About Authors:
B. A. Baviskar1, S. S. Khadabadi2, S. L. Deore1*, R. P.Marathe1
1Government College of Pharmacy, Kathora Naka,
Amravati – 444604, MS, INDIA.
2Government College of Pharmacy, Aurangabad Opp. Govt. Polytechnic, Osmanpura, Aurangabad-431005,
Maharashtra INDIA
*sharudeore_2@yahoo.com
Abstract: Topoisomerase is an enzyme that alters/regulates the super coiling of double-stranded DNA by transiently cutting one or both strands of the DNA and hence important target for anticancer activity. The present review articles is focusing a light on points like What is topoisomerase, its types, mode of action of cancer inhibition, Top-I targeted anticancer drugs, Top-II targeted anticancer drugs and Topoisomerase Cellular resistance.
Reference Id:PHARMATUTOR-ART-1302
Introduction:
Cancer is one of the most dreaded diseases of the 20th century and spreading further with continuance and increasing incidence in 21st century. Cancer, due to anomaly in mitotic cell cycle, is the second common cause of death in developed countries. In the United States, as the leading causes of death, it accounts for 25% of all the deaths in humans presently. In industrialized countries, approximately one in five die of cancer. According to WHO, out of an estimated total of 50 million deaths annually in the world, more than 5 million are attributed to Cancer and the number of deaths from cancer throughout the world is increasing. Approximately 500,000 new cases of cancer occur every year in India. As the human life span is increasing in India, more cases of cancer are observed here also. Cancer is thus regarded as an emerging health problem in India.
DNA topology:
There are three main types of topology: supercoiling, knotting and catenation to keep DNA as compact as possible essential for the survival of eukaryotic and prokaryotic cells. However, when transcription or replication occurs, DNA must be free, and these states seriously hinder the processes. (1) During replication, the newly replicated duplex of DNA and the original duplex of DNA become intertwined and must be completely separated in order to ensure genomic integrity as a cell divides. One of the most essential topological problem occurs at the very end of replication, when daughter chromosomes must be fully disentangled before mitosis occurs. Topoisomerase IIA plays an essential role in resolving these topological problems. Topoisomerases are enzymes that interconvert different topological states of DNA. Topoisomerases are isomerase enzymes that act on the topology of DNA.[2] Topoisomerases catalyze and guide the unknotting of DNA by creating transient breaks in the DNA using a conserved Tyrosine as the catalytic residue.[3]
TOPOISOMERASE
Topoisomerase enzyme was originally termed as gyrase. It is an enzyme that alters/regulates the supercoiling of double-stranded DNA by transiently cutting one or both strands of the DNA. [4] This is called as transient breaking and rejoining of DNA strands. DNA Topoisomerase aids the transcription and replication of DNA. As well as DNA topoisomerase I and DNA topoisomerase II, there is DNA topoisomerase III and DNA topoisomerase IV. DNA Topoisomerase III may regulate recombination. DNA Topoisomerase IV regulates the segregation of newly replicated chromosomes.[5] These enzymes are essential for DNA replication and are important targets for anti viral, anti bacterial and anti tumor drugs. These breaks in DNA accumulate, ultimately leading to programmed cell death, or apoptosis by hijacking the natural ability of topoisomerase to create breaks in chromosomal DNA. However these enzymes are structurally and mechanistically different. [6]
What is topoisomerase-I:
It is nomenclatures as type I: EC 5.99.1.2. Topoisomerase type I cuts one strand of DNA. Type I topoisomerases are additionally divided into two subtypes: A and B. Enzymes belonging to the subtype A have a complex mechanism of action that involves passage of the uncut strand through the enzyme-bridged cleavage of the other strand. Interestingly, while acting on DNA with nicks or with single-stranded regions, type IA topoisomerases can cleave the continuous strand and allow the passage of a segment of duplex DNA of the same or another DNA molecule through the cut strand. Topoisomerases of the subtype IB act by a simpler mechanism that involves free rotation of DNA at the transient nick site. [7]
· Type IA topoisomerases, which share many structural and mechanistic features with the type II topoisomerases, type IA topoisomerases form a covalent intermediate with the 5' end of DNA,
· Type IB topoisomerases, which utilize a controlled rotary mechanism. IB topoisomerases form a covalent intermediate with the 3' end of DNA.
· Type IC topoisomerase has been identified, called topo V. While it is structurally unique from type IA and IB topoisomerases, it shares a similar mechanism with type IB topoisomerase.
Topoisomerase I has several unusual features. Unlike type II topoisomerases, topoisomerase I does not require ATP hydrolysis to catalyze the complex topological rearrangements of DNA for which it is responsible. Whereas most enzymes involved in complex rearrangements of DNA are oligomeric, topoisomerase I appears to be a fully functional monomer. [8]
Top-I targeted anticancer drugs:
Top1 inhibitors can be grouped into two main classes: top1 poisons and top1 suppressors. Top1 poisons kill cells by trapping cleavage complexes rather than inhibiting top1 catalytic activity. [9]
Anticancer Mode of Action of topoisomerase-I
Topoisomerase-I is active during S phase, forms and rejoins single strand breaks in DNA which reduce torsion strain ahead of the DNA replication fork. Camptothecin and topotecan inhibit Topoisomerase I and induces DNA strand breaks localized near replication forks. Toisomerase I inhibitors are camptothecin and topotecan The mechanism of action of topoisomerase I inhibitors is Induce DNA strand breaks localized near replication forks by inhibiting religation of the broken DNA strand. [10]
What is topoisomerase-II:
It is nomenclature as type II: EC 5.99.1.3. Topoisomerase type II cuts both strands of DNA. Topoisomerase II is is a major nuclear protein present in a large number of copies. There are two isoenzymes of topoisomerase II (alpha and beta) which are coded on separate chromosomes. It is not clear what advantage derives from this arrangement. In bacteria this function is discharged by topoisomerase IV. It is also split into two subclasses: type IIA and type IIB topoisomerases, which share similar structure and mechanisms. [11]
Top-II targeted anticancer drugs:
These agents stimulate the topoisomerase II-cleavable complex, which is a transient configuration of topoisomerase II on DNA in which topoisomerase II is covalently attached to DNA. This causes the accumulation of cytotoxic nonreversible DNA doublestrand breaks generated by the processing of such complexes by DNA metabolic processes. As of present, the clinical use of catalytic topoisomerase inhibitors as antineoplastic agents is limited to aclarubicin and MST-16. Both of these compounds are preferentially active toward hematological malignancies and show limited activity toward solid tumors. [12]
Anticancer Mode of Action of topoisomerase-II
Topoisomerase II is responsible for cleavage; unwinding and rejoining double stranded DNA during DNA and RNA synthesis. Topoisomerase II inhibitors like doxorubicin and etoposide can intercalate in DNA and form covalent bonds with both DNA and Topoisomerase II leading to double strand breaks in DNA. The function of topoisomerase II is Cleavage, unwinding and rejoining double stranded DNA during DNA and RNA synthesis. Topoisomerase II inhibitors are doxorubicin and etoposide The mechanism of action of topoisomerase II inhibitors is Intercalate in DNA and form covalent bonds with both DNA and topoisomerase II leading to double strand breaks. Although the biological functions of TOP2 are important for ensuring genomic integrity, the ability to interfere with TOP2 and generate enzyme-mediated DNA damage is an effective strategy for cancer chemotherapy. [13]
Table 1 Anti cancer drugs that cleavages DNA by enzyme topoisomerases [14-17]
|
Topoisomerase II |
Drugs |
|
Anthracyclines
Demethylepipodophyllotoxins
Acridines 9-Anilinoacridines Acridinecarboxamides
Actinomycins Anthracene derivatives Anthracene-9, lo-diiones Anthracene bishydrazones Anthrapyrazoles Ellipticines Quinolones$.
Flavonoids Isoflavones Flavones Terpenoides Benzisoquinolinediones Z-Nitroimidazoles$ Naphtacene-5, 12-diones Quinoline-5, I-diones Indoloquinolinedionest
Topoisomerase I
Non-DNA-binders
Intercalators
Minor groove binders
|
Doxorubicin; daunorubicin (DNR); 4-demethoxy-DNR (idarubicin) VP-16 (etoposide); VM-26 (teniposide)
mAMSA N-[2(Dimethylamino)ethyl]- acridine-4-carboxamide
Actinomycin Dt
Mitoxantrone Bisantrene Piroxantrone 2-Methyl-9-hydroxyellipticine CP-l15,953;CP-67,804
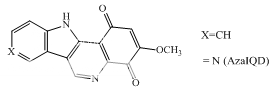
Genistein; orobol Quercetin; fisetin Terpentecin; clerocidin Amonafide; nafidimide RO-15-0216 Saintopint Streptonigrin AzalQD
Camptothecins 9-Aminocamptothecin, Lapachones NSC 314662 Chebulagic acid Indolinoquinolinediones: AzaIQD
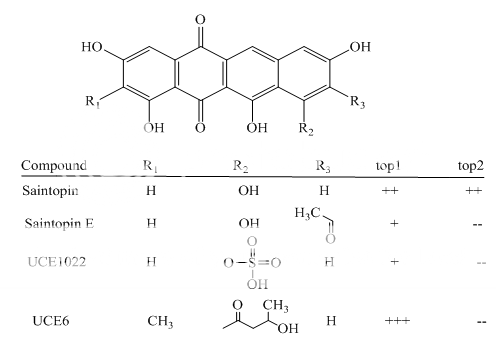
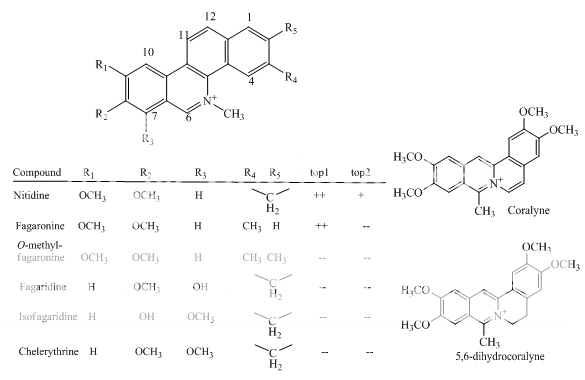
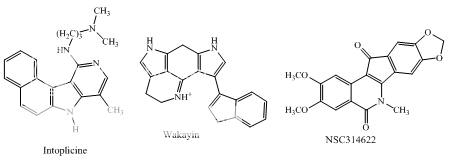
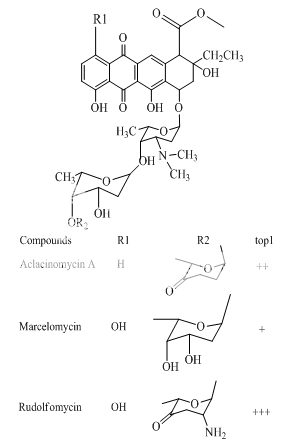
Benzoanthracenes: saintopins, UCE6 Benzophenanthridines: nitidine, fagaronine, coralyne Intoplicine Indolocarbazoles: NB-506, KT6006, rebeccamycin Anthracyclines: morpholinodoxorubicin, aclacinomycin, rudolfomycin Aclacinomycin Peptides: actinomycin D, NU/ICRF 505
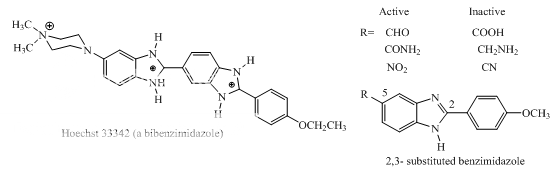
Benzimidazoles: Hoechst 33342, 2,5-substituted benzimidazoles Distamycin Bulgarein Lexitropsins |
Benzoanthracenes
Benzophenanthridines
Indolinoquinolinediones
Other polycyclic aromatics
Benzimidazole
Anthracyclines
Other compounds with minor groove interaction
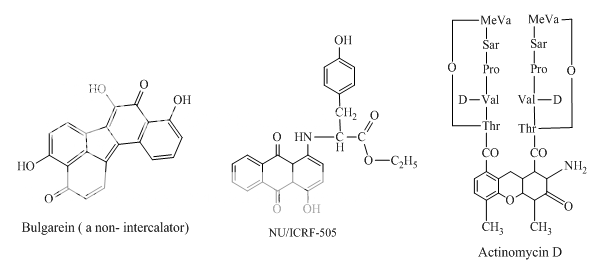
Fig 1 Topoisomerae- I inhibitors
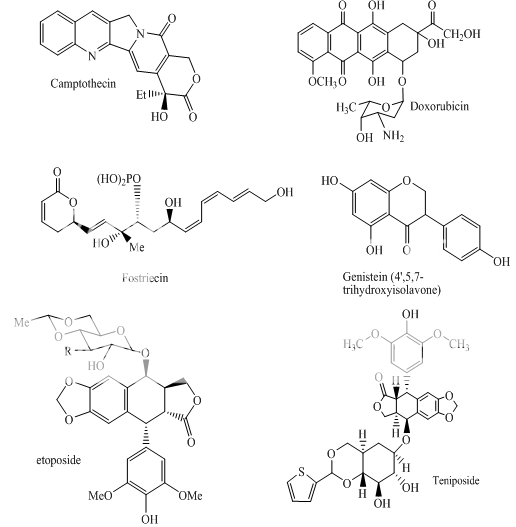
Fig 2: Topoisomerae- II inhibitors
Dual topoisomerase I and II poisons
These compounds are characterized by their ability to induce cleavable complexes with eukaryotic topoisomerase I and II, although to different extents. Actinomycin D, an intercalator with dual poisoning activity, and some anthracycline derivatives, like morpholinyldoxorubicin and nogalamycin, are topoisomerase I poisons that, upon appropriate chemical substitutions, can exhibit topoisomerase I or II activity. Since their cytotoxicity precludes clinical developments, they are regarded merely as interesting probes for investigating the nature of cleavable complex. Dual poisons are all, with the possible exception of the indoloquinolinedione azaIQD, j58 DNA intercalators, but it is unclear how this is related to their topoisomerase I activity. What is lacking at the present moment is an unambiguous correlation between a drug's mode of DNA binding and topoisomerase I or II inhibition.
Their ability to target both enzymes at the same time is intriguing for the possible combination of cytotoxic effects, although experiments using "classical" topoisomerase I and II poisons simultaneously show conflicting results. A synergistic effect on chromosome damage and cytotoxicity was observed using camptothecin with either m-AMSA or etoposide, but data from other groups indicated an antagonistic rather than additive effect. This antagonism results probably from the block of cell cycle progression by topoisomerase II drugs, that in turn lowers the population of S-phase cells, which are the preferential target of camptothecin. On the other hand, it has been observed that simultaneous inhibition of topoisomerase I and II by dual inhibitors enhances the cytotoxicity of those compounds whose molecular interactions within the cleavable complex appear different from those of the "classical" poisons. [18-22]
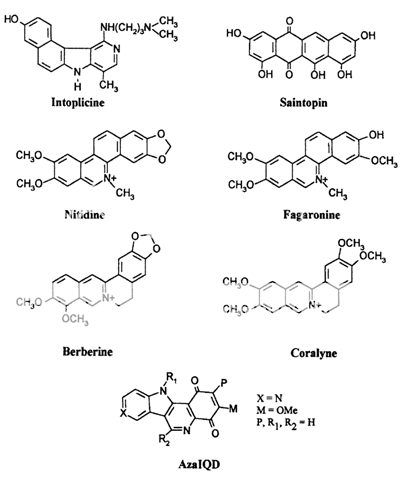
Fig 3 Dual topoisomerase I and II poisons.
CONCLUSIONS:
This review has tried to discuss the various characteristics of enzyme topoisomerases, their inhibitors and anticancer mechanism of action. There are many anticancer and antibacterial drugs operate through Enzyme topoisomerases. From its discovery in 1971, it is fascinating researchers due to its target efficiency and role in topology. Present research is directed not only to discover new therapeutic moieties that are targeting this enzyme but to evaluate role of this enzyme in many biological processes including degenerative diseases.
REFERENCES:
1. Bauer, W. R., Crick, F. H. C. and White, J. H., Scientific American, 243, (1980), 118-133.
2. Benham, C. J., Annu. Rev. Biophys. Biophys. Chem., 14, (1985), 23-45.
3. Benham, C. J., Nucleic. Acids. Res., 15, (1987), 9985-9995.
4. Warren E. Ross., Biochemical Phramcology, 34 (1985), 4191-4195.
5. Yves Pommier, Philippe Pourquier, Yi Fan, Dirk Strumberg et al., Biochimica. Biophysica Acta., 1400 (1998) 83-106.
6. Hsieh T., Current Opinion in Cell Biology, (1990), 2X11-463.
7. Barbara Gatto and Leroy Fong Liu., Advances in DNA Sequence-Specific Agents, 3, (1998), 39-65.
8. James M. Berger, Biophysica Acta., 1400, (1998), 3-18.
9. Beverly A. Teicher, Biochemical Pharmacology, 75, (2008), 1262 – 1271.
10. Smitha Antony, Glenda Kohlhagen, Keli Agama, Muthusamy Jayaraman, Shousong Cao, Farukh A. Durrani, Youcef M. Rustum, Mark Cushman and Yves Pommier, Mol Pharmacol., 67, (2005), 523–530.
11. Yves Pommier, Philippe Pourquier,Yoshimasa Urasaki, Jiaxi Wu, Gary S. Laco, Drug Resistance Updates, 2, (1999), 307–318.
12. Schwartsmann, M.J. Ratain, G.M. Cragg, J.E. Wong, N. Saijo, D.R. Parkinson, Y. Fujiwara, R. Pazdur, D.J. Newman, R. Dagher and L. Di Leone, Journal of Clinical Oncology, 20, (2002), 47-59.
13. Giovanni Capranico and Franco Zunino, Eur. J Cancer, Vol. ZXA, No. 12, (1992), pp. ZOSS-ZOa).
14. Taudou, G., Portemer, C., Jaxel, C., Duguet, M., Biochem. Pharmacol., 45, (1993), 331.
15. Cortes, E., Pinero, J., Cancer Chenu~ther. Pharmacol. 1994, 34, 411.
16. Kaufmann, S. H., Cancer Res., 51, (1991), 1129.
17. Masumoto, N., Nakano, S., Esaki, T., Tatsumoto, T., Fujishima, H., Baba, E., Nakamura, M., Niho, Y., Anticancer Res., 15, (1995), 405.
18. Yves Pommier and Mark Cushman The Indenoisoquinolines Non-Camptothecin Topoisomerase I Inhibitors: Update and Perspectives Mol Cancer Ther. 2009 May; 8(5): 1008–1014.
19. Teicher BA. Next generation topoisomerase I inhibitors: Rationale and biomarker strategies. Biochem Pharmacol. 2008;75:1262–127
20. Jody Plank, Tao-shih Hsieh Helicase-appended Topoisomerases: New Insight into the Mechanism of Directional Strand Transfer, November 6, 2009 The Journal of Biological Chemistry, 284, 30737-30741.
21. Nicola J. Sunter, Ian G. Cowell, Elaine Willmore, Gary P. Watters, and Caroline A. Austin Role of Topoisomerase II in DNA Damage Response following IR and Etoposide Journal of Nucleic AcidsVolume 2010 (2010), Article ID 710589, 8 pages
22. Pommier Y, Leo E, Zhang H, Marchand C DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010 May 28;17(5):421-33.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









