 About Authors: Anjali Gureja; 4th B.Pharm Student
About Authors: Anjali Gureja; 4th B.Pharm Student
N jawahar; Lecturer Department of Pharmaceutics
Mihir Trivedi; 4th B.Pharm Student
Anusha K; 4th B.Pharm Student
Prashant Subhashrao Wake; M.Pharm Student
Reference ID: PHARMATUTOR-ART-1072
Abstract
Patient compliance is necessary for optimum therapeutic outcome and the extent of adverse effects,convenience and cost of the drug determine the success of a medicine In recent years, there has been increasing interest on the use of bioadhesive polymers to control the delivery of biologically active agents systemically or locally The present study includes in the increased interest and use of bioadhesive polymers in bucco adhesive drug delivery system.Which is useful for drugs which are succeptible to extensive gastrointestinal degradation and first pass metabolism. Buccal administration as a method of preventing presystemic metabolism
1. As it provides.highy vascular site 2. Regional variation in permeability to drugs 3. Passive diffusion of unionized drugs 4. Escapes first pass metabolism.
A bioadhesive system plays a major role, due to its potential leads to high demand for buccal drug delivery system.
[adsense:336x280:8701650588]
Buccal Drug Delivery System
In recent years, there has been increasing interest on the use of bioadhesive polymers to control the delivery of biologically active agents systemically or locally. These bioadhesive systems are useful for the administration of drugs, which are susceptible to extensive gastrointestinal degradation and first pass metabolism. Buccal bioadhesive system appears to be attractive because it avoids significant limitations of traditional routes and first pass metabolism. Buccal delivery necessitates the use of muco-adhesive polymer as these dosage forms should ideally adhere to the mucosa and withstand salivation, tongue movement and swallowing for a significant period of time.
Advantages of delivering a drug through the oral mucosa, these drugs are viable candidates for delivery via this route. Many investigators have studied the potential of transmucosal delivery through the oral cavity, and the oral mucosa is increasingly being considered as an effective route for many drug classes.
A bioadhesive system plays a major role, due to its potential. Besides acting as platforms for sustained release dosage forms, bioadhesive polymers can themselves exert some control over the rate and amount of drug release and thus contribute to the therapeutic efficacy of bioadhesive drug delivery systems. Bioadhesion is an interfacial phenomenon in which two materials, at least one of which is biological, are held together by means of interfacial forces. The attachment could be between an artificial material and biological substrate.
Administration of the drug via the mucosal layer is a novel method that can render treatment more effective and safe, not only for the topical diseases but also for systemic ones. These unique dosage forms, which can be applied on a wet tissue, are formulated by utilizing the adhesive properties of some water - soluble polymers. The mucosal layer lines a number of regions of the body including the gastrointestinal tract, buccal cavity, airways, ear, nose, eye, urogenital tract, vagina and rectum are covered with a thick gel like structure known as mucin, therefore all bio-adhesives must interact with the mucin layer during the process of attachment, these represent the potential sites for attachment of any bioadhesive system. (Khanna R., 1998 and Chowdary K.P.R., 2000)
Drug delivery across the oral mucosa, can be divided into three different types. (Wani M.S., 2007)
i. Sublingual delivery, consisting of administration through the membrane of the ventral surface of the tongue and the floor of the mouth.
ii. Buccal delivery, consisting of administration through the buccal mucosa, mainly composed of the lining of the cheeks and
iii. Local delivery, consisting of administration through all areas other than former two regions.
These sites differ anatomically in their permeability to drugs, rate of drug delivery, and ability to maintain a delivery system for the time required for drug release out of the delivery apparatus and into the mucosa.
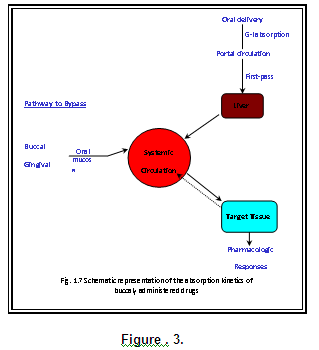
Buccal administration as a method of preventing presystemic metabolism (Mc Elnay J.C., 1990)
The buccal cavity provides a highly vascular mucous membrane site for the administration of drug. The epithelial lining of the oral cavity differs both in type (keratinised and non-keratinised) and in thickness in different areas and the differences give rise to regional variation in permeability to drugs.
Buccal Drug Delivery (Wani M.S., 2007)
The buccal mucosa lines the inner cheek, and buccal formulations are placed in the mouth between the upper gingivae (gums) and cheek to treat local and systemic conditions. The buccal route provides one of the potential routes for typically large, hydrophilic and unstable proteins, oligonucleotides and polysaccharides, as well as conventional small drug molecules. The oral cavity has been used as a site for local and systemic drug delivery.
Advantages of Drug Delivery via the Buccal Lining (Wani M.S., 2007)
Bypass of the gastrointestinal tract and hepatic portal system, increasing the bioavailability of orally administered drugs that otherwise undergo hepatic first-pass metabolism. In addition the drug is protected from degradation due to pH and digestive enzymes of the middle gastrointestinal tract.
i. Improved patient compliance due to the elimination of associated pain with injections; administration of drugs in unconscious or incapacitated patients; convenience of administration as compared to injections or oral medications.
ii. Sustained drug delivery.
iii. A relatively rapid onset of action can be achieved relative to the oral route, and the formulation can be removed if therapy is required to be discontinued.
iv. Increased ease of drug administration
v. Though less permeable than the sublingual area, the buccal mucosa is well vascularized, and drugs can be rapidly absorbed into the venous system underneath the oral mucosa.
Limitations of Buccal Drug Delivery (Wani M.S., 2007)
Depending on whether local or systemic action is required the challenges faced while delivering drug via buccal drug delivery can be enumerated as follows.
i. For local action the rapid elimination of drugs due to the flushing action of saliva or the ingestion of foods stuffs may lead to the requirement for frequent dosing.
ii. The non-uniform distribution of drugs within saliva on release from a solid or semisolid delivery system could mean that some areas of the oral cavity may not receive effective levels.
Structure of the human oral mucosa (Smart J.D., 2005)
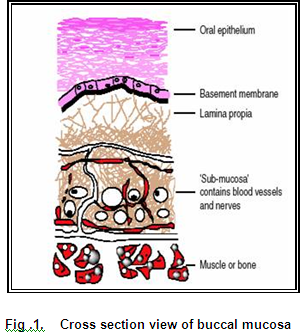
The oral mucosa is composed of an outermost layer of stratified squamous epithelium. Below this lies a basement membrane, lamina propria followed by the submucosa as the innermost layer. The epithelium is similar to stratified squamous epithelia found in rest of the body in that it has a mitotically active basal cell layer, advancing through a number of differentiating intermediate layers to the superfacial layers, where cells are shed from the surface of the epithelium (Gandhi and Robinson, 1988). The mucosae of the gingivae and hard plate are keratinized and the mucosae of the soft palate, the sublingual and the buccal regions, are not keratinized (Harris and Robinson, 1992).
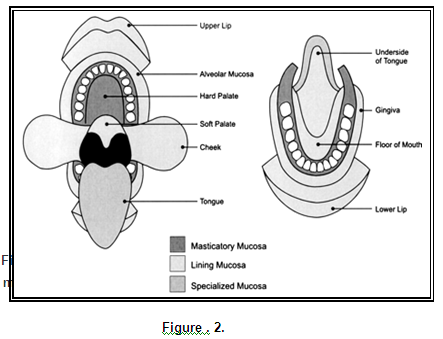
Table: 1
Thickness of epithelium in different regions of oral mucosa
|
Region |
Average Epithelial Thickness (mm) |
|
Skin (mammary region) |
100-120 |
|
Hard palate |
250 |
|
Attached gingiva |
200 |
|
Buccal mucosa |
500-600 |
|
Floor of mouth |
100-200 |
Biochemical composition
A notable feature of the oral mucosa is the large amount of protein present in the form of monofilaments in the cells of layers, in both Keratinised and nonkeratinized epithelia. Comparatively little is known about the lipid composition of the oral mucosae. The keratinized oral epithelium shows a lipid pattern of mainly neutral lipids, i.e. ceramides, whereas the non-keratinized epithelium contains few neutral but polar lipids, particularly cholesterol sulphate and glucosylceramides.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE. SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Characteristics of Mucus
Mucus is a glycoprotein, chemically consisting of a large peptide backbone with pendant oligosaccharide side chains whose terminal end is either sialic or sulfonic acid or L–fructose. The oligosaccharide chains are covalently linked to the hydroxy amino acids, serine and threonine, along the polypeptide backbone. About 25% of the polypeptide backbone is without sugars, the so-called ‘naked’ protein region, which is especially prone to enzymatic cleavage. The remaining 75% of the backbone is heavily glycosylated. The terminal sialic groups have a pKa value of 2.6 so that the mucin molecule should be viewed as a polyelectrolyte under neutral or acid condition. At physiological pH the mucin network may carry a significant negative charge because of the presence of sialic acid and sulfate, residues and this high charge density plays an important role in mucoadhesion.
A primary function of the oral mucosa is to provide a barrier. At the same time, the oral mucosa shares with the gut the ability to maintain a moist surface. The permeability of the oral mucosa in general is probably intermediate between that of the epidermis and that of the intestinal mucosa. Galey (1976) estimated the permeability of the buccal mucosa to be 4 – 4000 times greater than that of the skin. In general, the permeability of the oral mucosa decreases in the order: sublingual > buccal > palatal. This type dependent absorption was shown by Pimlot and Addy (1985).
Membrane storage during buccal absorption of drugs
The absorption of a drug from the mouth is not synonymous with drug entry into the systemic circulation. Instead, the drug appears to be stored in the buccal membranes due to drug binding in or on the oral epithelium. Evidence for the existence of a storage compartment is easily found in the published literature because drug lost from solutions placed in the mouth could be recovered from the buccal mucosa by rinsing the mouth with a buffer of the appropriate pH. Due to this phenomenon, buccal partitioning has been suggested as a more accurate term to describe the diffusion of drugs across the oral mucosa.
Bioadhesion in drug delivery
The term bioadhesion (Longer and Robinsons, 1986) defined as attachment of synthetic or natural macromolecules to mucus and / or an epithelial surface. In the case of polymer attached to the mucin layer of mucosal tissue the term “Mucoadhesion” is employed. In most instances the bioadhesive polymer is in contact with a soft tissue (buccal, intestinal, nasal etc.) and thus the tissue layer responsible for formation of the adhesive interface is mucus.
Mechanism of bioadhesion
The process of bioadhesion can be viewed as occurring in two steps. First intimate contact between the polymer and membrane followed by formation of bonds. The bonding occurs chiefly through both physical or mechanical bonds results from entanglement of the adhesive material and the extended mucus chains. Secondary chemical bonds may be due to electrostatic interactions, hydrophobic interactions, hydrogen bonding and dispersion forces. Electrostatic interactions and hydrogen bonding appear to be important as a result of the large number of charged and hydrophilic species, e.g.,hydroxylic(-OH),carboxylic (-COOH), sulfate (SO3H) and amino (-NH2) groups. Several theories of bioadhesion have been proposed to explain fundamental mechanisms of attachment.
Adsorption theory
In the adsorption theory, a bioadhesive polymer adheres to mucus because of secondary surface forces such as Van der Waals forces, hydrogen bonds or hydrophobic interactions. For a bioadhesive polymer with a carboxyl group, hydrogen bonding is considered to be the dominant force at the interface. On the other hand, hydrophobic interactions can explain the fact that a bioadhesive polymer may bind to a hydrophobic substrate more tightly than to a hydrophilic surface (Kaelble, 1977).
Wetting Theory
Wetting theory is predominantly applicable to liquid bioadhesive systems and analyses adhesive and contact behaviour in terms of the ability of a liquid or a paste to spread over a biological system.
The work of adhesion (expressed in terms of surface and interfacial tension), Y being defined as the energy per cm2 released when an interface is formed. The work of adhesion is given by:
Wa = YA+YB-YAB
Where ‘A’ and ‘B’ refer to the biological membrane and the bioadhesive formulation respectively. The work of cohesion is given by:
WC = 2 YA or YB
For a bioadhesive material B spreading on a substrate A, the spreading coefficient is given by:
SB/A = YA- (YB+YAB)
SB/A should be positive for a bioadhesive material to adhere to a biological membrane.
Fracture theory
This theory attempts to relate the difficulty of separation of two surfaces after adhesion. Fracture theory equivalent to adhesive strength is given by:
G= (E=∑/L) 1/2
Where,
E is the Young’s modulus of elasticity.
∑ is the fracture energy, and
L is the critical crack length when two surfaces are separated.
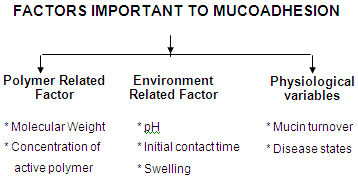
I. Polymer related factor
Molecular weight
The optimum molecular weight for maximum bioadhesion depends on the type of bioadhesive polymer. It is generally understood that the threshold required for successful bioadhesion is atleast 100,000 molecular weight. For example, polyethylene glycol (PEG) with a molecular weight of 20,000 has little adhesive character, whereas PEG with 200,000 molecular weight has improved, and a PEG with 400,000 has superior adhesive properties. The fact that bioadhesiveness improves with increasing molecular weight for a linear polymer implies two things:
1) Interpenetration is more critical for lower molecular weight polymers to be a good bioadhesive and
2) Entanglement is important for higher molecular weight polymers. Adhesiveness of a nonlinear structure, by comparison follows a quite different trend. The adhesive strength of dextran, with a very high molecular weight of 19,500,000 is similar to that of 200,000. The reason for this similarity may be that the helical conformation of dextran may shield many of the adhesive groups, which are primarily responsible for adhesion, unlike the conformation of PEG (Duchene et al., 1988).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE. SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Concentration
There is an optimum concentration of a bioadhesive polymer to produce maximum bioadhesion. In highly concentrated system, beyond the optimum level, however, the adhesive strength drops significantly because the coiled molecules become separated from the medium so that the chains available for interpenetration become limited (Duchene et al., 1988).
II. Environment related factors
pH
PH can influence the formal charge on the surface of mucous as well as certain ionizable bioadhesive polymers.
Initial contact time
Bioadhesive strength increases as the initial contact time increases.
Swelling
Swelling characteristics are related to the bioadhesive itself and its environment. Swelling depends on the polymer concentration, ionic strength as well as the presence of water.
POLYMERS IN BUCCAL DRUG DELIVERY
Polymers remain the most versatile class of biomaterials, being extensively applied in medicine and biotechnology as well as in the food and cosmetic industries.
Polymers used as biomaterials can be naturally occurring, synthetic or a combination of both. The characteristics of the main groups of natural polymeric materials according to their origin, properties and principal fields of application can be summarized as follows. (Nela Angelova and David Hunkeler, 1990).
Table-2
|
Polymers |
Main applications and comments |
|
Natural Polymers Protein and protein based polymers |
Absorbable, biocompatible, nontoxic, naturally available, typically elastic materials used as implants and in tissue engineering. |
|
Collagen |
Absorbable sutures, sponge wound dressing, drug delivery microspheres. |
|
Albumin |
Used in cell and drug micro encapsulation |
|
Poly (amino acids) |
Usually poly (a, l- amino acids) used as oligomeric drug carriers. |
|
Derivatives. From Vegetable sources. Carboxy methyl cellulose |
Cell immobilization via a combination of ionotropic gelation and polyelectrolyte complex formation |
|
Cellulose sulphate |
Components of polyelectrolyte complexes for immunoisolation. |
|
Agarose |
Largely used as supporting materials in clinical analysis and as an immobilization matrix. |
|
Alginate |
Excellent gel-formation properties. Used as immobilization matrices for cells and enzymes |
|
Carrageenan |
Excellent thermo reversible properties. Used for microencapsulation. |
|
From human and animal source. Hyaluronic acid |
Excellent lubricant, potential therapeutic agent. |
|
Heparin |
Antithrombotic and anticoagulant properties. Extensively used in surgery |
|
Microbial polysaccharide Dextran and its derivatives |
Excellent rheological properties. Plasma expander. Widely used as a drug carrier |
|
Chitosan and its derivatives |
Biocompatible, non toxic, excellent gel and film forming ability, natural, polycation. Widely used in controlled delivery systems |
Polymers that adhere to the mucin-epithelial surface can be conveniently divided into three broad categories:
Polymers that become sticky when placed in water and owe their bioadhesion to stickiness.
Polymers that adhere through non-specific, non-covalent interactions, which are primarily electrostatic in nature.
Polymers that bind to specific receptor sites on the cell surface.
Characteristics of an ideal polymer for mucoadhesive drug delivery system. (Bandyopadhyay A.K., 2006)
An ideal polymer should possess the following characteristics:
1. The polymer and its degradation products should be non-toxic and non-absorbable from the GI tract.
2. It should be non-irritant to the mucous membrane.
3. It should preferably form a strong non-covalent bond with mucin-epithelial cell surfaces.
4. It should preferably adhere quickly to moist tissue and should possess some site specificity.
5. It should allow easy incorporation of the drug and offer no hindrance to its release.
6. The polymer must not decompose on storage or during the shelf life of the dosage form.
7. The cost of the polymer should not be high so that the prepared dosage form remains competitive.
Gums and mucilages
Gums and mucilage’s are complex polysaccharides containing monosaccharides or their derivatives linked in a bewildering Varity of linkages and structures. They are condensation polymers. The term gum refers to polysaccharide hydrocolloids, which do not form a part of cell wall, but are exudates or slimes and are pathological products. Mucilage’s are part of cell and are physiological products. These polymers have invoked tremendous interests in the fields of pharmacy, medicine and food technology.
Classification
The polysaccharide are classified into the following groups according to their nature or occurrence:
i. Exudates: eg. Gum acacia
ii. Seed gums: eg. Guar gum, carob gum
iii. Plant extract; eg. Pectin, starch, cellulose
iv. Seed mucilage’s: eg. Platago, ocimum, tamarindus
v. Seaweed extracts eg. Agar, alginates
vi. Animal polysaccharides: eg. Chitin, chitosan
vii. Microbial exudates: eg. Dextran, xanthan
Mucoadhesive dosage forms can be made of either natural or synthetic polymers. The natural polymers offer certain specific advantages over synthetic polymers such as:
Easy availability
Non –toxicnature
Biocompatibility
Biodegradability
Pollution –free procrssing
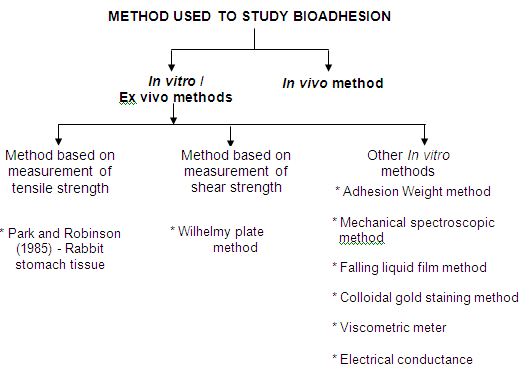
Tensile strength measureme
Park and Robinson, 1985 employed a method in which the force required to separate the bioadhesive sample from freshly excised rabbit stomach tissue was determined using a modified tensiometer. A section of the tissue, having the mucus side exposed, was secured on a weighed glass vial placed in a beaker containing USP simulated gastric fluid. Another section of the same tissue was placed over a rubber stopper again with the mucus side exposed and secured with a vial cap and a small quantity of polymer was placed between the two mucosal tissues. The force was used to detach the polymer from the tissue was then recorded.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE. SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Wilhelmy plate method
In this method, the plates are coated with a polymer to be tested and immersed in a temperature controlled mucus solution. The force required to pull the plate out of the solution is determined under constant experimental conditions.
In vivo methods
In vivo techniques for measuring the bioadhesive strength are relatively few. Some of the reported methods are based on the measurement of the residence time of bioadhesives at the application site. The three main in vivo techniques to monitor bioadhesion include
• Gamma scintinography
• Isolated loop techniques
• Transit with radiolabelled or fluorescent coupled dosage forms.
Reference:
• Ahuja A., Dogra M., And Agrawal S.P., 1995. Indian J. Pharm sci. 57(1), 2630.
• Anders R., and Merkle H.P., 1989. Evaluation of Laminated Muco- Adhesive Patches for Buccal Drug Delivery. International Journal of Pharmaceutics.49 (3), 231-240.
• Anonymus., 1989. In the Wealth of India, Raw Materials. Vol 8, 250-252.
• Azeez O.S., 2000.Production of Gum from Cashew Tree Latex. Leonardo Electronics.
• Bandyopadhyay A.K., Yajaman S., Ketousetuo K., 2006. Buccal Bioadhesive Drug Delivery – A Promising Option For Orally Less Efficient Drugs. Journal of Controlled Release 114:15-40.
• Banker G.S., Anderson N. R., Tablets In. Lachman., Liberman H.A.Kanigj L., (Eds.) 1987.The Theory and Practice of Industrial Pharmacy. Vargese Publishing House, Mumbai, 293-345.
• Barrer R.M., Barrie J.A., Wong P.S.L., 1968. The Diffusion and Solution of Gases in Highly Cross Linked Copolymers. 9:609–627.
• Beckett A.H. and Hossie R.D., 1971. Handbook of Experimental Pharmacology. 28; 25-46.
• Bernkop-Schnurch A., 2005. Mucoadhesive Systems In Oral Drug Delivery, 2(1).
• Campion, R.P., 1975. The Influence of Structure on Autohesion (Self–Tack) and Other Forms of Diffusion into Polymers. J. Adhesion 7:1 – 23.
• Celebi, N., Kislal,O., 1995. Development and Evaluation of A Buccoadhesive Propranolol Tablet Formulation. Pharmazie. Jul; 50(7): 470-2
• Chen, S.Y., Squier,C.A., 1984. The Structure and Function of Oral Mucosa. 7-30.
• Chowdary K.P.R., Srinivas L., 2000. Mucoadhesive Drug Delivery Systems: A Review of Current Status. Indian Drugs. 37 (9): 400-406.
• Claudia Colonna., 2006. 5-Methyl –Pyrrolidinone Chitosan Films As Carriers For Buccal Administration Of Proteins. AAPS PharmSciTech .7 (3) ,1-7.
• Claudia Valenta., Constantia E., Irene Harich., Andreas Bernkop-Schnurch., 2001 . Development and In vitro Evaluation of Mucoadhesive Delivery System for Progesterone. Journal Of Controlled Release 77:323-332.
• Deryaguin, B.V., Toporov, Y.P., Mueler, V.M., Aleinikova, I.N., 1997. On The Relationship between the Electrostatic and Molecular Component of The Adhesion Of Elastic Particles To A Solid Surface. J Colloid Interface Sci. 58:528-533.
• Desai, K.G.H., Kumar, T.M.P., 2004. Development And Evaluation Of Noval Buccal Adhesive Core-In-Cup Tablets Of Propranolol Hydrochloride. Indian Journal Of Pharmaceutical Sciences.66(4),438.
• Duchene D., Touchard, F., Peppas, N.A., 1988. Pharmaceutical and Medical Aspects of Bioadhesive Systems for Drug Administration. Drug Dev. Ind. Pharm. 14:283.
• Duvoix A., Blasius R.,Delhalle S., Schnekenburger M., Moceau F., Henry E., Diederich M.,2005. Chemopreventive And Therapeutic Effect Of Curcumin. Cancer Letters 223:181-190.
• Evans W.C.,Trease and Evans Parmacognosy, 1996., 14th edition . W.B. Saunders Company Ltd, London.
• Galey W.R., Lonsdale, H.K., Nacht, S.Y., 1976. Permeability Studies On Buccal Mucosae. Invest. Dermatol. 67:713-717
• Gandhi R.E., Robinson, J.R., 1988. Bioadhesion in Drug Delivery. Ind. J. Pharm. Sci. 50:145-152
• Ghaly E.S., Ruiz G., 2006. Mucoadhesive Delivery Systems Using Carrageenan And Eudragit RLPO.31-39.
• Goodman and Gilman's., 1991. The Pharmacological Basis of Therapeutics. 8th Edition ,vol. 1.
• Gorden R.E.,Rashanke T.W., Fonner D.E., Anderson N.R., Banker G.S., 1999. Pharmaceutical Dosage Forms-Tablets. Vol.2 Marcel Dekker, New York, 245-335.
• Goud K.H., Desai T. M., Pramod Kumar., 2004. Preparation and Evaluation of A Novel Buccal Adhesive System. AAPS. Pharm. Sci. Tech, 5(3).1-4.
• Grabovac V., 2005. Comparision Of The Mucoadhesive Properties Of Various Polymers. Adv. Drug Delivery Reviews 57:1713-1723
• Grieve M., 1995. http/www.botanical.com
• Gupta A., Garg S., Khar R K., 1992. Mucoadhesive Buccal Drug Delivery Systems, Indian drugs. 29 (13); 586-592.
• Harding S.E., 2003. Mucoahesive Interactions. Biochemical Society Transactions Volume 31, Part 5.
• Harris D., Robinson J.R., 1992. Drug delivery via the mucous membranes of the oral cavity. J. Pharm.Sci, 81:1-10.
• Helfand, E., Tagami, Y., 1972. Theory of the Intern between Immiscible Polymers. J. Chem. Phys. 57:1812-1813.
• Hirofumi Takeuchi., 2005. Novel Mucoadhesion Tests For Polymers And Polymer – Coated Particles To Design Optimal Mucoadhesive Drug Delivery System. Adv. Drug delivery reviews 57:1583-1594.
• Indian Pharmacoepia. 1996. Vol. II Pub. The Controller of Publication. 694.
• Jacobsen J.,Nielsen E.B., 1999. Filter Grown TR146 Cells As An In vitro Model Of Human Buccal Epithelial Permeability.Eur J Oral Sci107:138-146
• Jain S., Shrivastava S., Nayak S., Sumbhate S., .2007. Resent Trend In Curcuma Longa .Pharmacognosy Rev. 1:119-128.
• Jasti B., Xiaoling Li., 2003. Resent Advances in Mucoadhesive Drug Delivery System .Pharmatech.194-196.
• Jens T. Cartensen, 1997. Pharmaceutics of Solid and Solid Dosage Forms, Hoh wiley and Sons, Inc .248.
• Jin Whan Lee., Jae Han Park., Joseph R. Robinson., 2000. Bioadhesive-Based Dosage Forms: The Next Generation. J. Pharm. Sci. 89(7):850-865.
• John D Smart., 2005. Buccal Drug Delivery. Expert Opin. Drug Delivery 2(3).
• Johnson, J.B., Davis, D.A., Davies F., 1958. Gray’s Anatomy 32nd edition; Longmans Green And Co: London. 737.
• Juan Manuel Liabot, Ruben Hilario Manzo and Daniel Alberto Allemandi., 2002. Double-Layered Mucoadhesive Tablets Containing Nystatin. AAPS Pharm Sci Tech.3 (3).
• Keiko Tsutsumi., Yasuko Obato., Tsuneji Nagai., Thorsteinn Loftsson., Kozo Takayama., 2002. Buccal Absorption of Ergotamine Tartrate Using the Bioadhesive Tablet System in Guinea-Pigs. International Journal of pharmaceutics. 238,161-170.
• Khanna R, Agarwal S P, Ahuja A., 1998. Mucoadhesive Buccal Drug Delivery: A Potential Alternative to Conventional Therapy. Indian J.Pharm.Sci; 60(l):1-11.
• Kok Khiang Peh., 1999. Polymeric Films As Vehical For Buccal Delivery: Swelling, Mechanical And Bioadhesive Properties. J Pharm Pharmaceut Sci 2(2):53-61.
• Kokate, C.K., Purohit, A.P., Gokhale, S.B., 1995. Pharmacognosy. 130-135.
• Kulkarni G.T., Gowthamarajan K., Suresh B., 2004.Stability Testing Of Pharmaceutical Products: An Overview. Ind. J. Pharm.Educ.38 (4)
• Langoth N., Jocben K., and Bernkop-Schnurc A., 2005. Development of a mucoadhesive and permeation enhancing buccal delivery system for PACAP (Pituitary adenylate cyclase- activating polypeptide). International Journal Of Pharmaceutics.296,103-111.
• Leonie Asfora Sarubbo, Lucianna Alves De Oliveira, Ana Lucia Figueiredo Porto., 2004. Partition Of Proteins In Aqueous Two-Phase Systems Based On Cashew –Nut Tree Gum and Poly (ethylene glycol). Brazilian Archives Of Biology And Technology, vol.47 (5)685-691.
• Longer M.A., Robinson, J.R., 1986. Fundamental Aspects of Bioadhesion. Pharm. Int. 7: 114-117.
• Maheshwari R.K., Singh A.K.,Gaddipati J., Srimal R.C., 2006. Multiple Biological Activities Of Curcumin : A Short Review .Life Sciences 78:2081-2087.
• Mahmood A.M., and Hung-Seng Chng, 1995. Design Of A Dissolution Apparatus Suitable For In Situ Release Study Of Triamcinolone Acetonide From Bioadhesive Buccal Tablets, International Journal Of Pharmaceutics.121 (2), 129-139.
• Marcia Cruz-Correa., 2006. Combination Treatment With Curcumin And Quercetin Of Adenomas In Familial Adenomatous Polposis. Clinical Gastroenterology and Heopatology Vol. 4(8).
• Mashru R., Sutariya V., Sankalia M., 2005. Transbuccal Delivery Of Lamotrigine Across Porcine Buccal Mucosa: in vitro Determination
• Mc Elnay J C, Swarbick J, Boylan J C, Eds, 1990. Encyclopedia of Pharmaceutical Technology, Marcel Dekkar (New York) 2:189
• Miquel J., Bernd A., Sempere J.M., 2002. The Curcuma Antioxidant: Pharmacological Effect and Prospects For Future Clinical Use: A Review. Archives Of Gerontology And Geriatrics 34 :37-46
• Mitra A. K., Alur H. H., Johnston, 2002. Peptides and Protein- Buccal Absorption, Encyclopedia of Pharmaceutical Technology, Marcel Dekker Inc, Edition 2081-2093.
• Mortezza Rafiee Tehzan., Ghiassedin Jazayeri., Tayegeh Toliya., Khossrow Baynti., Kazem., Kdurosh., Parid.,2002. Development and In vitro Evaluation of Novel Buccoadhesive Tablet Formulation Of Prednisolone. Acta . Pharm. 32 ,121-127.
• Muhammed M., Lakshmi P, curcuminoids bioprotectant compounds from turmeric, Sabinsa corporation, New Jerssy, USA.www.sabinsa.com/paper_curcumin.htm
• Mukherjee P. K., Maiti K., 2007. Curcumin –Phospholipid Complex:Preparatio, Thetrapeutic Evaluation And Pharmacokinetic Study In Rats. Int. J. Pharm.330:155-163.
• Nicholas H .G .H. and Leslie Z .B, 1995. Pharmacokinetics and Pharmacodynamics : Rational Dose Selection And The Time Course Of Drug Action, In: Basic and Clinical Pharmacology, Bestran Gkatzang, Ed Prentice Hall International Inc, , 59-66.
• Panigrahi L., Pattnaik S., Ghosal S.K., 2005. Design and Characterization of Mucoadhesive Buccal Patches of Diclofenac Sodium.Indian J Pharm.Sci 67(3):319-326.
• Park H., Robinson JR., 1985. Journal of control release. 2:47.
• Park K., Robinson R., 1984. Int.J. Pharm.19:107.
• Parrot e.l., 1985. Experimental Pharmaceutics, 4th edition .Surjeet Publications, New Delhi.
• Patel V.M.,Bhupender G.,2007 Formulation ,Evaluation ,And Comparison Of Bilayered And Multilayered Mucoadhesive Buccal Devices of Prppranolol Hydrochloride. AAPS PharmSciTech 8(1).1-8.
• Patel, V.M., Parajapat, B.G., Patel, J.K., Patel, M.M., 2006. Physicochemical Characterization and Evaluation of Buccal Adhesive Patches Containing Propranolol Hydrochloride. CurrentDrug Delivery.3 (3), 325-331.
• Pharmaceutical excipients., 1986. American Pharmaceutical Association Publication, Washington D.C.
• Phrmacoppeia of India. 1996, Controller Of Publications, New Delhi.
• Pimlott, S.J., Addy, M., 1985. Site Dependent Absorption Study on Buccal Mucosae. Oral Surg. Oral Med. Oral Pathol. 59:145-148.
• Pramod Kumar T.M., Shiva Kumar H.G., Desai K.G., 2004. Oral Transmucosal Drug Delivery Systems. Indian drugs ,41(2): 63-75.
• Quality Control Methods for Medicinal Plant Materials, 1998 W.H.O.,Geneva.
• Ringman J.M., Frautschy S. A., Cole G. M., Masterman D. L., Cummings J. L., 2005. A Potential Role of the Curry Spices Curcumin In Alzheimer Disease. Current Alzheimer Research ( 2) :1-6.
• Senel S., Kremer M., Nagy K.,and Squier C., 2001. Delivery of Bioactive Peptides and Proteins across Oral (Buccal) Mucosa. Current Pharmaceutical Biotechnology, 2, 175-186.
• Sharma R. A., Gescher A.J., Steward W. P., 2005. Curcumin : The Story So Far. European Journal Of Cancer 41:1955-1968.
• Sharma R. A.,Heather R.M., 2001., Pharmacodynamic And Pharmacokinetic Study Of Oral Curcuma Extract In Patient With Colorectal Cancer. Clinical cancer research (7)1894-1900.
• Shojaei A.H., 1998. Buccal Mucosa as A Route for Systemic Drug Delivery. Journal of Pharmacy and Pharmaceutical Sciences.1 (1); 15-30.
• Singh B. and Ahuja.N., 2002. Development of Controlled-Release Buccoadhesive Hydrophilic Matrices of Diltiazem Hydrochloride: Optimization of Bioadhesion, Dissolution and Diffusion Parameters. Drug development and industrial pharmacy.28 (4),431-442.
• Singh B. Kaur S. and Ahuja N., 2006. Formulation and Optimization of Controlled Release Mucoadhesive Tablets of Atenolol Using Response Surface Methodology. AAPS PharmSciTech .7 (1) .1-4.
• Smart, J.D., 1991. An In vitro Assessment of Some Mucosa-Adhesive Dosage Forms. International Journal of Pharmaceutics. 73(1), 69-74.
• Squier, C.A., Cox, P., Wertz, P.W., 1991. Lipid Content and Water Permeability of Skin and Oral Mucosa. The J. Invest, Dermat, 96:123-126.
• Squier, C.A., Nany., D. 1985. Arch. Oral.Biol. 30:313-318.
• The Indian Materia Medica. 811-816.
• Thimma shetty J, Suresh Babu C, Udupa N.,1996. Preparation and Evaluation Of Buccal Dosage Forms Of Insulin. Pharmag. 8-14.
• Tripathi, K.D., 1999. Essentials of Medical pharmacology. 4th edition.
• Wani M.S., 2007.,Current Status In Buccal Drug Delivery. Pharmainfo.net.









