perivascular nerves(Julio & Richard, 1999). nNOS can be expressed in sarcoplasmic reticulum of cardiomyocytes. eNOS is expressed in predominantly in coronary vascular and endocardial endothelial cells and also in cardiomyocytes but at very low level. The major advantage of iNOS is that it can be expressed in cardiomyocytes, infiltrating inflammatory cells, coronary vascular smooth muscle, fibroblast and other areas (Hwa et al., 2008).
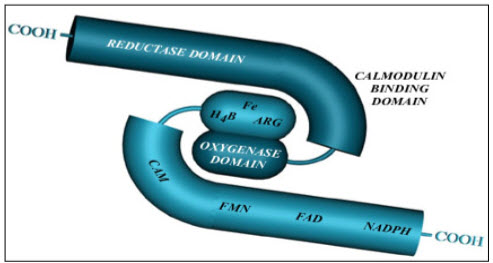
Figure: 6 Common structure of NOS (Reproductive and cardiovascular disease research group, 2009)
NOS contain 2 sub-unit which have 3 domain: Calmodilin binding domain, Reductase domain and Oxygenase domain.Reductase subunit conatain the FMN and FAD which help in transfer electron from NADPH to Oxygenase domain. As calmidulin binding is very important for NOS induction, this site plays very importnat in the NO production. Whereas oxygenase domain served as a binding site for the L-arginine (Reproductive and cardiovascular disease research group, 2009).
iNOS can be expressed in almost all type of cells with on proper stimulation and first identified in macrophages. eNOS and nNOS are the Ca2+ regulated isoform whereas iNOS is Ca2+ independent isoform (Hwa et al., 2008). iNOS activity is not depends on the intracellular Calcium because it has a calmodulin bound as an internal subunit. iNOS promoter contains two transcriptional regulatory regions, an enhancer and a basal promoter (Jianjun et al., 1997). Unlike eNOS and nNOS, iNOS is a soluble enzyme and does not requiredincrease intracellular Ca2+ concentration for it activation. Another advantage of iNOS is that it can produces much more amount which last longer compare to NO produced from other type of NOS (Reproductive and cardiovascular disease research group, 2009).
1.4b Mechanism of iNOS expression: As NO is very play both as defence and cytotoxic molecules its regulation is very important which is achieved by cellular distribution of NO, changes in gene expression, enzymatic activation (Reproductive and cardiovascular disease research group, 2009). Macrophages take part in the early immune response in response to micro organism and produce NO as a defence substance. Treatment of murine macrophages to the LPS, cytokines (including interferon-y [IFN-y], interleukin-l [IL-l], and tumor necrosis factor [TNF]), lead to synthesis of NO in microphages (Weinberg, et al., 1995).Many inflammatory cytokines like IL-1beta, TNF-alpha, IFN-gamma, are known to plays role in regulation of iNOS gene expression. NF-kB, interferon regulatory factor (IRF)-1 and cAMP responsive element binding protein (CREB) has been found to play key role in transcriptional regulation of iNOS gene expression. Other experiment also suggests that Intracellular mitogen-activated protein (MAP) kinase and protein kinase (PKC) phosphorylation pathways also play role in iNOS expression. (Joseph loscalzo, 2000). NO production is take place through Janus kinas (JAK) family and signal transducers and activators of transcription (STAT) protein via IFN-g receptor signals. After binding of cytokines to receptors it shows receptor dimerization and leads to phosphorylation of STATs. These activated STATs then dimerize and translocate to nuclear and shows increase expression of transcription factor, IRF-1 which further bind to specific DNA elements of iNOS gene promoter region and increase iNOS expression (Inductible Nitric Oxide, 2008).One study suggests that an interferon-g-activated site (GAS) is very important for the complete expression of iNOS gene. STAT (signal transducer and activator of transcription) is a cytoplasmic protein, when treatment IFN-g leads to phosphorylation of STAT a which is then translocate into nucleus where it show transcription of GAS- containing genes(Jianjun Gao, 1997).The functional promoter elements for the regulation of iNOS expression is located at upstream of -4.7 kilobases (kb) with unique enhancer region having 4 functional nuclear factor kappa B elements (Taylor & Geller, 2000).One study also suggests that iNOS gene expression in macrophages is regulated by NF-κB. In normal cells NF-κB is located in the cytosol as a homodimer or heterodimer and this is linked with inhibitory IκB protein (IκB). Phosphorylation of IκB leads to activation of NF-κB. This leads to degradation of NF-κB and translocation of NF-κB in the nucleus. NF-κB is involved in the regulation of inflammatory response by increasing specific gene expression. There are two types of NF-κB has been identified in macrophages (Hwa et al., 2008). Disturbance in the NOS pathway is occurs by alteration in signal transduction by lipoprotein, increase in superoxide anion production and decrease in affinity of NOS for L-arginine(Claudio & Louis, 2001).
1.5 Glutathione and Diethyl maleate Glutathione (gamma- Glutamylcysteineglycine or GSH) is produced in liver and acts as intracellular antioxidant with numerous and composite biological functions. GSH’s protein is made of three amino acids like cysteine, glutamic acid and glycerine. GSH is known to be in two forms: Reduced GSH (active form) and Oxidisized GSH (inactive form).Oxidized GSH get converted to reduced form by glutathione reductase (Glutathione Health Article). GSH maintain the constructive redox potention to defend the cell from oxidative stress. GSH play important role in the revival of sulfahydryl and antioxidants. GSH act as a substrate for the seleno-protein which convert hydrogen peroxide to water. It also converts hydroperoxide to water and alcohols. Both of these actions inhibit formation of hydroxyl, peroxyl and alkoxyl radicals. GSH maintain the level of cysteine production which is useful for glutathione as well for the other protein (Glutathione in Cell Culture)Glutathione has got some important functions like invovled in the protein synthesis, DNA synthesis and also protecting cells from harmful effect of free radicals by its antioxidant propertie which allow it to binds to various peroxides or oxygen free radicals. After binding with oxidants, GSH get converted to oxidised glutathione which is then converted to reduced glutathione by glutathione reductase(Hothersall, et al., 1997). GSH act as cofactor for iNOS thereby it converted to S-nitrosoglutathione(GSNO) by combining with NO and control the release of NO (Marina et al., 2000).DEM is a electrophilic agent and it binds to glutathione to form the thioester conjugate which is catalyse by the glutathione s- transferase enzyme(Candice & Barry, 1997). It has been seen that DEM reduces the cytotoxicity induced by the LPS like hyporeactivity,hypotension and has a protective effect in the local skin inflammation, reduces the ling injury and also increase the servival rate in the cerebral ischemia-reperfusion. Glutathione plays very important role in the regulation of iNOS expression in Macrophages (Keon, 1999).
Aim of the project: Aim of the project is to determine the effect of Diethyl maleate on nitrite production and iNOS expression and there by finding out the possible mechanism by which DEM effect on Nitrite production.
2. Material and method
2.1 Cell culture technique: is a technique in which the cells are grown under managed condition.
2.1a Preparation of the culture media: In the aseptic condition, Culture media is prepared with the use of the DMEM(Dulbecco’s modified eagle’s medium), FBS and penicillin.
2.1b Recovery of Frozen Cell Lines: Most of all cells culture are available in the frozen condition and need to recover their normal growth pattern before using them in the experiment.
Prepare culture media with use of DMEM (500ml), 10%FBS (50ml), and penicillin (10mg/ml). Label the flask with cell line name, passage number and date. Take the ampoule of the cells from the liquid nitrogen storage and put in the water bath and warm it at 37°C temperature. Spray ampoule with 70% IMS and take it to cabinet. Transfer the cells from the ampoules to the T-75 flask slowly, drop wise and add sufficient ml of the culture media. Incubate the flask at 37° and at 5% CO2. Examine the cells microscopically after 24 hours and sub-culture as necessary.
2.1c Subculture of Adherent Cell Lines: Cells are growing in the culture media should be subculture as after few days depletion of the constituent can be observed in the culture media. So to avoid culture dieing, subculturing is very important. When cell lines covers around 80-90 % of the flask surface it is ready for the sub culturing.
Take off the entire medium. Wash the cell lines with the 10% PBS for 5 minutes with the slight sacking. Add 2.5ml of the culture medium to the flask. Scrap the cell lines with the scraper. Take 1.25ml of the scrap cells medium and transfer to the T-75 flask and make up the volume up to 20ml with the culture medium. Repeat the step 5 in the T-75. Incubate the cells at 37°. Examine the cells next day.
2.1d Haemocytometer or cell counting: In a sterile condition make the cells suspension as explain above and take 10µl of the cell suspension and mix with the equal volume of the trypan blue. Mix the mixture properly. Clean the haemocytometer properly. Slightly moisten the cover slip with exhaled breath or water and then coverslip is slip over the chamber back. Load around 5-10µl of the cell suspension on the both side of the chamber. Observe under the microscope using x20 magnification. Count the cells (both viable and non-viable) in the 4 squares (viable seen as bright and non-viable look like stained blue). Take the average of the 4 squares and calculate the total number of viable cells and total number of cells with the use of below equation.
A stands for mean number of viable cells counted, B stands for mean number of non-viable cell counted, C is the dilution factor and D is correction factors.
Concentration of viable cells (cells/ml) = A x C x D
Concentration of non-viable cells (cells/ml) = B x C x D
Total number of viable cells = concentration of viable cells x volume
Total number of cells = number of viable + number of dead cells
Percentage Viability = (No of viable cells x 100) divided by Total No of cells.
For the nitrite and MTT assay, we required around 3x10-6cells/ml so calculate the required concentration of cells suspension to make 25 ml of medium which will have 3x10-6cells/ml. Prepare 96 well with use of cell suspension.
2.2 Nitrite assay: The nitrite assay is very simple and sensitive assay used for the quantitevely determination of the nitrite or nitrate in urine, blood, saliva, and serum, cell lysate and tissue culture medium. When nitrite is treated with griese reagent it leads to production of purple colour azo compounds. So by detecting the colour intensity we can find out how much nitrite are present in the sample.
2.2a Standard nitrite assay: Prepare 1mM stock solution of the sodium nitrite with the use of DMEM.Now prepare different concentration of the sodium nitrite i.e. 0µl, 10µl, 20µl, 30µl, 40µl, 50µl, 100µl with the use of DMEM. Add 100µl of each of the standard in triplicate to 96 well plate. Add 100µl of the Griese reagent mixture to each of the wells. Leave it for 30 minute at the room temperature. Read the absorbance at 540nm on the multiskan plate reader.
2.2b Nitrite assay with the Drugs (DEM): Prepare the 96 well plates with the use of the (3x10-6cells/ml) cell suspension in the sterile condition. Incubate for 24-48hrs to make it confluent. Take out the media from the 96 wells and add different concentration of the drug (0µM, 1µM, 2.5µM, 5µM, 10µM, 25µM) as triplicate and label first 5 wells as a control and remaining 5 as an active. Incubate the plate for 1 hrs. Take out the media from the 96 wells add different concentration of the drug (0-25µM) and LPS (1 µM/ml), first 5 wells as a control (without LPS) and remaining 5 as an active (with LPS). Incubate the plate for 24 hrs. Take 100 µl of the medium from the each well and use it for the nitrite assay. Add 100 µl of the griese reagent and leave it for 30 minutes at the room temperature. Read the absorbance at 540nm on the multiskan plate reader.
2.3 MTT assay: MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide) assay is quantitative determination of enzyme activity and cell proliferation or cell viability. MTT is a yellow colour and it gets reduced to purple colour in the living cells. Ethanol is used to dissolve the insoluble formazan crystals. A death cell does not give any colour so this assay use for determination of the cell viability (MTT Assay).
Procedure:Prepare the 96 well plate with cell suspension (3x10-6cells/ml) and add different drug concentration (0µl, 1µl, 2µl, 5µl, 5µl, 10µl, 25µl) as like nitrite assay. Incubate plate for 1 hrs and then add different drug concentration to the control and add drug + LPS (1µm) to the active. Incubate it for 24 hrs. Take out the entire medium from the wells and add 20µl of 5mg/ml of MTT to each well and incubate for 2 hrs at 37°. Remove the solution from the well and add 100µl of isopropranol. Incubate for 10 minutes on the shaker and read the plate at 540nm (Proliferation Assay, MTT assay, 2009).
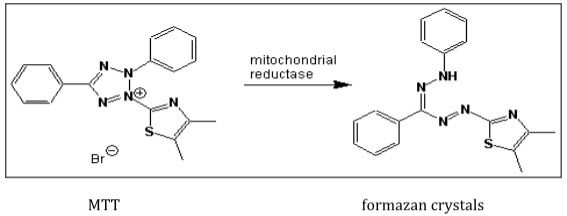
Figure 7: mechanism of MTT assay (Proliferation Assay, MTT assay, 2009).
2.4 Cell lysis: Plate the macrophages cells (3x10-6cells/ml) in the 24-well culture plate and incubate it for 24hrs. Remove the media and add different drug concentration (0µl, 1µl, 2.5µl, 5µl, 10µl, 25µl) to the wells (control and active). Incubate for 1 hr and then add again different drug concentration in the control and add drug+LPS (1µg/ml) in the active and incubate for 24 hrs. After incubation period take out the media from the wells and wash each well with ice-cold 10% PBS. Add lysis buffer to lyses the cells and place on the ice for 5 minutes. Now take the lysate out carefully from the wells to the apendoph tubes and heat at 95° for 5 minutes. Then centrifuge the lysate at 10000rpm for 5 minutes and store at 4 c.
2.5 SDS-PAGE: Wash the gel plates with the water and then with 70% IMS and dry it properly and join the gel plates collectively to form the cassette. Add few ml of the distilled water to the gel plates and leave it for 5 minute to check leaking of the plate. Remove water properly and Prepare 15% SDS gel mixture and then de-gas the mixture and add TEMED. Carefully add 3-3.5 ml of 15% SDS gel mixture to the plate cassette from the side of the cassette and then add few 500µl of distilled water on the top of the each gel to make the surface uniform. Leave it for the set. When it get set remove the water with the use of filter paper and prepare 4.05% of stacking gel, de-gas it before adding TEMED. Add few ml of the stacking gel on the 15% SDS gel mixture and put the well forming comb to the cassette until solution reaches the cutaway edge of the plate. Leave it for set. When it gets set carefully remove the well forming comb and accumulate the cassette in the electrophoresis tank. Fill up central reservoir with the electric buffer. Prepare the loading sample by mixing the sample with the sample blue (1:1) and fill the samples lysates and the protein ladder in the wells. Now carefully fill out the electrophoresis tank up to ¾ with the electrode buffer. Connect to the power pack and gel is now run at 200V. After some time gel samples band can been seen to move downwards and switch off the power pack when the gel runs up to the bottom. Carefully take the cassette out the electrophoresis tank and take out the gel carefully from the plate. Put the gel in the blotting solution and can be use for the western blotting.
2.6 Western blotting: After electrophoresis, wash the gels with the blotting buffer to remove salts and detergents. Wash the nitrocellulose membrane with the methanol for 5 minutes and then wash with blotting buffer for 15 minutes. Saturate the fibre pad and filter paper in the blotting buffer for 30 minutes. Place the fibre pad on the clear panel (+ve) side, then place filter paper, membrane, gel ,filter paper, and fibre pad in the same order to make a “sandwich”. Then carefully close the gel holder and place the gel in the tank in the way that black panel in the side of cathode or on the black side. Put some ice box to keep the tank cool during the transfer process. Fill out the tank with the blotting buffer up to 800mls.now put the lid in place and turn on the power pack at 350 mA for around 2 hours(Michael & Fiona, 1998).
2.7 Immunodetection of blots: Leave the membrane paper for 24 hrs in the blocking solution at 4 d to block the remaining binding sites on the nitrocellulose membrane. Pour all blotting solution and wash membrane 6×5 times with washing solution. Prepare mouse anti-iNOS antibody with the use of blocking solution (1:2500) and add 10 ml of solution to the membrane. Leave it for 24 hrs. After 24 hrs take out primary antibody solution and wash the membrane 6×5 times with washing solution. Prepare the secondary antibody of horseradish peroxidase-conjugated antimouse IgG (1:5000) and anti biotin (1:5000) with washing solution. Add 10 ml of the secondary antibody solution to the membrane and leave it for 1 hrs. Wash the membrane 6×5 with the washing solution and then visualize the bands using the ECL detection.
Results
Nitrite assay: For the determination of the effect of DEM on the nitrite production, Nitrite assay is used here. Nitrite standard curve is obtained with the help of the sodium nitrite reagent with the gradually increase in the concentration. In nitrite standard curve (figure 1) there is a gradually increase in the nitrite concentration can be seen with increase in the sodium nitrite concentration. The R2 value was found to be 0.998.
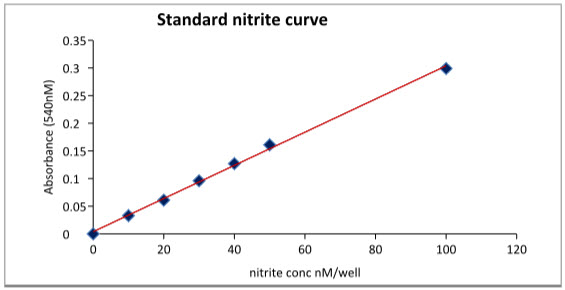
Figure 8: Standard nitrite curve
|
Active |
Control |
||
|
Drug concentraion |
Nitrite production |
Drug concentraion |
Nitrite production |
|
μM |
µg/protein |
μM |
µg/protein |
|
0 |
0.45 |
0 |
0.04 |
|
1 |
0.39 |
1 |
0.04 |
|
2.5 |
0.3 |
2.5 |
0.03 |
|
5 |
0.25 |
5 |
0.03 |
|
10 |
0.21 |
10 |
0.03 |
|
25 |
0.17 |
25 |
0.03 |
Table 1: Nitrite results for control and active
The Nitrite curve for the different concentration of the DEM with LPS (active) and without LPS (control) is obtained (figure2) for the comparison. Here there is increased concentration of the nitrite can be seen in the active group at 0µM DEM concentration as compare to control. There is a significant decreased in the nitrite production can be seen with the increase in the DEM concentration whereas there is no effect can be seen in the control.
|
Active |
||
|
DEM concentration |
Nitrite Production |
SED |
|
µM |
µg/protein |
|
|
0 |
0.45 |
0.021 |
|
1 |
0.39 |
0.002 |
|
2.5 |
0.3 |
0.01 |
|
5 |
0.25 |
0.009 |
|
10 |
0.21 |
0.004 |
|
25 |
0.17 |
0.007 |
Table 2: Nitrite production in active
|
Active |
||
|
DEM concentration |
Nitrite Production |
SED |
|
µM |
µg/protein |
|
|
0 |
0.45 |
0.021 |
|
1 |
0.39 |
0.002 |
|
2.5 |
0.3 |
0.01 |
|
5 |
0.25 |
0.009 |
|
10 |
0.21 |
0.004 |
|
25 |
0.17 |
0.007 |
Table 2: Nitrite production in active
|
Control |
||
|
Drug concentraion |
Nitrite production |
SED |
|
µM |
µg/protein |
|
|
0 |
0.04 |
0.01 |
|
1 |
0.04 |
0.01 |
|
2.5 |
0.03 |
0.007 |
|
5 |
0.03 |
0.009 |
|
10 |
0.03 |
0.005 |
|
25 |
0.03 |
0.009 |
Table 3: Nitrite production in control
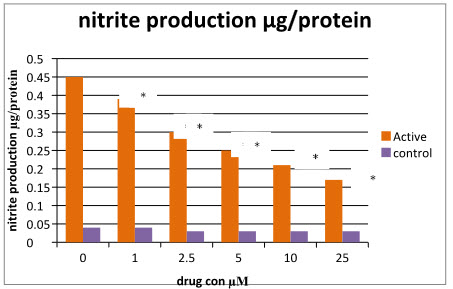
Figure 9: Nitrite curve of control and active.
MTT assay: as mention earlier MTT is used for the determination of the cell viability or cell proliferation. In figure, it can be seen that DEM does not show any significant effect on cell viability in the control group. There is a significant decrease in the cell viability can be seen in the active at 0µM DEM. There is a increase in the cell viability can be seen with increase in the concentration up to 5µM of drug concentration after that it does not show any significant increase in cell viability with increase in concentration.
|
MTT assay |
||||
|
Drug concentration µM |
Control |
% cell viability |
Active |
% cell viability |
|
0 |
0.461 |
100 |
0.3308 |
72 |
|
1 |
0.434 |
94 |
0.364 |
79 |
|
2.5 |
0.482 |
105 |
0.3898 |
85 |
|
5 |
0.47 |
102 |
0.421 |
91 |
|
10 |
0.42 |
91 |
0.3866 |
84 |
|
25 |
0.379 |
82 |
0.374 |
81 |
Table 4: MTT results of Active and Control
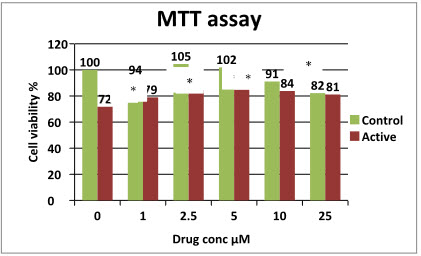
Figure 10: MTT results of control and active.
BCA assay: As protein content in the samples can interfere with the nitrite assay its required to obtained the standard BCA curve (figure 4) which is used in the determination of the different protein content in the samples (active and control). Standard curve shows the gradually increase in the protein concentration with increase in protein content.
|
Standard |
||
|
Concentration (µM) |
Absorbance |
Actual absorbance |
|
0 |
0.099 |
0 |
|
12.5 |
0.103 |
0.004 |
|
25 |
0.107 |
0.008 |
|
50 |
0.116 |
0.017 |
|
100 |
0.13 |
0.031 |
|
150 |
0.138 |
0.039 |
|
200 |
0.15 |
0.051 |
|
250 |
0.16 |
0.061 |
Table 5: BCA standard curve results
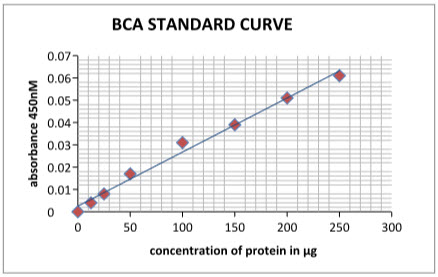
Figure 11: BCA standard curve
Western blotting: Western blot analysis for the iNOS expression is done in both active and control group of macrophages.
In LPS-DEM treated macrophages (Active) there is clear bands for the iNOS can been seen which indicate inhibition of iNOS expression. The intensity of the colour depends on the extend of inhibition. Here at 0µM DEM concentration there is a clear band for iNOS which indicates iNOS induction with LPS treatment. When different concentration of DEM (1µM, 2.5µM, 5µM, 10µM,25µM) are added there is clear decreased in the colour intensity of iNOS band can seen which indicates DEM has inhibitor effect on iNOS. At 25µM DEM concentration there is complete inhibition of iNOS is observed.
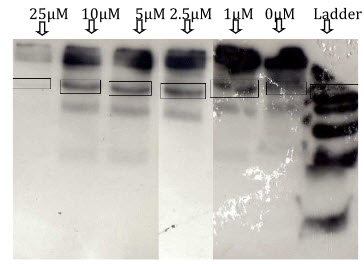
Figure12: Determination of iNOS expression in LPS+DEM treated macrophages (active).

Figure 13: Decreased in the intensity of iNOS band colour in LPS+DEM treated macrophages with increase in DEM concentration(active).
|
Western blotting of iNOS |
|
|
DEM concentration |
% Expression |
|
0 |
100 |
|
1 |
84 |
|
2.5 |
26 |
|
5 |
14 |
|
10 |
9 |
|
25 |
3 |
Table 6: Western blotting of iNOS
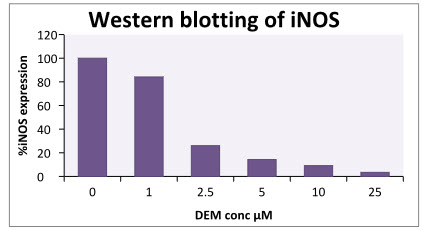
Figure 14: Determination of %iNOS expression in LPS+DEM treated macrophages.
In control there is no band for iNOS expression is observed which indicate that in control DEM does not shows any effect on iNOS. (In control there is band for iNOS at 2.5µM which is mainly due to some experimental error).
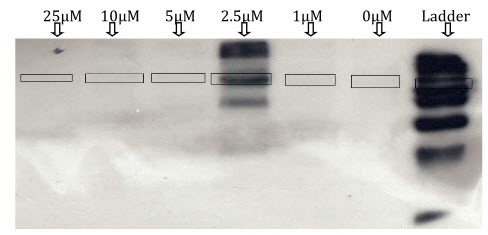
Figure 15: determination of iNOS expression in the control.

Figure 16: western blotting of iNOS without LPS treatment (control).
Discussion:
The main purpose of present study is to determine the effect of the DEM on the iNOS expression in the LPS treated and without LPS treated macrophages. For this Nitrite assay, MTT assay (cell viability) and western blotting was performed.
LPS is a nitrite synthase inducer and when macrophages are activated with the LPS it shows increased in the nitrite production by the calcium independent NOS enzyme (iNOS) (Candice & Barry, 1997). When Macrophages cells are treated LPS and INF-γ its leads to production of large amount of nitrogen (nitrite) and reactive oxygen species because of their microbicidal and tumoricidal activity (Yolande, et al., 1995). Here LPS concentration of 1µg/ml is used for the activation of the cells because this concentration shows good activation of macrophages cells and the less toxicity. In control, macrophages are not treated with the LPS, thereby cells are not activated with LPS and so there was no iNOS expression in the control and very less amount of nitrite production can be seen which is due to experimental errors. Moreover there is not significant decrease in the nitrite production is seen with different concentration of DEM in control. Whereas in LPS activated macrophages, there is a macrophages cells activation can be seen at the 0µM DEM concentration and therefore there is a significant increased in the nitrite production is seen compare to control. There is a significant decrease in the nitrite production with the increase in the DEM concentration was seen which indicates that DEM has effect on the nitrite production in the LPS activated macrophages. To know whether decrease in the NO production involved iNOS expression, western blotting of iNOS was done. Western blotting of iNOS shows that, with increase in the concentration of DEM in LPS activated macrophages inhibits iNOS expression. This indicate that decreased in the NO production is mainly due to inhibition of iNOS expression by DEM. This results supports to the previous work by Young et al.,1999 that DEM is a glutathione depleting agent which decreased nitrite production and iNOS expression both in the macrophages and hepatocyte after activation with LPS. This indicates there is a relation between the glutathione and iNOS expression (NO production).
Study by keon and his colegue shows, DEM was found to significanttly decrease the glutathione level in the LPS treated macrophages. In their study they found that DEM signicantlly decrease the nitrite production, glutathione level and interleukin-1b (IL-1b) level but fail to show any effect on the tumor necrosis factor-a (TNF-a ). These results shows that iNOS inhibition is mainlly due to inhibition of the interleukin-1b not due to tumor necrosis factor-a(Young et al., 1999). NO and glutathione required NADPH as a cofactor and when NO production is inhibited there is also decreased in NADPH concentration, so there may be NADPH play crucial role in understanding the relation between glutathione and NO production (Hothersall, et al., 1997). When Nitrite production is increase, it produces oxidative stress and increase in the GSH level has been observed (Chun, et al., 2005). With this study there is decrease in the GSH level with increase in nitrite production was also observed in the other study by walker and his colleague (1995).
When macrophages are activated with the LPS, it show significant increase in the nitrite production with that it also shows significant decrease in the cell viability as compare to control which means excessive NO production is act as cytotoxic agent. Significant increase in the cell viability can be observed at the DEM concentration of 1μM, 2.5μM, 5μM. Whereas at DEM concentration of 10μM, and 25μM did not show significant changes in cell viability. These means inhibition of NO production provides the protective effect to the cells and this results support to work that glutathione depleting agents like DEM play important role in the reduction of the cell toxicity by LPS there by it reduces the lung injury (Nathew et al., 1996) and also local skin inflammation (Jones et al., 1997). Increase in the cell viability may be due to decrease in the cytotoxicity by decrease in NO production by DEM.
Conclusion:
Results shows that glutathione depleting agent DEM decreases the NO production and iNOS expression in LPS treated macrophages which indicates there is a corelation between glutathione and NO production. DEM also shows increse in the cell viability which may be due to reduction in production of NO which serve as cytoprotective action.
Future work: Glutathione depleting agent DEM has protective effect in LPS induce hyporeactivity and hypotension, local skin inflammation, reduces the lung injury and increase in survival rate the cerebral ischemia-reperfusion. In this study it can be seen that DEM reduces the NO production and iNOS expression which can be useful in the treatment of many disease. So underlying exact mechanism involved in the iNOS expression by Glutathione need to be study which will play very important role in treatment of atherosclerosis and other diseases.
References
- Atherosclerosis. (2009, Sep 29). Retrieved from American heart association: http://www.americanheart.org/presenter.jhtml?identifier=4440
- Balligand, J. L., Longrois, D. U., Simmons, W. W., Pimental, D., Malinski, T. A., Kapturczak, M., et al. (1994). Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J. Biol. Chem , 269 (44), 27580-27588.
- Eckardstein, A. v. (2004). Atherosclerosis: diet and drugs. Zurich: Springer publication.
- Gao, J., Morrison, D. C., Parmely, T. J., Russell, S. W., & Murphy, W. J. (1997). An Interferon--activated Site (GAS) Is Necessary for Full Expression of the Mouse iNOS Gene in Response to Interferon- and Lipopolysaccharide. The journal of biological chemistry , 272 (2), 1226-1230.
- Glutathione Health Article. (n.d.). Retrieved from Health line: http://www.healthline.com/galecontent/glutathione
- Glutathione in Cell Culture. (n.d.). Retrieved from Sigma-Alorich: http://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/glutathione.html
- Harrison, D., Griendling, K. K., Landmesser, U., & Drexler, H. (2003,). Role of oxidative stress in atherosclerosis. The American Journal of Cardiology , 91 (3), 7-11.
- Heitzer, T., Schlinzing, T., Krohn, K., Meinertz, T., & Munzel, T. (2001). Endothelial Dysfunction, Oxidative Stress, and Risk of Cardiovascular Events in Patients With Coronary Artery Disease. American heart association , 104, 2673-2678.
- Helmut, S. (1997). OXIDATIVE STRESS: OXIDANTS AND ANTIOXIDANTS. Experimental physiology , 82, 291-295.
- Hothersall, J. S., Cunha, F. Q., Neild, G. H., & Norohna-Dutra, A. A. (1997). Induction of nitric oxide synthesis in J774 cells lowers intracellular glutathione: effect of modulated glutathione redox status on nitric oxide synthase induction. Biochemical journal , 322, 477-481.
- Inductible Nitric Oxide. (2008). Retrieved from SIGMA-ALORICH: http://www.sigmaaldrich.com/life-science/cell-biology/learning-center/pathway-slides-and/inductible-nitric-oxide-synthase-inos.html
- Jones, J. J., Macgilvray, I. D., Nathens, A. B., Bitar, R., & Rotstein, O. D. (1997). Glutathione Depletion Prevents Lipopolysaccharide-Induced Local Skin Inflammation. Arch Surg , 132 (11), 1165-1170.
- Kang, K. K., Pak, Y. M., & Kim, N. D. (1999). Diethylmaleate and Buthionine Sulfoximine, Glutathione-Depleting Agents, Differentially Inhibit Expression of Inducible Nitric Oxide Synthase in Endotoxemic Mice. Nitric oxide , 3 (3), 265-271.
- Klabunde, R. E. (2008, september 23). nitric oxide. Retrieved from cardiovascular physiological concepts: http://www.cvphysiology.com/Blood%20Flow/BF011.htm#NO_Biosynthesis
- Lee, H. J., Jeong, H. S., Kim, D. J., Noh, Y. H., Yuk, D. Y., & Hong, J. T. (2008). Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264.7 cells. Archives of Pharmacal Research , 342-349.
- Lia, C. Q., Wrighta, T. L., Donga, M., Dommelsa, Y. E., Trudela, L. J., Dedona, P. C., et al. (2005). Biological role of glutathione in nitric oxide-induced toxicity in cell culture and animal models. free radical biology and medicine , 39 (11), 1489-1498.
- Loscalzo, J., & Vita, J. A. (2000). Nitric oxide and the cardiovascular system. (J. A. Joseph Loscalzo, Ed.) united state of america: humana press.
- Moncada, S., & Higgs, A. (1993). The L-Arginine-Nitric Oxide Pathway. The new england journal of medicine , 329, 2002-2012.
- M.W. Walker, M.T. Kinter, R.J. Roberts and D.R. Spitz, Nitric oxide-induced cytotoxicity: involvement of cellular resistance to oxidative stress and the role of glutathione in protection, Pediatr. Res. 37 (1995), pp. 41–49.
- MTT Assay. (n.d.). Retrieved from Absute Astronomy: http://www.absoluteastronomy.com/topics/MTT_assay#encyclopedia
- Napolia, C., & Ignarro, L. J. (2001). Nitric Oxide and Atherosclerosis. Nitric oxide , 5 (2), 88-97.
- Nathens, A. B., Marshall, J. C., Watson, R. W., Dackiw, A. P., & Rostein, O. D. (1996). Diethylmaleate attenuates endotoxin-induced lung injury. Arch Surg. , 120 (2), 360-366.
- Nikulina, M. A., Andersen, H. U., Karlsen, A. E., Darville, M. I., Eizirik, D. L., & Poulsen, T. M. (2000). Glutathione depletion inhibits 1l-1b-stimulated nitric oxide production by reducing inducible nitric oxide synthase gene expression. Cytokine , 12, 1391-1394.
- Nitric Oxide. (2009, Sep 26). Retrieved from Daily strength: http://images.google.co.uk/imgres?imgurl=http://static.dailystrength.org/userfiles/0/7/3/4/204370/pg_1377454253.jpg&imgrefurl=http://www.dailystrength.org/people/204370/photos-videos/item/545863&usg=__Z0uNwaz0sHh7N94bNeENJizIdGg=&h=284&w=372&sz=25&hl=en&s
- Panza, J. A., & Cannon, R. O. (1999). Endothelium, nitric oxide, and atherosclerosis. (R. O. Julio A. Panza, Ed.) New york: Future publication limited.
- Proliferation Assay, MTT assay. (2009, march 24). Retrieved from Wallert and Provost Lab: http://www.mnstate.edu/provost/MTT_Proliferation_Protocol.pdf
- Reproductive and cardiovascular disease research group. (2009). Retrieved from Nitric oxide: http://www.sgul.ac.uk/depts/immunology/~dash/no/synthesis.htm
- Ross, R. (1999). Atherosclerosis — An Inflammatory Disease. The new england journal of medicine , 115-126.
- Rouiller, Y. B., Corradin, S. B., Smith, J., Schneider, P., Ransijn, A., Jongeneel, V., et al. (1995). Role of Glutathione in Macrophage Activation: Effect of Cellular Glutathione Depletion on Nitrite Production and Leishmanicidal Activity. cellular immunology , 164 (1), 73-80.
- Seis, H. (1997). OXIDATIVE STRESS: OXIDANTS AND ANTIOXIDANTS. Experimental physiology , 82, 291-295.
- Smith, F. S., & Titheradge, M. A. (1998). In M. A. Titheradge (Ed.), Nitric Oxide Protocols (Vol. 100, pp. 171-180). humana press.
- Taniyama, Yoshihiro, Griendling, & K. K. (2003). Reactive Oxygen Species in the Vasculature. American heart association , 42, 1075-1081.
- Taylor, B., & Geller, D. (2000). Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. NCBI , 13, 413-24.
- Tonkin, A. (2003). Atherosclerosis and Heart Disease. (A. Tonkin, Ed.) london: Martin Dunitz publication.
- Vanhoutee, P. M., Rubanyi, G. M., Miller, V. M., & Houston, D. S. (1986). Modulation of Vascular Smooth Muscle Contraction by the Endothelium. Annual Review of Physiology , 48, 307-320.
- Wakulich, C. A., & Tepperman, B. L. (1997). Role of glutathione in nitric oxide-mediated injury to rat gastric mucosal cells. European journal of pharmacology , 319 (2-3), 333-341.
- Weinberg, J. B., Misukonis, M., Shami, P., Sauls, D., Dittman, W., Wood, E., et al. (1995). Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood- Journal of the american society of hematology , 86 (3), 1184-1195.
- Yolande Buchmuller-Rouiller, S. B. (1995). Role of Glutathione in Macrophage Activation: Effect of Cellular Glutathione Depletion on Nitrite Production and Leishmanicidal Activity. cellular immunology , 164 (1), 73-80.









