![]() About Author:
About Author:
Prathipati padmaja
vathsalya college of pharmacy, JNTU
prathipatipadmaja@gmail.com
INTRODUCTION
Buccal drug delivery was introduced by Orabase1 in 1947, when gum tragacanth was mixed with dental adhesive powder to supply penicillin to the oral mucosa (Sudhakar et al., 2006). In recent years, delivery of therapeutic agents through various transmucosal routes has gained significant attention.
Buccal delivery of drugs provides an attractive alternative to the oral route of drug administration, particularly in overcoming deficiencies associated with the latter mode of dosing. Buccal mucosa consist of stratified squamous epithelium supported by a connective tissue lamina propia (Squire and Wertz, 1996) was investigated as a site for drug delivery several decades ago and the interest in this area for the trasmucosal drug administration is still growing.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1620
Definition:
Buccal delivery is defined as drug administration through the mucosal membranes lining the cheeks (buccal mucosa).
The main impediment to the use of many hydrophilic macromolecular drugs as potential therapeutic agents is their inadequate and erratic oral absorption. The future challenge of pharmaceutical scientists is to develop effective nonparenteral delivery of intact proteins and peptides to the systemic circulation2.
Based on our current understanding of biochemical and physiological aspects of absorption and metabolism of many biotechnologically- produced drugs, they cannot be delivered effectively through the conventional oral route. Because after oral administration many drugs are subjected to presystemic clearance extensive in liver, which often leads to a lack of significant correlation between membrane permeability, absorption, and bioavailability (Sanders, 1990).
Difficulties associated with parenteral delivery and poor oral availability provided the impetus for exploring alternative routes for the delivery of such drugs. These include routes such as pulmonary, ocular, nasal, rectal, buccal, sublingual, vaginal, and transdermal. In absence of external stimuli to facilitate absorption, use of these alternative routes had limited success.
[adsense:468x15:2204050025]
LITERATURE REVIEW
* Ganesh P, et.al., have presented the Buccal drug delivery system as the effective drug delivery system, which eliminates the problems of hepatic first pass metabolism and drug degradation in the gastro-intestinal tract. This paper also discusses the evaluation of buccal drug delivery by the assessment of swelling index and bioadhesion study.
* Patel K.V. et.al., presents Buccal administration of drugs provides a convenient route of administration for both systemic and local drug actions. Key advantages and limitations related to the buccal drug delivery system has also been discussed in the review. In the development of these buccal drug delivery systems, mucoadhesion of the device is a key element. Mucoadhesive polymers have been utilized in many different dosage forms in efforts to achieve systemic delivery of drugs through the buccal mucosa. Recent innovations in the dosage form development and in vivo and in vitro mucoadhesion testing methods has also been focused.
* Navneet Verma, et.al., presented on the theories of mucoadhesion for the buccal drug delivery system. Among the various transmucosal routes, buccal mucosa has excellent accessibility, an expanse of smooth muscle and relatively immobile mucosa, hence suitable for administration of retentive dosage form. Direct access to the systemic circulation through the internal jugular vein bypasses drugs from the hepatic first pass metabolism leading to high bioavailability. Furthermore, films have improved patient compliance due to their small size and reduce thickness, compared for example tablets. Also presented the ideal properties of polymers and the preparation methods of films.
* Ganesh G.N.K, et.al., Prepared buccal tablets were comparatively evaluated for their physicochemical parameters like weight variation, hardness, thickness and friability test. The surface pH, swelling index, bio-adhesive strength, in-vivo residence time are also carried out which has been important. In vitro drug release rate has been studied.
* Asha S.John, et.al, have studied on the bilayered mucoadhesive tablets and evaluated the physcochemical properties for the buccal drug delivery like drug content, swelling study, matrix erosion, surface PH study etc, bioadhsion time etc.,
* Rahamatullah Shaikh, et.al., presented on Mucoadhesion, which is commonly defined as the adhesion between two materials, at least one of which is a mucosal surface. Over the past few decades, mucosal drug delivery has received a great deal of attention. Mucoadhesive dosage forms may be designed to enable prolonged retention at the site of application, providing a controlled rate of drug release for improved therapeutic outcome. Application of dosage forms to mucosal surfaces may be of benefit to drug molecules not amenable to the oral route, such as those that undergo acid degradation or extensive first-pass metabolism. The mucoadhesive ability of a dosage form is dependent upon a variety of factors, including the nature of the mucosal tissue and the physicochemical properties of the polymeric formulation. This review article aims to provide an overview of the various aspects of mucoadhesion, mucoadhesive materials, factors affecting mucoadhesion, evaluating methods.
* John D.Smart, presents the paper on the mechanism of drug delivery via the oral mucosa. The anatomy of oral mucosa also has been presented. The buccal route has been used for many years to deliver drugs such as certain steroids that are subjected to first-pass metabolism. Further recent interest in this route has been generated with regard to the non-parenteral delivery of new peptide and protein drugs produced as a result of advances in the biotechnology.
* Hitesh patel, prented a paper on the buccal drug delivery includes the factors affecting the drug delivery via the oral mucaosa, like molecular weight, flexibility, hydrogen-bonding capacity, cross-linking density, charge, concentration, hydration (swelling), and certain environmental factors. This paper also adds a note on the buccal mucoadhesive dosage forms like buccal films, buccal tablets, buccal gels and ointments, and buccal patches.
* A. Puratchikody, et.al., presents the future challenges and opportunities in the buccal drug delivery system. The recent innovations and applications are well explained in this paper. The commercially available buccal mucoadhesive dosage forms are listed in this paper. The formulation design also has been explained. The pharmaceutical, physiological, and the pharmacological considerations for the formulation design are well explained.
* Pranshu Tangri, et,al., presented a paper on the on the principles of mucoadhesive drug delivery systems based on adhesion to biological surfaces that are covered by mucus. An overview of the last decade’s discoveries on mucoadhesion and applications of mucoadhesive hydrogels as drug carriers is given. Techniques that are frequently used to study the adhesion forces and physicochemical interactions between hydrogel, mucus, and the underlying mucosa are reviewed. Mucoadhesive drug delivery systems is one of the most important novel drug delivery systems with its various advantages and it has a lot of potential in formulating dosage forms for various chronic diseases.
BUCCAL DRUG DELIVERY SYSTEM
Oral mucosa:
Anatomy of the oral mucosa
Light microscopy reveals several distinct patterns of maturation in the epithelium of the human oral mucosa based on various regions of the oral cavity.
Three distinctive layers of the oral mucosa are:
* the epithelium,
* basement membrane, and
* connective tissues.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The oral cavity is lined with the epithelium, below which lies the supporting basement membrane. The basement membrane is, in turn, supported by connective tissues (Fig. 1).
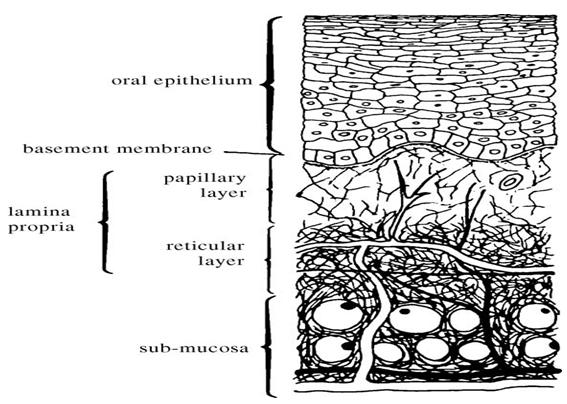
Fig. 1. Anatomy of the oral mucosa (Squier et al., 1976).
The epithelium, as a protective layer for the tissues beneath, is divided into:
(a) non-keratinized surface in the mucosal lining of the soft palate, the ventral surface of the tongue, the floor of the mouth, alveolar mucosa, vestibule, lips, and cheeks, and
(b) keratinized epithelium which is found in the hard palate and non-flexible regions of the oral cavity (Chen and Squier, 1984).
The epithelial cells, originating from the basal cells, mature, change their shape, and increase in size while moving towards the surface. The thickness of buccal epithelium in humans, dogs, and rabbits has been determined to be approximately 500–800 Am (Harris and Robinson, 1992)3.
The basement membrane forms a distinctive layer between the connective tissues and the epithelium. It provides the required adherence between the epithelium and the underlying connective tissues, and functions as a mechanical support for the epithelium. The underlying connective tissues provide many of the mechanical properties of oral mucosa.
The buccal epithelium is classified as a nonkeratinized tissue (Meyer and Gerson, 1964). It is penetrated by tall and conical-shaped connective tissues. These tissues, which are also referred to as the lamina propria, consist of collagen fibers, a supporting layer of connective tissues, blood vessels, and smooth muscles (Gandhi and Robinson, 1994).
The rich arterial blood supply to the oral mucosa is derived from the external carotid artery. The buccal artery, some terminal branches of the facial artery, the posterior alveolar artery, and the infraorbital artery are the major sources of blood supply to the lining of the cheek in the buccal cavity (Stablein and Meyer, 1984).
A gel-like secretion known as mucus, which contains mostly water-insoluble glycoproteins, covers the entire oral cavity. Mucus is bound to the apical cell surface and acts as a protective layer to the cells below (Allen et al., 1984). It is also a visco-elastic hydrogel, and primarily consists of 1–5% of the above-mentioned waterinsoluble glycoproteins, 95–99% water, and several other components in small quantities, such as proteins, enzymes, electrolytes, and nucleic acids. This composition can vary based on the origin of the mucus secretion in the body (Lehr, 1996,Haas and Lehr, 2002)4.
Oral mucosa, a barrier to permeability:
The effective permeability coefficient (Peff) values reported in the literature across the buccal mucosa for different molecules range from a lower limit of 2.2×10_9 cm/s for dextran 4000 across rabbit buccal membrane to an upper limit of 1.5×10_5 cm/s for both benzylamine and amphetamine across rabbit and dog buccal mucosa, respectively (Gandhi and Robinson, 1994).
This range clearly demonstrates the presence of a permeability barrier in the oral mucosa, which is mostly imposed by the oral epithelium acting as a protective layer for the tissues beneath, and as a barrier to the entry of foreign material and microorganisms. However, this range is estimated to be 4–4000 times more permeable than that of skin (Galey et al., 1976).
The permeability barrier property of the oral mucosa is predominantly due to intercellular materials derived from the so-called “membrane coating granules” (MCGs) (Gandhi and Robinson, 1994). MCGs are spherical or oval organelles that are 100–300 nm in diameter and found in both keratinized and non-keratinized epithelia. These organelles have also been referred to as “small spherically shaped granules”, “corpusula”, “small dense granules”, “small lamellated bodies”, “lamellated dense bodies”, “keratinosomes”, “transitory dense bodies”, and “cementsomes” ((Hayward, 1979) and references therein).
However, most of these descriptive names have not fully defined the functions of this cellular species. MCGs were first named as such because it was believed that they were subject to exocytosis from the cytoplasm of the stratum spinosum of keratinized epithelia following thickening of these cells. Nonetheless, it is actually the contents of MCGs that are subject to exocytosis prior to the onset of membrane thickening.
MCGs are found near the upper, distal, or superficial border of the cells, and a few occur near the opposite border ((Hayward, 1979)and references therein). Several hypotheses have been suggested to describe the functions of MCGs, including a membrane thickening effect, cell adhesion, production of a cell surface coat, cell desquamation, and permeability barrier.
Hayward has reviewed the literature related to these functions, and it appears that the permeability barrier is most often attributed to MCGs. They discharge their contents into the intercellular space to ensure epithelial cohesion in the superficial layers, and this discharge forms a barrier to the permeability of various compounds.
Cultured oral epithelium devoid of MCGs has been shown to be permeable to compounds that do not typically penetrate oral epithelium (Squier et al., 1978).In addition, permeation studies conducted using tracers of different sizes have demonstrated that these tracer molecules did not penetrate any further than the top 1–3 cell layers.
When the same tracer molecules were introduced sub-epithelially, they penetrated through the intercellular spaces. This limit of penetration coincides with the level where MCGs are observed. This same pattern is observed in both keratinized and nonkeratinized epithelia (Gandhi and Robinson, 1994). which indicates that keratinisation of the epithelia, in and of itself, is not expected to play a major role as a barrier to permeation (Squier and Hall, 1984)4.
The main mechanisms responsible for the penetration of various substances include:
* simple diffusion (paracellular, transcellular),
* carrier-mediated diffusion,
* active transport, and
* pinocytosis or endocytosis.
Recent evidence has shown that passive diffusion is the primary mechanism for the transport of drugs across the buccal mucosa, although carrier-mediated transport has been reported to have a small role.
Two routes of passive transport are available in the buccal epithelium; one involves the transport of compounds through the intercellular spaces between the cells (paracellular), and the other involves passage into and across the cells (transcellular).
Depending on the nature of the permeant, i.e. the overall molecular geometry, lipophilicity, and charge, either of the transport pathways across buccal epithelium can be selected.
While considerable evidence has been presented to document that most compounds diffuse through the buccal mucosa by passive diffusion or simple Fickian diffusion3,4 (Siegel, et al., 1971) some are transported by a carriermediated process across the buccal mucosa.
Glucose (Oyama et al., 1999)monocarboxylic acids and salicylic acid (Utoguchi et al., 1997)and nicotinic acid (Evered and Vadgama, 1981), are examples of substances which utilize a carrier-mediated diffusion mechanism for permeation across buccal epithelium.
Another barrier to drug permeability across buccal epithelium is enzymatic degradation. Saliva contains no proteases, but does contain moderate levels of esterases, carbohydrases, and phosphatases (Robinson and Yang,2001).
However, several proteolytic enzymes have been found in the buccal epithelium (Veuillez et al., 2001) Walker et alreported that endopeptidases and carboxypeptidases were not present on the surface of porcine buccal mucosa, whereas aminopeptidases appeared to be the major enzymatic barrier to the buccal delivery of peptide drugs. Aminopeptidase N and A (plasma membrane-bound peptidases) and aminopeptidase B (cytosolic enzyme) have been found in the buccal tissue (Kashi and Lee, 1986).The use of mucoadhesive polymers as enzyme inhibitor agents has been developed to overcome this obstacle in peptide and protein delivery.
BUCCAL DRUG DELIVERY SYSTEM
Definition: Buccal delivery is defined as drug administration through the mucosal membranes lining the cheeks (buccal mucosa).
The main impediment to the use of many hydrophilic macromolecular drugs as potential therapeutic agents is their inadequate and erratic oral absorption. The future challenge of pharmaceutical scientists is to develop effective nonparenteral delivery of intact proteins and peptides to the systemic circulation5.
Based on our current understanding of biochemical and physiological aspects of absorption and metabolism of many biotechnologically- produced drugs, they cannot be delivered effectively through the conventional oral route. Because after oral administration many drugs are subjected to presystemic clearance extensive in liver, which often leads to a lack of significant correlation between membrane permeability, absorption, and bioavailability (Sanders, 1990).
Difficulties associated with parenteral delivery and poor oral availability provided the impetus for exploring alternative routes for the delivery of such drugs. These include routes such as pulmonary, ocular, nasal, rectal, buccal, sublingual, vaginal, and transdermal. In absence of external stimuli to facilitate absorption, use of these alternative routes had limited success6.
The oral cavity is an attractive site for drug delivery due to ease of administration, avoidance of possible drug degradation in the gastrointestinal tract, and first-pass metabolism. Within the oral mucosal cavity, delivery of drugs is classified into three categories: (i) sublingual delivery, which is systemic delivery of drugs through the mucosal membranes lining the floor of the mouth (ii) buccal delivery, which is drug administration through the mucosal membranes lining the cheeks (buccal mucosa), and (iii) local delivery, which is drug delivery into the oral cavity.
The buccal region of the oral cavity is an attractive target for administration of the drug of choice. Buccal delivery involves the administration of the desired drug through the buccal mucosal membrane lining of the oral cavity. Unlike oral drug delivery, which presents a hostile environment for drugs, especially proteins and polypeptides, due to acid hydrolysis and the hepatic first-pass effect, the mucosal lining of buccal tissues provides a much milder environment for drug absorption. Other routes, such as nasal, ocular, pulmonary, rectal, and vaginal drug administration, have provided excellent opportunities for the delivery of a variety of compounds.
THEORIES OF BDDS
Mucoadhesion/bioadhesion
Definition
In 1986, Longer and Robinson defined the term “bioadhesion” as the “attachment of a synthetic or natural macromolecule to mucus and/or an epithelial surface” (Longer, and Robinson, 1986)7.
The general definition of adherence of a polymeric material to biological surfaces (bioadhesives) or to the mucosal tissue (mucoadhesives) still holds.
Mucoadhesion theories of polymer attachment:
Mucoadhesion is a complex process and numerous theories have been presented to explain the mechanisms involved.
These theories include:
* mechanical-interlocking,
* electrostatic,
* diffusion– interpenetration,
* adsorption and
* fracture processes.
Whilst undoubtedly the most widely accepted theories are founded surface energy thermodynamics and interpenetration/diffusion (Madsen et al., 1998).
These numerous theories should be considered as supplementary processes involved in the different stages of the mucus/substrate interaction, rather than individual and alternative theories (Bodde, 1989),
1.The wettability theory
The wettability theory is mainly applicable to liquid or low viscosity mucoadhesive systems and is essentially a measure of the ‘‘spreadability” of the API delivery system across the biological substrate. This theory postulates that the adhesive component penetrates surface irregularities, hardens and anchors itself to the surface.
The adhesive performance of such elastoviscous liquids may be defined using wettability and spreadability; critical parameters that can be determined from solid surface contact angle measurements. This process defines the energy required to counter the surface tension at the interface between the two materials allowing for a good mucoadhesive spreading and coverage of the biological substrate (Ugwoke et al., 2005)8. Therefore the contact angle (/), which may be easily determined experimentally, is related to interfacial tension (c), of both components using
γSG=γSL+ γLGcos Ø
S = γSG-(γSL- γLG),
Where, γLGis liquid–gas surface tension,
γSLis solid–liquid surface tension and
γSGis solid–gas surface tension.
Mucoadhesive polymer systems that exhibit similar structure and functional groupings to the mucus layer will show increased miscibility; this in turn will result in a greater degree of polymer spreadability across the mucosal surface.
Lower water: polymer contact angles of such systems will facilitate hydration of the polymer chains and thus promote intimate contact between polymeric delivery platform and the mucus substrate.
In the case of an extremely hydrophilic polymer however, the water contact angle will be much lower than that of the mucosal surface, thus discouraging such an intimate contact due to a high interfacial surface free energy (Shojaei and Li, 1997).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2. The electronic theory
This theory describes adhesion occurring by means of electron transfer between the mucus and the mucoadhesive system arising through differences in their electronic structures.
The electron transfer between the mucus and the mucoadhesive results in the formation of a double layer of electrical charges at the mucus and mucoadhesive interface9. The net result of such a process is the formation of attractive forces within this double layer (Dodou et al., 2005).
Controversy has surrounded this theory arising from the statement that electrostatic forces are an important cause of bond adhesion, rather than merely a result of high joint strength (Kinloch, 1980).
3. The fracture theory
According to this theory, the adhesive bond between systems is related to the force required to separate both surfaces from one another. This ‘‘fracture theory” relates the force for polymer detachment from the mucus to the strength of their adhesive bond.
The work fracture has been found to be greater when the polymer network strands are longer or if the degree of cross-linking within such as system is reduced (Ahagon and Gent, 1975).
This theory allows the determination of fracture strength (r) following the separation of two surfaces via its relationship to Young’s modulus of elasticity (E), the fracture energy (e) and the critical crack length (L) through the following equation9: (Gu et al., 1988)
σ = (E×?/L)½
4. The adsorption theory
In this instance, adhesion is defined as being the result of various surface interactions (primary and secondary bonding) between the adhesive polymer and mucus substrate.
Primary bonds due to chemisorption result in adhesion due to ionic, covalent and metallic bonding, which is generally undesirable due to their permanency (Kinloch, 1980) .Secondary bonds,arise mainly due to van der Waals forces, hydrophobic interactions and hydrogen bonding. Whilst these interactions require less energy to ‘break’ (Table 1) they are the most prominent form of surface interaction in mucoadhesion processes as they have the advantage of being semi-permanent bonds10 (Ahagon and Gent, 1975; Jiménez-Castellanos., et al., 1993).
|
Type Bond energy (kJ mol_1) |
|
Primarybonding
Ionic 590- 1050 Covalent 63-710 Metallic 113-347
Secondary bonding
Hydrogen bonding 10–42 Other dipole dipole 4–21 Dipole-induced dipole Deybe forces <2 Dispersion (London) forces 0.08–42 |
Typical bond types and energies, modified from Kinloch (Kinloch, 1980).
Table no.1: Typical bond types and energies
5. The diffusion-interlocking theory
This theory proposes the time-dependent diffusion of mucoadhesive polymer chains into the glycoprotein chain network of the mucus layer. This is a two-way diffusion process with penetration rate being dependent upon the diffusion coefficients of both interacting polymers.
Although there are many factors involved in such processes, the fundamental properties that significantly influence this inter-movement are molecular weight, cross-linking density, chain mobility/flexibility and expansion capacity of both networks (Lee et al., 2000). Furthermore, temperature also has been noted as important environmental factor for this process (Jabbari and Peppas, 1995).
Whilst it is acknowledged that longer polymer chains may diffuse, interpenetrate and ultimately entangle to a greater extent with surface mucus, it should be recognised that a critical chain length of at least 100,000 Da is necessary to obtain interpenetration and molecular entanglement.
Additionally excessive chain cross-linking will act to decrease the polymer mobility and thus interfacial penetration (Ludwig, 2005). Another significant contributory factor in determining interpenetration is the miscibility of both systems with one another. It is reasonable to postulate then that maximum diffusion and bioadhesive strength may be achieved when the solubility parameter (d) of the bioadhesive polymer and the mucus glycoprotein is similar (Vasir et al., 2003)12.
The time at which maximum adhesion occurs between two substrates during interpenetration has been supported by experimental evidence in recent studies using AFTFTIR and rheological techniques (Madsen et al., 1998).and may be determined using the depth of interpenetration (I), and the diffusion coefficient (Db) 11(Mikos and Peppas, 1986).
t = I2/ Db
MECHANISM OF BDDS
Drug delivery via the oral mucosa
Absorption of drug via the mucous membranes of the oral cavity can occur in either the sublingual, buccal, or local regions. The local region includes all areas other than the former two regions.
In general, the oral mucosa is classified as a somewhat leaky epithelium with a permeability rank order of sublingual>buccal>palatal, based on the thickness and degree of keratinization of the tissues (Harris and Robinson, 1992)13.
Different regions of the oral cavity vary greatly in terms of their composition and their potential utility in drug delivery. The thin and highly permeable membrane of the sublingual tissue is a perfect target if a prompt onset is desired. Considerable surface area and high blood flow to this region provide a means for rapid access to the systemic circulation. However, if a retentive, sustained-release system is desired, the sublingual membrane fails to be an appropriate target tissue.
Sustained-release systems, which are able to provide sustained drug concentrations in the systemic circulation due to delayed release of the drug from the formulation, are suitable dosage forms for the buccal region of the oral cavity. The lower permeability of this region compared to the sublingual site is ideal for controlled-release systems14.
Additionally, drug delivery via this site avoids extensive enzyme degradation and first-pass metabolism seen with oral administration, which is desired outcomes for the delivery of therapeutic proteins and peptides. However, the low permeability of this site is not always an attractive feature and, depending on the choice of drug, can be a major limitation. Use of sub-toxic levels of penetration enhancers and targeted delivery may potentially overcome this problem in the buccal region of the oral cavity.
Local delivery in the oral cavity has had particular applications in the treatment of toothache, periodontal diseases, and bacterial infections. However, because of its specificity, local delivery does not have the broad range of applications that sublingual and buccal drug administration provides.
ADVANTAGES
Advantages of BDDS:
* Among the various transmucosal routes, buccal mucosa has excellent accessibility, an expanse of smooth muscle and relatively immobile mucosa, hence suitable for administration of retentive dosage forms.
* Direct access to the systemic circulation through the internal jugular vein bypasses drugs from the hepatic first pass metabolism leading to high bioavailability15.
* Low enzymatic activity, suitability for drugs or excipients that mildly and reversibly damages or irritates the mucosa, painless administration, easy drug withdrawal, facility to include permeation
* Enhancer/enzyme inhibitor or pH modifier in the formulation and versatility in designing as multidirectional or unidirectional release systems for local or systemic actions etc opts buccal adhesive drug delivery systems as promising option for continued research (Alur et al., 2001).
* However, the effect of salivary scavenging and accidental swallowing of delivery system; barrier property of buccal mucosa stands as the major limitations in the development of buccal adhesive drug delivery systems16.
* In addition the drug is protected from degradation due to pH and digestive enzymes of the middle gastrointestinal tract.
* Improved patient compliance due to the elimination of associated pain with injections; administration of drugs in unconscious or incapacitated patients; convenience of administration as compared to injections or oral medications.
* Sustained drug delivery.
* A relatively rapid onset of action can be achieved relative to the oral route, and the formulation can be removed if therapy is required to be discontinued.
* Increased ease of drug administration
* Though less permeable than the sublingual area, the buccal mucosa is well vascularized, and drugs can be rapidly absorbed into the venous system underneath the oral mucosa.
* In comparison to TDDS, mucosal surfaces do not have a stratum corneum. Thus, the major barrier layer to transdermal drug delivery is not a factor in transmucosal routes of administration. Hence transmucosal systems exhibit a faster initiation and decline of delivery than do transdermal patches.
* Transmucosal delivery occurs is less variable between patients, resulting in lower intersubject variability as compaired to transdermal patches.
* The large contact surface of the oral cavity contributes to rapid and extensive drug absorption.
DISADVANTAGES
Disadvantages of BDDS:
* The disadvantages associated with this route of drug delivery are the low permeability of the buccal membrane (Rojanasakul et al., 1992) specifically when compared to the sublingual membrane, (Harris and Robinson, 1992; Gandhi and Robinson, 1994) and a smaller surface area15.
* The total surface area of the membranes of the oral cavity available for drug absorption is 170 cm2 (Collins and Dawes, 1987) of which ~50 cm2 represents non-keratinized tissues, including the buccal membrane (Lee, 2000).
* The continuous secretion of saliva (0.5–2 l/day) leads to subsequent dilution of the drug (Gandhi and Robinson, 1994).
* Swallowing of saliva can also potentially lead to the loss of dissolved or suspended drug and, ultimately, the involuntary removal of the dosage form16.
Moreover, the hazard of choking by involuntarily swallowing the delivery system is a concern, in addition to the inconvenience of such a dosage form when the patient is eating or drinking.
Limitations of Buccal Drug Delivery:
Depending on whether local or systemic action is required the challenges faced while delivering drug via buccal drug delivery can be enumerated as follows:
* For local action the rapid elimination of drugs due to the flushing action of saliva or the ingestion of foods stuffs may lead to the requirement for frequent dosing.
* The non-uniform distribution of drugs within saliva on release from a solid or semisolid delivery system could mean that some areas of the oral cavity may not receive effective levels.
* For both local and systemic action, patient acceptability in terms of taste, irritancy and ‘mouth feel’ is an issue15,16.
MARKETED PRODUCTS
Commercial buccal adhesive drug delivery systems (Batchelor, 2004)
Recent reports suggest that the market share of buccal adhesive drug delivery systems are increasing in the American and European market with the steady growth rate of above 10%. Some of the commercially available buccal adhesive formulations are listed in Table 1.417.
|
Commercial name |
Bioadhesive polymer |
Company |
Dosage form |
|
Buccastem |
PVP, Xanthum gum, Locust bean gum |
Rickitt Benckiser |
Tablet |
|
Suscard |
HPMC |
Forest |
Tablet |
|
Gaviscon liquid |
Sodium alginate |
Rickitt Benckiser |
Oral liquid |
|
Orabase |
Pectin, gelatin |
ConvaTech |
Oral paste |
|
Corcodyl gel |
HPMC |
Glaxosmithkline |
Oromucosal gel |
|
Corlan pellets |
Acacia |
Celltech |
Oromucosal pellets |
|
Fentanyl Oralet™ |
CP 934, Sodium CMC |
Lexicomp |
Lozenge |
|
Miconaczole Lauriad |
Modified starch, CP-934 |
Bioalliance |
Tablet |
|
EmezineTM |
CP 934 and PVP K-30 |
BDSI's |
Tablet |
|
BEMA Fentanyl |
…. |
BDSI's |
Tablet |
|
Straint™ SR |
CP 974, HPMCK4M |
Ardana |
Tablet |
|
Zilactin |
…. |
Zila |
Buccal film |
|
Luborant |
Sodium CMC |
Antigen |
Artificial saliva |
|
Saliveze |
Sodium CMC |
Wyvern |
Artificial saliva |
|
Tibozole |
Polycarbophil and CP 934P |
Tibotec |
Tablet |
Table no.2: commercially available buccal dosage forms
FORMULATION OF BDDS
Formulation design
General criteria for selection of drug candidate
1. Buccal adhesive drug delivery systems with the size 1–3 cm2 and a daily dose of 25 mg or less are preferable18.
2. The maximal duration of buccal delivery is approximately 4–8 hr (Alur et al., 1999).
3. Drug must undergo first pass effect or it should have local effect in oral cavity.
4. Drugs with biological half life 2-8 hr will in general be good candidates for sustained release dosage forms.
5. Local drug irritation caused at the site of application is to be considered while selecting the drug.
Pharmaceutical considerations
Great care needs to be exercised while developing a safe and effective buccal adhesive drug delivery device. Factors influencing drug release and penetration through buccal mucosa, organoleptic factors, and effects of additives used to improve drug release pattern and absorption, the effects of local drug irritation caused at the site of application are to be considered while designing a formulation19-21.
Buccal adhesive polymers
Polymer is a generic term used to describe a very long molecule consisting of structural units and repeating units connected by covalent chemical bonds. The term is derived from the Greek words: polys meaning many, and meros meaning parts (Rathbone et al., 1996).
The key feature that distinguishes polymers from other molecules is the repetition of many identical, similar, or complementary molecular subunits in these chains. These subunits, the monomers, are small molecules of low to moderate molecular weight, and are linked to each other during a chemical reaction called polymerization.
Instead of being identical, similar monomers can have varying chemical substituents. The differencesbetween monomers can affect properties such as solubility, flexibility, and strength. The term buccal adhesive polymer covers a large, diverse group of molecules, including substances from natural origin to biodegradable grafted copolymers and thiolated polymers. Bioadhesive formulations use polymers as the adhesive component.
These formulations are often water soluble and when in a dry form attract water from the biological surface and this water transfer leads to a strong interaction. These polymers also form viscous liquids whenhydrated with water that increases their retention time over mucosal surfaces and may lead to adhesive interactions22.
Bioadhesive polymers should possess certain physicochemical features including hydrophilicity, numeroushydrogen bond-forming groups, flexibility for interpenetrationwith mucus and epithelial tissue, and visco-elastic properties (Batchelor et al., 2004).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Ideal characteristics
-
Polymer and its degradation products should be non-toxic, non-irritant and free from leachable impurities.
-
Should have good spreadability, wetting, swelling and solubility and biodegradability properties.
-
pH should be biocompatible and should possess good viscoelastic properties.
-
Should adhere quickly to buccal mucosa and should possess sufficient mechanical strength.
-
Should possess peel, tensile and shear strengths at the bioadhesive range.
-
Polymer must be easily available and its cost should not be high.
-
Should show bioadhesive properties in both dry and liquid state.
-
Should demonstrate local enzyme inhibition and penetration enhancement properties.
-
Should demonstrate acceptable shelf life.
-
Should have optimum molecular weight.
Classification
In general, adhesive polymers can be classified as synthetic vs. natural, water-soluble vs. water-insoluble, and charged vs. uncharged polymers. Examples of the recent polymers classified in these categories are listed in Table 2. ( Nazila Salamat-Miller et al.,2005)
|
Criteria |
Categories |
Examples |
|
Source |
Semi-natural /natural |
Agarose, chitosan, gelatin |
|
|
|
Hyaluronic acid |
|
|
|
Various gums (guar, hakea, xanthan, |
|
|
Synthetic |
Cellulose derivatives |
|
|
|
[CMC, sodium CMC, HEC, HPC, HPMC] |
|
|
|
Poly(acrylic acid)-based polymers |
|
|
|
[CP, PC, PAA] |
|
|
|
Others |
|
|
|
Poly(N-2-hydroxypropyl methacrylamide) (PHPMAm) |
|
|
|
PVA, PVP, thiolated polymers |
|
Aqueous solubility |
Water-soluble |
CP, HEC, HPC, HPMC (cold water) |
|
|
Water-insoluble |
Chitosan (soluble in dilute aqueous acids), EC, PC |
|
Charge |
Cationic |
Aminodextran, chitosan, trimethylated chitosan |
|
|
Anionic |
Chitosan-EDTA, CP, CMC, pectin, PAA, PC |
|
|
Non-ionic |
Hydroxyethyl starch, HPC, |
Table no.3: Mucoadhesive polymers in buccal delivery
Some representative polymers
1. Hydrogels
Hydrogels often called as “wet” adhesives because they require moisture to exhibit the adhesive property. They are usually considered to be cross linked water swollen polymers having water content ranging from 30% to 40% depending on the polymer used. These are hydrophilic matrices that absorb water when placed in an aqueous media. This may be supplied by the saliva, which may also act as the dissolution medium. They are structured in such a manner that the cross linking fibers present in their matrix effectively prevent them from being dissolved and thus help them in retaining water23.
When drugs are loaded into these hydrogels, as water is absorbed into the matrix, chain relaxation occurs and drug molecules are released through the spaces or channels within the hydrogel network. Polymers such as polyacrylates (carbopol and polycarbophil), ethylene vinyl alcohol, polyethylene oxide, poly vinyl alcohol, poly (N-acryloyl pyrrolidine), polyoxyethylenes, self cross linked gelatin, sodium alginate, natural gums like guar gum, karaya gum, xanthan gum, locust bean gum and cellulose ethers like methyl cellulose, hydroxyl propyl cellulose, hydroxy propyl methyl cellulose, sodium carboxy methyl cellulose etc. form part of the family of hydrogels (Mishra et al., 1996).
2. Copolymers
Researchers are currently working on carrier systems containing block copolymers rather than using single polymeric system. Copolymerization with two or more different monomers results in chains with varied properties.
A block copolymer is formed when the reaction is carried out in a stepwise manner, leading to a structure with long sequences or blocks of one monomer alternating with long sequences of the other. These networks when composed of hydrophilic and hydrophobic monomers are called polymer micelle. These micelles are suitable for enclosing individual drug molecules. Their hydrophilic outer shells help to protect the cores and their contents from chemical attack by aqueous medium.
Most micelle-based systems are formed from poly (ethylene oxide)-b-polypropylene-b-poly (ethylene oxide) triblock network. There are also graft copolymers, in which entire chains of one kind (e.g., polystyrene) are made to grow out of the sides of chains of another kind (e.g., polybutadiene), resulting in a product that is less brittle and more impact-resistant.
Thus, block and graft copolymers can combine the useful properties of both\ constituents and often behave as quasi-two-phase systems (Lowman and Peppas, 1997).
3. Multifunctional polymers
These are the bioadhesive polymers having multiple functions. In addition to the possession of bioadhesive properties, these polymers will also serve several other functions such as enzyme inhibition, permeation enhancing effect etc.
Examples are polyacrylates, polycarbophil, chitosan etc.
4. Thiolated polymers
These are the special class of multifunctional polymers also called thiomers. These are hydrophilic macromolecules exhibiting freethiol groups on the polymeric backbone. Due to these functional groups various features of well established polymeric excipients such as poly (acrylic acid) and chitosan were strongly improved (Hornof et al., 2003).
Thiolatedpolymers designated thiomers are capable of formingdisulphide bonds withcysteine-rich sub domains of mucus glycoproteins coveringmucosal membranes24.
Consequently, the bridging structure most commonly used in biological systems is utilized to bind drug delivery systems on the mucosal membranes. By immobilization of thiol groups the mucoadhesive properties of poly (acrylicacid) and chitosan, was improved to 100-fold to 250- fold R. (Saviae et al., 2003; Allen et al., 1999).
Thiomers are capable of forming intra- and inter chain disulphide bonds within the polymeric network leading to strongly improved cohesive properties and stability of drug delivery systems such as matrix tablets. Due to the formation of strong covalent bonds with mucus glycoproteins, thiomers show the strongest mucoadhesive properties of all so far tested polymeric excipients via thiol disulphide exchange reaction and an oxidation process.
Zinc dependent proteases such as aminopeptidases and carboxypeptidases are inhibited by thiomers. The underlying mechanism is based on the capability of thiomers to bind zinc ions and this property is highly beneficial for oral administration of protein and peptide drugs.
They also exhibit permeation-enhancing effects for the paracellular uptake of drugs based on a glutathione-mediated opening process of the tight junctions (Kast et al., 2003; Leitner et al., 2003).
5. Milk protein
A particular example is a milk protein concentrate containing a minimum of 85% of proteins such as Prosobel L85, LR85F at concentration of 15% to 50%, preferably 20% to 30% in a bioadhesive tablet showed good bioadhesive property (United States Patent, 2005).
Permeation enhancers
Membrane permeation is the limiting factor for many drugs in the development of buccal adhesive delivery devices. The epithelium that lines the buccal mucosa is a very effective barrier to the absorption of drugs. Substances that facilitate the permeation through buccal mucosa are referred as permeation enhancer (Chattarajee and Walker, 1995).
As most of the penetration enhancers were originally designed for purposes other thanabsorption enhancement, a systemic search for safe and effective penetration enhancers must be a priority in drug delivery25.
The goal of designing penetration enhancers, with improved efficacy and reduced toxicity profile is possible by understanding the relationship between enhancer structure and the effect induced in the membrane and of course, the mechanism of action.
However, the selection of enhancer and its efficacy depends on the physicochemical properties of the drug, site of administration, nature of the vehicle and other excipients.
In some cases usage of enhancers in combination has shown synergistic effect than the individual enhancers. The efficacy of enhancer in one site is not same in the other site because of differences in cellular morphology, membrane thickness, enzymatic activity, lipid composition and potential protein interactions are structural and functional properties.
Penetration enhancement to the buccal membrane is drug specific (Shojaei et al., 1998). Effective penetration enhancers for transdermal or intestinal drug delivery may not have similar effects on buccal drug delivery because of structural differences; however, enhancers used to improve drug permeation in other absorptive mucosa improve drug penetrationthrough buccal mucosa. These permeation enhancers should be safe and non-toxic, pharmacologically and chemically inert, non-irritant, and non-allergenic (Aungst et al., 1994).
However, examination of penetrationroute for transbuccal delivery is important because it is fundamental to select the proper penetration enhancer to improvethe drug permeability. The differentpermeation enhancers available are (Aungst et al., 1994; Kurosaki et al., 1889; Lee and Crit, 1991).
- Chelators: EDTA, citric acid, sodium salicylate, methoxy salicylates.
- Surfactants: sodium lauryl sulphate, polyoxyethylene, Polyoxyethylene-9-laurylether, Polyoxythylene-20-cetylether, Benzalkonium chloride, 23-lauryl ether, cetylpyridinium chloride, cetyltrimethyl ammonium bromide.
- Bile salts: sodium glycocholate, sodium deoxycholate, sodium taurocholate, sodium glycodeoxycholate, sodium taurodeoxycholate.
- Fatty acids: oleic acid, capric acid, lauric acid, lauric acid/ propylene glycol, methyloleate, lysophosphatidylcholine, phosphatidylcholine.
- Non-surfactants: unsaturated cyclic ureas.
- Inclusion complexes: cyclodextrins.
- Others: aprotinin, azone, cyclodextrin, dextran sulfate, menthol, polysorbate 80, sulfoxides and various alkyl glycosides.
- Thiolated polymers: chitosan-4-thiobutylamide, chitosan- 4-thiobutylamide/GSH, chitosan-cysteine, Poly (acrylic acid)-homocysteine, polycarbophil-cysteine, polycarbophil- cysteine/GSH, chitosan-4-thioethylamide/GSH, chitosan- 4-thioglycholic acid.
Mechanisms of action of permeation enhancers:
Mechanisms by which penetration enhancers are thought to improve mucosal absorption are as follows (Ganem et al., 1996; Siegel et al., 1985). Changing mucus rheology: Mucus forms viscoelastic layer of varying thickness that affects drug absorption.
Further, saliva covering the mucus layers also hinders the absorption. Some permeation enhancers act by reducing the viscosity of the mucus and saliva overcomes this barrier.
- Increasing the fluidity of lipid bilayer membrane: The most accepted mechanism of drug absorption through buccal mucosa is intracellular route.
Some enhancers disturb the intracellular lipid packing by interaction with either lipid packing by interaction with either lipid or protein components.
- Acting on the components at tight junctions: Some enhancers act on desmosomes, a major component at the tight junctions there by increases drug absorption25.
- By overcoming the enzymatic barrier: These act by inhibiting the various peptidases and proteases present within buccal mucosa, thereby overcoming the enzymatic barrier. Inaddition, changes in membrane fluidity also alter the enzymatic activity indirectly.
- Increasing the thermodynamic activity of drugs: Some enhancers increase the solubility of drug there by alters the partition coefficient. This leads to increased thermodynamic activity resulting better absorption.
- Surfactants such as anionic, cationic, nonionic and bile salts increases permeability of drugs by perturbation of intercellular lipids whereas chelators act by interfering with the calcium ions,fatty acids by increasing fluidity of phospholipids and positively charged polymers by ionic interaction with negative charge on the mucosal surface (Senel et al., 2000).
Chitosan exhibits several favorable properties such as biodegradability, biocompatibility and antifungal/antimicrobial properties in addition to its potential bioadhesion and absorption enhancer (Senel et al., 2000; Schipper et al., 2004).
DOSAGE FORMS & ITS METHOD OF PREPARATION
Mucoadhesive dosage forms for buccal administration
1. General considerations in dosage form design
1.1. Physiological aspects
Constant flow of saliva and mobility of the involved tissues challenge drug delivery to the oral cavity. The residence time of drugs delivered to the oral cavity is typically short, in the range of 5–10 min (Lee et al., 2000). Buccal mucoadhesive formulations are expected to overcome this problem.
Bioadhesive polymers offer a means by which a delivery system is attached to the buccal mucosa, and hence, provide substantially longer retention times at the absorption site. They also provide a means to confine and maintain high local concentrations of the drug and/or excipient(s) to a defined, relatively small region of the mucosa in order to minimize loss to other regions and limit potential side effects.
The buccal mucosa is a very suitable region for bioadhesive system application because of its smooth and relatively immobile surface, as well as direct accessibility. However, there are some inherent limitations associated with buccal drug delivery, including short residence time, small absorption area, and barrier properties of thebuccal mucosa.
The size of a buccal dosage form is restricted by the very limited area available for application of the delivery system. This size restriction, in turn, limits the amount of drug that can be incorporated in the dosage forms.
In general, a buccal delivery device that is 1–3 cm2 in size (Anders et al., 1989)and a drug with a daily dose requirement of 25 mg or less (Gandhi et al., 1994)would be preferred.
In addition, an ellipsoid shape appears to be most acceptable (Anders et al., 1989), and the thickness of buccal delivery devices is usually limited to a few millimeters (Rathbone et al., 1994). The mucus layer covering the buccal mucosa is necessary for bioadhesive systems. Unfortunately, it not only forms a physical barrier to drug permeation, but also prevents long-term bioadhesion and sustained drug release by its short turnover time26,27.
Interestingly, the presence of bioadhesive polymers on a mucous membrane might alter theturnover of mucin, since the residence time of mucoadhesives are usually longer than the reported mucin turnover time (Lee et al., 2000). Nevertheless, the maximum duration for buccal drug delivery is usually limited to approximately 4–6 hr, since meal intake and/or drinking may require dosage form removal (Mitra et al., 2002).
1.2. Pathological aspects
Many diseases can affect the thickness of the epithelium, resulting in alteration of the barrier property of the mucosa. Some diseases or treatments may also influence the secretion and properties of the Mucus (Khanvilkar et al., 2001), as well as the saliva. Changes at the mucosal surface due to these pathological conditions may complicate the application and retention of a bioadhesive delivery device.
Therefore, understanding the nature of the mucosa under relevant disease conditions is necessary for designing an effective buccal delivery system. In addition, drugs with the potential of changing the physiological conditions of the oral cavity may not be suitable for buccal delivery28.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
1.3. Pharmacological aspects
A buccal dosage form may be designed to deliver a drug to the systemic circulation, or merely indicated for local therapy of the oral mucosa. Selection of dosage forms is affected by the intended application, target site of action, drug characteristics, and the site to be treated (periodontal pockets, gingival, teeth, buccal mucosa, or systemic)28.
1.4. Pharmaceutical aspects
Regardless of dosage form types, the drug must be released from the delivery system and subsequently taken up by the oral mucosa. Poor drug solubility in saliva could significantly retard drug release from the dosage form.
Cyclodextrin has been used to solubilize and increase the absorption of poorly water-soluble drugs delivered via the buccal mucosa (Jain et al., 2002). Other factors affecting both drug release and penetration through buccal mucosa must also be considered in the formulation design.
In addition to the physicochemical characteristics required for desirable drug release and absorption, organoleptic properties of the drug or the delivery device should also be considered, since the buccal delivery systems are to be exposed to a highly developed sensory organ. Some excipients may be incorporated to enhance the effectiveness and acceptability of the dosage forms. Selection of formulation excipients is yet another important consideration, since acidic compounds can stimulate the secretion of saliva, which enhances not only drug dissolution, but also drug loss by involuntary swallowing.
Besides, addition of a separate additive for each function could complicate and enlarge the dosage form, which might be problematic for buccal applications. Therefore, as mentioned previously, polymers with multiple functions seem promising. Permeability characteristics of the buccal mucosa may be continually changed by the rapid turnover of the buccal epithelium (3–8 days compared to about 30 days for the skin (Gandhi et al., 1994). Generally, the buccal mucosa is considerably less permeable, and hence, does not provide rapid absorption and good bioavailability seen with sublingual administration29.
Permeability of the buccal mucosa can be increased by various penetration enhancers capable of increasing cell membrane fluidity, extracting the structural intercellular and/or intracellular lipids, altering cellular proteins, or altering mucus structure and rheology (Veuillez et al., 2001). At present, bile salts, fatty acids, and sodium lauryl sulfate are the most commonly investigated penetration enhancers. As one example, incorporation of unsaturated fatty acids into the mucoadhesive polymers has been shown to be effective in buccal delivery of drugs. Unsaturated fatty acids such as oleic acid (18:1), eicosapentaenoic acid (20:5), and docosahexaenoic acid (22:6), when incorporated into PluronicR F-127 (PF-127) gel, have shown to be effective in buccal delivery of insulin (Morishita et al., 2001). The penetration enhancing effect of this class of fatty acids has also been shown in a formulation for the rectal delivery of insulin (Barichello et al., 1999).
Additionally, long chain unsaturated fatty acids are found to be permeation enhancers in the small intestine(Muranishi et al., 1990). The mechanism for the permeability enhancement by unsaturated fatty acids is through increasing the fluidity of the membrane phospholipids. This class of permeation enhancers reversibly alters the physical structure of the membrane by incorporating themselves into the phospholipid membrane.
Unfortunately, penetration enhancers always raise the concerns regarding their irritation and toxicity even though the oral mucosa is likely to be less sensitive to irreversible irritation or damage than other mucosal membranes, since it is routinely exposed to a multitude of foreign compounds (Murkle et al., 1986). The significant enhancement in drug permeation across the buccal mucosa provided by chitosan renders this bioadhesive polymer a very attractive excipient (Senel et al., 2000). Even though the enzyme activity in the buccal mucosa is relatively low and, as a result, drug inactivation is slower and less extensive than in other mucosal routes (De Vries et al., 1991), susceptible drugs, especially peptides and proteins, can still be degraded by the enzymes in saliva and buccal mucosa.
Therefore, enzyme inhibitors may be incorporated in the dosage forms to increase drug bioavailability. As previously mentioned, some bioadhesive polymers, such as poly (acrylic acid), polycarbophil, and carbopol, can also inhibit certain proteolytic enzymes (trypsin, a chymotrypsin, carboxypeptidases A and B, and leucine aminopeptidase(Lueben et al., 1999). However, cysteine protease (pyroglutamyl aminopeptidase) may not be inhibited by polycarbophil and carbopol(Lueben et al., 1999).
The pH-partitioning theory characteristic of passive diffusion also governs the transcellular permeability of ionizable drugs across the buccal mucosa, similar to other epithelial membranes. Maximal permeation occurs at the pH at which these drugs are predominantly in the unionized form. Control of pH is critical for successful buccal delivery of ionizable drugs.
Saliva has a weak buffering capacity to maintain pH value within local regions. It might be desirable to include some pH modifiers in the formulation in order to temporarily modulate the microenvironment at the application site for better drug absorption. It is worth noting that pH can also influence the charge on the surface of the mucus, as well as certain ionizable groups of the polymers, which might affect the strength of mucoadhesion.
In addition, it has been shown that the pH of the medium influences the degree of hydration of cross-linked poly (acrylic acid), e.g. polycarbophil (Ch’ng et al., 1985; Park et al., 1985). Therefore, the pH needs to be carefully chosen to optimize both drug permeation and mucoadhesion.
A formulation may be evaluated both in vitro and in vivo. Unfortunately, buccal drug administration to animals is difficult, and only rabbits and pigs have a non-keratinized mucosal lining similar to that in humans. As a result, only a small numbers of absorption studies have been studied in vivo. The most popular diffusion models are those in vitro experiments in which excised buccal mucosa is mounted in a diffusion apparatus. However, it is very difficult to maintain the integrity and viability of the excised animal tissues. Although in vitro experiments can prove useful for predicting possible trends in vivo, caution must be exercised when extrapolating in vitro data to in vivo situations.
As an example of an investigation aimed at assessing in vitro/in vivo correlation, Junginger et al. have evaluated the in vitro permeation of FITC-labeled, high-molecular-weight dextrans across excised porcine buccal mucosa, and compared these results with the in vivo administration of a buccal device to the oral cavity of pigs (Junginger et al., 1999). The results obtained demonstrated a less than optimal correlation between the in vitro and in vivo studies, even in the same species.
However, it should be noted that similar trends were observed in both experiments, where FITC-dextran with a molecular weight of 4000 was easily permeable across both membranes, and the permeability of this compound increased in the presence of a permeation enhancer, sodium glycodeoxycholate.
Buccal mucoadhesive dosage forms
Buccal mucoadhesive dosage forms can be categorized into three types based on their geometry.
Type I is a single layer device with multidirectional drug release. This type of dosage form suffers from significant drug loss due to swallowing.
In type II devices, an impermeable backing layer is superimposed on top of the drug-loaded bioadhesive layer, creating a double-layered device and preventing drug loss from the top surface of the dosage form into the oral cavity.
Type III is a unidirectional release device, from which drug loss is minimal, since the drug is released only from the side adjacent to the buccal mucosa. This can be achieved by coating every face of the dosage form, except the one that is in contact with the buccal mucosa29.
Buccal dosage forms can also be classified as either a breservoirQ or bmatrixQ type30:
In the reservoir type, an excessive amount of the drug is present in the reservoir surrounded by a polymeric membrane, which controls the drug’s release rate.
In the matrixtype systems, the drug is uniformly dispersed in the polymer matrix, and drug release is controlled by diffusion through the polymer network30.
In general, dosage forms designed for buccal drug delivery should be small and flexible enough to be acceptable for patients, and should not cause irritation. Other desired characteristics of a buccal mucosadhesive dosage form include high drug loading capacity, controlled drug release (preferably unidirectional release), good bioadhesive properties, smooth surface, tastelessness, and convenient application. Erodible formulations can be beneficial because they do not require system retrieval at the end of desired dosing interval.
A number of relevant buccal mucoadhesive dosage forms have been developed for a variety of drugs. Several peptides, including thyrotropin-releasing hormone (TRH), insulin, octreotide, leuprolide, and oxytocin, have been delivered via the buccal route, albeit with relatively low bioavailability (0.1–5%) owing to their hydrophilicity and large molecular weight(Veuillez et al., 2001), as well as the inherent permeation and enzymatic barriers of the buccal mucosa.
Buccal mucoadhesive dosage forms include tablets, patches, films, and semisolids (gels and ointments), and will be discussed briefly. Buccal dosage forms can be used to treat both local and systemic conditions.
A promising example of buccal mucoadhesive formulations for systemic use is the buccal delivery of salmon calcitonin (sCT) using thin-film composite containing40 Ag of sCT (200 IU)(Cui et al., 2002). Interestingly, in vivo studies in female New Zealand white rabbits demonstrated a relative bioavailability of 43.8F10.9%, and the reduction in plasma calcium level after the buccal administration of sCT was comparable to that observed when sCT was administered by the intravenous route. These results indicate that therapeutically effective amounts of salmon calcitonin can be delivered to the systemic circulation via the buccal mucosa. Buccal mucoadhesive dosage forms can also used for local therapy.
Van Roey and Haxaire (Van Roey et al., 2004)have developed buccal mucoadhesive tablets containing low dose (10 mg) of an antifungal drug, miconazole nitrate. When used once daily for 7–14 days in 357 HIV-positive patients suffering from oropharyngeal candidiasis, the clinical response was comparable to systemic therapy with 400 mg ketoconazole once daily.
In addition, the mucoadhesive tablet was generally well-tolerated and caused fewer incidences of gastrointestinal disorders and drug-related adverse events than those observed when ketoconazole was administered systemically. The authors suggested that this particular dosage form is the first and only once-daily topical treatment option for this condition (Van Roey et al., 2004). Although numerous buccal mucoadhesive dosage forms have been investigated, only a few products are commercially available. Striantkis a testosterone buccal system (tablet-like gum patch) recently approved by the United States Food and Drug Administration (FDA). It is indicated for replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone.The tablet is applied to the gum region twice daily. Interestingly, Striantk is designed to stay in position for as long as 12hr, which is the time required for the next regularly scheduled dose. Patients are recommended to check if Striantk is in place following tooth brushing, use of mouthwash, and consumption of food or alcoholic/ non-alcoholic beverages29.
Another commercially available product is the nitroglycerin buccal extended-release tablet (NitrogardR), used to treat angina in three ways:
* to relieve the pain of an angina attack (applied when the attack begins),
* to prevent expected attacks from occurring (when applied just before an attack is expected to occur),
* or to reduce the number of attacks (when applied regularly on a long-term basis).
The dosage for adults is 1 tablet every 3–5 hr while awake. It is worth mentioning that although Striantk and NitrogardR are classified as buccal dosage forms, they are intended for application between the upper lip and gum above the left and right incisors (the teeth just to the left and right of the two front teeth).
1. Buccal tablets
Tablets have been the most commonly investigated dosage form for buccal drug delivery to date (Table 3). Buccal tablets are small, flat, and oval, with a diameter of approximately 5–8 mm (Rathbone et al., 1994). Unlike conventional tablets, buccal mucoadhesive tablets allow for drinking and speaking without major discomfort. They soften, adhere to the mucosa, and are retained in position until dissolution and/or release is complete. These tablets can be applied to different sites in the oral cavity, including the palate, the mucosa lining the cheek, as well as between the lip and the Fm. Successive tablets can be applied to alternate sides of the mouth31.
The major drawback of buccal bioadhesive tablets is their lack of physical flexibility, leading to poor patient compliance for long-term and repeated use.
Preparation:
Bioadhesive tablets are usually prepared by direct compression, but wet granulation techniques can also be used. Tablets intended for buccal administration by insertion into the buccal pouch may dissolve or erode slowly; therefore, they are formulated and compressed with sufficient pressure only to give a hard tablet.
In order to achieve unidirectional release, every face of the tablet, except the one that is in contact with the buccal mucosa, can be coated with water impermeable materials, such as ethylcellulose, hydrogenated castor oil, etc., using either compression or spray coating31.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Multilayered tablets may be prepared by sequentially adding and compressing the ingredients layer by layer. If necessary, the drug may be formulated in certain physical states, such as microspheres, prior to direct compression in order to achieve some desirable properties, e.g. enhanced activity and prolonged drug release (Giunchedi et al., 2002).
Some newer approaches use tablets that melt at body temperatures (Rudnic et al., 2000). The matrix of the tablet is solidified while the drug is in solution. After melting, the drug is automatically in solution and available for absorption, thus eliminating dissolution as a rate-limiting step in the absorption of poorly soluble compounds.
|
Active ingredient |
Polymers used |
Investigators [Ref.] |
|
Propranolol HCl |
HPMC and PC |
Akbari et al., 2004 |
|
Piroxicam |
HPMC and CP 940 |
Jug et al., 2004 |
|
Pindolol |
CP 934, sodium CMC,HPMC and HPC |
|
|
Pentazocine |
CP 974P and HPMC |
Agarwal et a.,1999 |
|
Omeprazole |
Sodium alginate and HPMC |
Choi et al., 2000 |
|
Nifedipine |
CMC and CP |
Varshosaz et al., 2002 |
|
Nicotine |
CP 934 and HPC |
Park et al., 2002 |
|
Miconazole nitrate |
Mixtures of HPMC, sodium CMC, CP 934P, and sodium alginate |
Mohammed et al., 2003 |
|
Metronidazole |
HEC, HPC, HPMC, or NaCMC combined with CP 940, |
Perioli et al.,2004 |
|
|
CP 971, or PC |
|
|
Lignocaine HCl |
CP 934P, sodium CMC, and PVP K30 |
Parvez et al.,2002 |
|
Lactoferrin |
Sodium alginate |
Kuipers et al.,2002 |
|
Insulin |
CP 934 and HPC |
Ishida et al.,1981 |
|
Diltiazem HCl |
CP 934 with either HPC, |
Ahuja et al.,1995 |
|
Chlorpheniramine maleate |
Polyoxyethylene |
Tiwari et al.,1999 |
|
Acitretin |
CP 934P and HPMC |
Gaeta et al.,2003 |
Table no.4. List of investigated buccal mucoadhesive tablets
2. Buccal patches
Patches are laminates consisting of an impermeable backing layer, a drug-containing reservoir layer from which the drug is released in a controlled manner, and a bioadhesive surface for mucosal attachment. Buccal patch systems are similar to those used in transdermal drug delivery.
Preparation:
Two methods used to prepare adhesive patches include
* solvent casting
* direct milling.
In the solvent casting method, the intermediate sheet from which patches are punched is prepared by casting the solution of the drug and polymer(s) onto a backing layer sheet, and subsequently allowing the solvent(s) to evaporate31.
In the direct milling method, formulation constituents are homogeneously mixed and compressed to the desired thickness, and patches of predetermined size and shape are then cut or punched out. An impermeable backing layer may also be applied to control the direction of drug release, prevent drug loss, and minimize deformation and disintegration of the device during the application period. The drugs and polymers that have been used to develop buccal mucoadhesive patches are listed in Table 4.
|
Active ingredient |
Polymers used |
Investigators [Ref.] |
|
Acyclovir |
Copolymers of acrylic acid |
Shojaei et al.,1998,1994 |
|
Buprenorphine |
CP 934P, polyisobutylene, |
Guo.,1994; Guo et al.,1996 |
|
Cetylpyridinium chloride |
PVA, HEC, or chitosan |
Nafee et al.,2003 |
|
Metoprolol tartrate |
EudragitR NE40D with HPMC, |
Wong et al.,1993 |
|
Miconazole nitrate |
Sodium CMC, chitosan, |
Nafee et al.,2003 |
|
Oxytocin |
CP 974P |
Li et al.,1996 |
|
Terbutaline sulfate |
CP 934, CP 971, HPMC, HEC, |
Mohamed et al.,2000 |
|
Triamcinolone acetonide |
CP, poloxamer, and HPMC |
Chun et al.,2003 |
Table no.5. List of investigated buccal mucoadhesive patches
3. Buccal films
Films are the most recently developed dosage form for buccal administration (Table 6). Buccal films may be preferred over adhesive tablets in terms of flexibility and comfort.
In addition, they can circumvent the relatively short residence time of oral gels on the mucosa, which are easily washed away and removed by saliva. Moreover, in the case of local delivery for oral diseases, the films also help protect the wound surface, thus helping to reduce pain and treat the disease more effectively32.
An ideal film should be flexible, elastic, and soft, yet adequately strong to withstand breakage due to stress from mouth movements. It must also possess good bioadhesive strength in order to be retained in the mouth for the desired duration of action. Swelling of film, if it occurs, should not be too extensive in order to prevent discomfort.
Preparation:
Bioadhesive films are similar to laminated patches in terms of their flexibility and manufacturing process. They are usually manufactured by a solvent casting method. The drug and polymer(s) are first dissolved in a casting solvent or solvent mixture. The solution is then cast into films, dried, and finally laminated with a backing layer or a release liner. The backing layer helps retard the diffusion of saliva into the drug layer, thus enhancing the adhesion time and reducing drug loss into the oral cavity.
The solvent casting method is simple, but suffers from some disadvantages, including long processing time, high cost, and environmental concerns due to the solvents used. These drawbacks can be overcome by the hot-melt extrusion method recently reported by Repka et al. (Repka et al., 2002).
|
Active ingredient |
Polymers used |
Investigators [Ref.] |
|
Acyclovir |
Chitosan HCl and PAA sodium salt |
Rossi et al.,2003 |
|
Glibenclamide |
Chitosan and PVP |
Ilango et al.,1997 |
|
Insulin |
Gelatin and CP 934P |
Ritschel et al.,1989 |
|
Lidocaine |
HPC |
Okamoto et al., |
|
Nifedipine |
Sodium alginate, MC, PVP, and PEG |
Save et al.,1994 |
|
Testosterone |
PC and EudragitR S-100 |
Jay et al.,2002 |
Table no.6. List of investigated buccal mucoadhesive films
4. Buccal gels and ointments
Semisolid dosage forms, such as gels and ointments, have the advantage of easy dispersion throughout the oral mucosa. However, drug dosing from semisolid dosage forms may not be as accurate as from tablets, patches, or films32. Poor retention of the gels at the site of application has been overcome by using bioadhesive formulations (Table 6).
Certain bioadhesive polymers, e.g. poloxamer 407 (Miller et al., 1982), sodium carboxy methylcellulose (Wong et al., 1999), carbopol (Kumar et al., 1994), hyaluronic acid (Gurny et al., 1990), and xanthan gum (Meseguer et al.,1993)33, undergo a phase change from a liquid to a semisolid. This change enhances the viscosity, which results in sustained and controlled release of drugs. Hydrogels are also a promising dosage form for buccal drug delivery.
Preparation:
They are formed from polymers that are hydrated in an aqueous environment and physically entrap drug molecules for subsequent slow release by diffusion or erosion (Martin et al., 2003).
A major application of adhesive gels is the local delivery of medicinal agents for the treatment of periodontitis, which is an inflammatory and infectious disease that causes formation of pockets between the gum and the tooth, and can eventually cause loss of teeth. It has been suggested that mucoadhesive polymers might be useful for periodontitis therapy when incorporated in antimicrobial-containing formulations that are easily introduced into the periodontal pocket with a syringe (Ikinci et al., 2002).
Bioadhesive ointments have not been described in the literature as extensively as other dosage forms, especially when compared to tablets and patches (Ishida et al., 1983). HPMC has been used as an adhesive ointment ingredient (Ahuja et al., 1997).
|
Active ingredient |
Polymers used |
Investigators [Ref.] |
|
Diclofenac sodium |
Hydroxyethyl methacrylate |
Cassidy et al.,1993 |
|
Ergotamine tartrate |
PVA |
Tsutsumi et al.,2002 |
|
Flurbiprofen |
HEC, PVP, and PC |
Jones et al.,1999 |
|
Lidocaine |
PEG, CP 934P, |
Tan et al.,2000 |
|
Tetracycline |
HEC, PVP, and PC |
Jones et al.,1996 |
|
Triamcinolone |
Poloxamer 407 and |
Shin et al.,2000 |
Table no.7. List of investigated buccal mucoadhesive gels
FACTORS AFFECTING BDDS
Factors affecting mucoadhesion in the oral cavity
Mucoadhesive characteristics are a factor of both the bioadhesive polymer and the medium in which the polymer will reside. A variety of factors affect the mucoadhesive properties of polymers, such as molecular weight, flexibility, hydrogen bonding capacity, cross-linking density, charge, concentration, and hydration (swelling) of a polymer, which are briefly addressed below.
1. Polymer-related factors
1.1. Molecular weight
In general, it has been shown that the bioadhesive strength of a polymer increases with molecular weights above 100,000 (Chen and Cyr, 1970)34.
As one example, the direct correlation between the bioadhesive strength of polyoxyethylene polymers and their molecular weights, in the range of 200,000 to 7,000,000, has been shown by Tiwari et al. (Tiwari et al., 1999)35.
1.2. Flexibility
Bioadhesion starts with the diffusion of the polymer chains in the interfacial region. Therefore, it is important that the polymer chains contain a substantial degree of flexibility in order to achieve the desired entanglement with the mucus.
A recent publication demonstrated the use of tethered poly(ethylene glycol)–poly(acrylic acid) hydrogels and their copolymers with improved mucoadhesive properties (Huanget al., 2000) The increased chain interpenetration was attributed to the increased structural flexibility of the polymer upon incorporation of poly(ethylene glycol)36.
In general, mobility and flexibility of polymers can be related to their viscosities and diffusion coefficients, where higher flexibility of a polymer causes greater diffusion into the mucus network (Gu et al., 1998)37
1.3. Hydrogen bonding capacity
Hydrogen bonding is another important factor in mucoadhesion of a polymer. Park and Robinson found that in order for mucoadhesion to occur, desired polymers must have functional groups that are able to form hydrogen bonds (Park and Robinson, 1987). They have also confirmed that flexibility of the polymer is important to improve this hydrogen bonding potential38.
Polymers such as poly(vinyl alcohol), hydroxylated methacrylate, and poly(methacrylic acid), as well as all their copolymers, are polymers with good hydrogen bonding capacity (Peppas and Buri, 1985)
1.4. Cross-linking density
The average pore size, the number average molecular weight of the cross-linked polymers, and the density of crosslinking are three important and interrelated structural parameters of a polymer network (Gu et al., 1998)39.
Therefore, it seems reasonable that with increasing density of cross-linking, diffusion of water into the polymer networkoccurs at a lower rate which, in turn, causes an insufficient swelling of the polymer and a decreased rate of interpenetration between polymer and mucin40.
Flory (Flory, 1953)has reported this general property of polymers, in which the degree of swelling at equilibrium has an inverse relationship with the degree of cross-linking of a polymer.
1.5. Charge
Some generalizations about the charge of bioadhesive polymers have been made previously, where nonionic polymers appear to undergo a smaller degree of adhesion compared to anionic polymers41.
Peppas and Buri have demonstrated that strong anionic charge on the polymer is one of the required characteristics for mucoadhesion (Peppas and Buri, 1985). It has been shown that some cationic polymers are likely to demonstrate superior mucoadhesive properties, especially in a neutral or slightly alkaline medium (Park et al., 1989)42.
Additionally, some cationic high-molecular-weight polymers, such as chitosan, have shown to possess good adhesive properties (Lehr et al., 1992).
1.6. Concentration
The importance of this factor lies in the development of a strong adhesive bond with the mucus, and can be explained by the polymer chain length available for penetration into the mucus layer. When the concentration of the polymer is too low, the number of penetrating polymer chains per unit volume of the mucus is small, and the interaction between polymer and mucus is unstable (Peppas and Buri, 1985)43.
In general, the more concentrated polymer would result in a longer penetrating chain length and better adhesion. However, for each polymer, there is a critical concentration, above which the polymer produces an bunperturbedQ state due to a significantly coiled structure. As a result, the accessibility of the solvent to the polymer decreases, and chain penetration of the polymer is drastically reduced44.
Therefore, higher concentrations of polymers do not necessarily improve and, in some cases, actually diminish mucoadhesive properties. One of the studies addressing this factor demonstrated that high concentrations of flexible polymeric films based on polyvinylpyrrolidone or poly(vinyl alcohol) as film-forming polymers did not further enhance the mucoadhesive properties of the polymer. On the contrary, it decreased the desired strength of mucoadhesion (Solomonidou et al., 2001).
1.7. Hydration (swelling)
Hydration is required for a mucoadhesive polymer to expand and create a proper bmacromolecular meshQ of sufficient size, and also to induce mobility in the polymer chains in order to enhance the interpenetration process between polymer and mucin.
Polymer swelling permits a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding and/or electrostatic interaction between the polymer and the mucous network (Gu et al., 1998)45.
However, a critical degree of hydration of the mucoadhesive polymer exists where optimum swelling and bioadhesion occurs (Peppas and Buri, 1985).
2. Environmental factors
The mucoadhesion of a polymer not only depends on its molecular properties, but also on the environmental factors adjacent to the polymer. Saliva, as a dissolution medium, affects the behavior of the polymer. Depending on both the saliva flow rate and method of determination, the pH of this medium has been estimated to be between 6.5 and 7.5 (Rathbone et al., 1994)46.
The pH of the microenvironment surrounding the mucoadhesive polymer can alter the ionization state and, therefore, the adhesion properties of a polymer. Mucin turnover rate is another environmental factor. The residence time of dosage forms is limited by the mucin turnover time, which has been calculated to range between 47 and 270 min in rats (Lehr et al., 1991)and 12–24 h in humans (Forstner, 1978).
Movement of the buccal tissues while eating, drinking, and talking, is another concern which should be considered when designing a dosage form for the oral cavity. Movements within the oral cavity continue even during sleep, and can potentially lead to the detachment of the dosage form. Therefore, an optimum time span for the administration of the dosage form is necessary in order to avoid many of these interfering factors (Ho et al., 1992)46.
Future challenges and opportunities
The main impediment to the use of many hydrophilic macromolecular drugs as potential therapeutic agents is their inadequate and erratic oral absorption. The relatively recent evolution of recombinant DNA research and modern synthetic and biotechnological methodologies allow the biochemist and chemist to produce vast quantities of variety of peptides and proteins possessing better pharmacological efficacy.
However, therapeutic potential of these compounds lies in our ability to design and achieve effective and stable delivery systems.
The future challenge of pharmaceutical scientists will not only be polypeptide cloning and synthesis, but also to develop effective non-parenteral delivery of intact proteins and peptides to the systemic circulation.
Buccal permeation can be improved by using various classes of transmucosal and transdermal penetration enhancers such as bile salts, surfactants, fatty acids and derivatives, chelators and cyclodextrins47.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Researchers are now looking beyond traditional polymer networks to find other innovative drug transport systems. Much of the development of novel materials in controlled release buccal adhesive drug delivery is focusing on the preparation and use of responsive polymeric system using copolymer with desirable hydrophilic/hydrophobic interaction, block or graft copolymers, complexation networks responding via hydrogen or ionic bonding and new biodegradable polymers especially from natural edible sources.
At the current global scenario, scientists are finding ways to develop buccal adhesive systems through various approaches to improve the bioavailability of orally less/inefficient drugs by manipulating the formulation strategies like inclusion of pH modifiers, enzyme inhibitors, permeation enhances etc.
Novel buccal adhesive delivery system, where the drug delivery is directed towards buccal mucosa by protecting the local environment is also gaining interest. Currently solid dosage forms, liquids and gels applied to oral cavity are commercially successful47.
The future direction of buccal adhesive drug delivery lies in vaccine formulations and delivery of small proteins/peptides. Microparticulate bioadhesivesystems are particularly interesting as they offer protection to therapeutic entities as well as the enhanced absorption that result from increased contact time provided by the bioadhesive component.
Exciting challenges remain to influence the bioavailability of drugs across the buccal mucosa. Many issues are yet to be resolved before the safe and effective delivery through buccal mucosa. Successfully developing these novel formulations requires assimilation of a great deal of emerging information about the chemical nature and physical structure of these new materials47.
The future challenge in the development of buccoadhesive dosage forms is to modify the permeability barrier of the mucosa using safe and effective penetration enhancers. Mucoadhesive drug delivery systems available in the market include aftach tablet (Triamcinolone acetonide), suradrin tablet (Nitroglycerin), Buccostem tablet (prochlormperazine maleate). Salcoat powder sprays (Beclomethazone dipropionate. Rhinocort powder spray (Beclomethazone Dipropionate) and sucralfate (Aluminum hydroxide). Though there are only a few mucoadhesive formulations available currently, it can be concluded that drug delivery using mucoadhesive formulations offers a great potential both for systemic and local use in the near future.
Various strategies are being employed to achieve oral absorption of peptides. These strategies include manipulation of the formulation (e.g. inclusion of penetration enhancers or protease inhibitors etc.), maximizing retention of the delivery system at the site of absorption, and alteration of the peptide so as to optimize affinity for endogenous transport systems, build in chemical and metabolic stability, minimize the size and optimize the balance between lipophilicity and hydrogen bonding potential.
EVALUATION OF BUCCAL DDS
Evaluation of bi-layered tablets:
All the above batches were evaluated for average thickness, average weight and weight variation, hardness, friability, swelling index, surface pH, in vitro drug release, mucoadhesive strength,residence time and in vivo bioavailability studies.
Weight variation:
Collect 10 tablets from each formulation of varying concentration of natural polymer. Weigh the tablets individually from all the selected formulations; calculate the average weight and comparing the individual tablet weights to the average48.
Thickness:
Collect 3 tablets from each batch of formulation and the thickness of the tablets were measured with the help of vernier caliper. The average thickness is calculated49.
Friability
Friability of the tablets was determined by using Roche friabilator. From each batch, 6 tablets were weighed accurately which was W1 then placed in the friabilator and rotated at 25 rpm for 4 min. After completing the rotation weight of tablets were weighed which is W2. The percentage friability was determined50.
Hardness:
Monsanto hardness tester was used for this purpose. The hardness of five tablets in each batch was measured and the average hardness was calculated51.
Drug content
Five tablets were accurately weighed and powdered. A quantity of the powder equivalent to 8.0 mg of Atorvastatin calcium was weighed accurately and extracted in 100 ml methanol by shaking for 20 min. After filtration through whatmann filter paper no.1 and sufficient dilution with methanol, samples were analyzed spectrophotometrically at 246 nm. This procedure was repeated thrice. Amount of drug present was determined from the standard curve of Atorvastatin calcium in methanol52.
Matrix erosion
After swelling study, the swollen tablets were dried at 60°c for 24 h in an oven and kept in descicator for 48 h and reweighed (W3). Matrix erosion was calculated using following formula (Ramana MV. et. al., 2007)53.
% Matrix erosion = [(W1-W3) ÷ W3] × 100
In vitro swelling studies:
The swelling rate of buccoadhesive tablets are evaluated using 2% w/v agar gel plate. For each formulation, 3 tablets are weighed and average weight of each 3 tablets are calculated (W1). The tablets are placed with the core facing the gel surface in Petri dishes which are placed in an incubator. The tablets are removed at time intervals of 0.5, 1, 2, 3, 4, 5 and 6 hours, excess water on surface is absorbed using filter paper and swollen tablets are weighed. The average weight (W2) is determined and then swelling index is calculated using the formula54.
% Swelling index = ((W2-W1)/W1) ×100
Determination of surface pH of tablets:
Buccoadhesive tablets are left to swell for 2hrs on surface of agar plate. The surface pH is measured using pH paper placed on core surface of the swollen tablet. The surface pH of the buccal tablets was determined in order to investigate the possibility of any side effects in-vivo. As the acidic or alkaline pH may cause irritation to the buccal mucosa, the pH was maintained to neutral as closely as possible. A combined glass electrode was used for this purpose. The tablet was allowed to swell by keeping it in contact with 1 ml of distilled water (pH 6.5 ± 0.05) for 2 h at room temperature. The pH was measured by bringing the electrode in contact with the surface of the tablet and allowing it to equilibrate for 1 min55.
In-vitro mucoadhesion studies:
Mucoadhesive strength of the buccal tablets was measured on the “Modified Physical Balance method” which is shown in figure 6. The method used porcine buccal membrane as the model mucosal membrane. The fresh porcine buccal mucosa was cut into pieces and washed with phosphate buffer pH 6.8. The both pans were balanced by adding an appropriate weight on the left- hand pan. A piece of mucosa was tied to the surface of the beaker and placed below the left pan which was moistened with phosphate buffer pH 6.856. The tablet was stuck to the lower side of left pan with glue. Previously weighed beaker was placed on the right hand pan and water (equivalent to weight) was added slowly to it until the tablet detach from the mucosal surface. The both pans were balanced by adding an appropriate weight on the left- hand pan. The weight required to detach the tablet from the mucosal surface gave the bioadhesive strength. The experiment was performed in triplicate and average value was calculated57.
Force of adhesion = (mucoadhesive strength/100)×9.81.
In vivo residence time:
The in-vivo residence time was examined in humanvolunteers. The placebo buccal tablets were prepared and given to the human volunteers and advised to administer the tablet in the buccal region. The time required for the tablet to detach from the buccal region is determined as residence time58.
In vitro release studies:
The drug release rate from buccal tab paddle method at 370c 0.5 layer were attached to the glass slide with any glue and the glass slide is dropped into 900 ml of phosphate buffer pH 6.8 containing 0.2% of sodium lauryl sulphate. Samples are withdrawn at 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7 and 8 hours and replaced with fresh dissolution medium. The amount of labetalol released is determined spectrophotometrically59.
Release kinetics
In-vitro dissolution has been recognized as an important element in drug development. Under certain conditions it can be used as a surrogate for the assessment of bioequivalence. Several theories/kinetic models describe drug dissolution from immediate and modified release dosage forms. There are several models to represent the drug dissolution profiles where ft is the function of t (time) related to the amount of drug dissolved from the pharmaceutical dosage system. In order to elucidate mode and mechanism of drug release, the in-vitro data was transformed and interpreted at graphical interface constructed using various kinetic models. The zero order release Eq. (1) describes the drug dissolution of several types of modified release pharmaceutical dosage forms, as in the case of transdermal systems, matrix tablets with low soluble drugs, coated forms, osmotic systems etc., where the drug release is independent of concentration60.
Qt = Qo + Kot------------ (1)
Where, Qt is the amount of drug released in time t,
Qo is the initial amount of the drug in the solution and
Ko is the zero order release constant The first order
Eq. (2) describes the release from the system where release is concentration dependent e.g. pharmaceutical dosage forms containing water soluble drugs in porous matrices.
log Qt = log Qo + K1 t /2.303------- (2)
Where, Qt is the amount of drug released in time t,
Qis the initial amount of drug in the solution and
K is the firstorder release constant.
Higuchi describedthe release of drug from insoluble matrix as a squareroot of time
Qt = KH √t------------ (3)
Where, Qt is the amount of drug released in time t,
KH is Higuchi’s dissolution constant.
The following plots were made: cumulative % drug release vs. time (zero order kinetic model); log cumulative of % drug remaining vs. time (first order kinetic model); cumulative % drug release vs. square root of time (higuchi model).
Ex vivo Permeation Study
In this study, porcine buccal mucosa was used as a membrane. Diffusion studies were carried out, to evaluate the permeability of drug across the porcine buccal mucosal membrane, by using glass surface Franz diffusion cell. Porcine buccal mucosa was obtained from local slaughter house and used within 2 hrs of slaughter. The tissue was stored in phosphate buffer pH 7.4 solution upon collection. The epithelium was separated from underlying connective tissues with surgical scissors clamped between donor and receiver chamber of diffusion cells for permeation studies. The smooth surface of mucosa should face the donor chamber and receiver chamber was filled with phosphate buffer of 7.4 pH. Whole assembly was placed in water bath maintained at 37}10C.
Buccal epithelium was allowed to stabilization for period of 1hr and hydrodynamic in receiver chamber was maintained by stirring with magnetic bead at 50 rpm. After the stabilization of buccal epithelium, the patch was kept on buccal epithelium and 3ml of phosphate buffer of 6.8 ph was added in donor chamber. The sample of 1 ml were withdrawn at the time interval of 1 hour upto 8 hrs and replaced with equal volume of fresh dissolution medium. The sink condition was maintained throughout the study. The withdrawn sample was diluted to 5ml.The amount of Labetalol was determined by UV-VIS Spectrophotometer at 302 nm61.
In vivo oral bioavailability studies
Albino white rabbits weighing about 1.5-2Kg were used for oral bioavailability studies. All animal experiments were approved by Institutional Animal Ethical Committee, J.S.S.CollegeofPharmacy, Ooty (Proposal no:JSSCP/IAEC/M.PHAR/PH.CEUTICS/02/2010-11). Allthe rabbits were fasted overnight before theexperiments but had free access to water.
GROUPINGS: 3 animals were taken
Group 1- Control
Group 2- Drug suspension
Group 3- Buccal tablet
Drug suspension (0.3% w/v CMC) was administered orally by oral feeding tube at dose of 3.8 mg calculated based on human dose of 50mg, conversion factor = 0.07 (50*0.07=3.5mg, and for 1.6 dose is 3.8mg) Another animal was given anesthesia and premoistened buccal tablet was administered in the gingival area of the buccal cavity by opening then mouth of rabbit with help of forceps. Blood (0.5mL) was collected from marginal ear vein at 0, 0.50, 1, 2, 3, 5, 7, 8,12 hours after administration. Blood samples were placed into eppendorf tubes containing 0.3 ml solution and centrifuged immediately. After centrifugation, the plasma obtained was stored at - 200C until further analysis62.
Evaluation of bioadhesive properties
The evaluation of bioadhesive properties is fundamental to the development of novel bioadhesive delivery systems. These tests are also important to screen large number of materials and their mechanisms. Numerous methods have been developed for studying mucoadhesion. Since no standard apparatus is available for testing bioadhesive strength, an inevitable lack of uniformity between test methods has arisen. Nevertheless, three main testing modes are recognized – tensile test, shear strength, and peel strength63.
The most popular technique used for the determination of force of separation in bioadhesive testing is the application of force perpendicularly to the tissue/adhesive interface, during which a state of tensile stress is set up. But during the shear stress, the direction of the forces is reoriented so that it acts along the joint interface. In both tensile and shear modes, an equal pressure is distributed over the contact area.
Peel test:
The peel test is based on the calculation of energy required to detach the patch from the substrate. The peel test is of limited use in most bioadhesive systems. However, it is of value when the bioadhesive system is formulated as a patch64.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Tensile test & shear strength:
In tensile and shear experiments, the stress is uniformly distributed over the adhesive joint, whereas in the peel strength stress is focused at the edge of the joint. Thus tensile and shear measure the mechanical properties of the system, whereas peel strength measures the resistant of the peeling force65.
Review of the literature confirmed that the most common technique used for the measurement of bioadhesion test is tensile strength method. McCarron et al. and Donnellyhave reported extensively on the use of a commercial apparatus, in the form of a texture profile analyzer operating in bioadhesive test mode, to measure the force required to remove bioadhesive films from excised tissue in vitro63-66.
Fig. no.2:Texture profile analyzer in bioadhesion test mode
The texture analyzer, operating in tensile test mode and coupled with a sliding lower platform, was also used to determine peel strength of similar formulations.
Fig. no.3:Simplified representation of a typical test set-up used to determine peel strength of bioadhesive films
Rheological techniques that study the flow and deformation of materials may be useful in predicting the mucoadhesive ability of a polymeric formulation67-69. A simple rheological approach for polymer solutions and gels was first suggested by Hassan and Gallo. In this method, rheological interaction between a polymer gel and mucin solution was determined. It was shown that a polymer gel and mucin solution mixture exhibited larger rheological responsethan the sum of the values of polymer and mucin. However, a wide variation in results is found in the literature that utilize rheological methods for mucoadhesion determination, which may be attributable to differences in mucin type and concentration,70-73 as well as polymer concentrations.72 Therefore, Hagerstrom73recommend that the rheological method should not be used as a stand-alone method for studying the mucoadhesive properties of the polymer gels.
In vivo aspects of mucoadhesive testing have recently been reported to monitor the mucoadhesion on tissue surface such as the GIT or the buccal cavity. However, there are only a limited number of in vivo studies reported in the literature in vitro work because of the time, cost, and ethical constrains. The most common in vivo techniques to monitor mucoadhesion include GI transit times of bioadhesive-coated particles and drug release from in situ bioadhesive devices.
Ch’ng74studied the in vivo transit time for bioadhesive beads in the rat GIT. A 51Cr-labeled bioadhesive was inserted at selected time intervals; the GITs were removed. The GIT of the rat was then cut into 20 equal segments and the radioactivity was measured.
Davis74 investigated the noninvasive in vivo technique to determine the transit of mucoadhesive agent. Therefore, in this study a formulation was used containing a gamma-emitting radionuclide. The release characteristics and the position polymer could be examined by gamma scintigraphy.
In recent times, magnetic resonance imaging (MRI) is another noninvasive technique that is widely used. Christian Kremser74 used MRI to detect the time and location of release of mucoadhesive formulation using dry Gd-DOTA powder.
RECENT INNOVATIONS
Related to dosage forms:
1. Biobadhesive Spray:
Buccoadhesive sprays are gaining popularity over other dosage forms because of flexibility, comfort, high surface area and availability of drug in solution form. The fentanyl Oralet ™ is the first FDA-approved (1996) formulation developed to take advantage of oral transmucosal absorption for the painless administration of an opioid in a formulation acceptable to children75.
In 2002, the FDA approved Subutex (buprenorphine) for initiating treatment of opioid dependence (addiction to opioid drugs, including heroin and opioid analgesics) and Suboxone (buprenorphine and naloxone) for continuing treatment of addicts. In 2005, Oral-lyn buccal spray was approved for commercial marketing and sales in Ecuador.
2. Gel Forming Liquids:
This type of a formulation is liquid upon instillation and undergoes a phase transition to form a viscoelastic gel in response to stimulus such as temperature, ionic strength or pH. Carbomers become more viscous upon increased pH. Poloxamers and smart hydrogel®( Adnaced medical solution) gel at approximately body temperature76.
Gellan gum and alginate both form gel in response to increased ionic strength (particularly with Ca+2 ions). Gel forming formulations are currently used for sustained ocular delivery. Recent work has examined the oesophageal retention of smart Hydrogel77, a liquid that gels in response to both high force and temperature78, with its gelling temperature at about 32°C.
CONCLUSION:
In conclusion, the mucosal adhesive dosage forms are now on the starting line. The advantages are tremendous which make further study in this field extremely important. The formulation of these drug delivery systems depends on the developments of suitable polymers with excellent mucosal adhesive properties, stability and biocompatibility. The buccal cavity provides a highly vascular mucous membrane site for the dministration of drugs. The epithelial lining of the oral cavity differs both in type (keratinized and non-keratinized) and thickness in different areas, and the differences give rise to regional variations in permeability to drugs. So far, the oral mucosa has been utilized for the delivery of small drug molecules, since their adsorption occurs more reproducibly and rapidly. The main advantages of the buccal route of administration over the traditional per oral route are that drug degradation in the stomach is avoided, first-pass metabolism is avoided, and therapeutic drug levels of drug can be achieved rapidly. Clearly these advantages are presently clinically relevant for only a limited number of drugs. However, with the recent developments of new formulation types, such as mucoadhesive preparations and the use of peptides as drugs, this number may increase in the future.
REFERENCES:
1. Scrivener C.A., Schantz C.W., J AM Dental Assoc, 35, 1947, 644-647.
2. Harris D., Robinson J.R., Bioadhesive polymers in peptide drug delivery. Biomaterials 11, 1990, 652–658.
3. Hao J., and Heng P.W.S., Buccal Delivery System, Drug Dev. Ind. Pharm., 2003;29(8):821?832.
4. Odeblad E., The discovery of different type of cervical mucus, Bull. Ovul. Method Res. Ref. Cent. Aust, 1994;21:3?35.
5. P. D. Nakhat, A.A.Kondawar,Studies on buccoadhesive tablets of terbutaline sulphate, Indian Journal of Pharmaceutical sciences, Julyaugust 2007 page no:505-510.
6. Doijid RC, Manvi FC, Malleswara Rao V.S.N, Patel PS, Buccoadhesive drug delivery system of isosorbide : Formulation and evaluation, Year:2006, Volume:68, Issue:6, page:744-748.
7. Bagul U., Gujar K. and Bhavsar, In Vitro study of mucoadhesion strength of Polymers for mucoadhesive drug delivery systems, Int. J. Current Pharm. Res., 2009;1(1):42?46.
8. Ugwoke M.I., Agu R.U. and Kinget R., Nasal mucoadhesive drug delivery: background, application trends and future perspective, Adv. Drug Deliv. Rev., 2005;57:1640?1665.
9. Gu J.M. and Robinson J.R., Binding of acrylic polymers to mucin/epithelial surfaces: structure properties relationship, Crit. Rev. Ther. Drug Carrier Syst.,1988;5:21?67.
10. Ahagon A. and Gent A.N., Effect of interfacial bonding on the strength of adhesion, J. Polym. Sci. Polym. Phys., 1975;13:1285? 1300.
11. Mikos A.G. and Peppas N.A., Sysrem for controlled release of drugs : Bioadhesive systems, STP Pharma. Sci., 1986;19:13?32.
12. Aditya G., Gudas G.K. and Rajesham V.V., Design and evaluation of Controlled release Mucoadhesive buccal Tablets of Lisinopril, Int. J. Current Pharm. Res., 2010;2(4):24?27.
13. Sanders L.M. (1990) drug delivery systems and routes of administrations of peptide and protein drugs, Eur. J. Drug metab. Pharmacokinet, 15(2) 95-102.
14. Davis, S.S. (1991) From peptide to product- Delivery systems for biopharmaceuticals, Proc. Int. Symp. Control. Release Bioact. Mater 18, 65-66.
15. Lalla J.K. and Gurnancy R.A., Polymers for mucosal Delivery-Swelling and Mucoadhesive Evaluation, Indian Drugs, 2002, 39(5).
16. Wani M.S., Parakh S.R., Dehghan M.H., Polshettiwar S.A., Chopade V.V., Pande V.V., Current Status In Buccal Drug Delivery System, Pharmainfo.net, 5(2), 2007.
17. A. Puratchikody, Prasanth V.V, Sam T. Mathew, Ashok Kumar B, Buccal Drug Delivery: Past, Present and Future, International Journal of Drug Delivery 3 (2011) 171-184.
18. James C, Boylan. Drug delivery buccal route. In: James Swarbrick, editor. Encyclopedia of Pharmaceutical Technology: Supplement 3, Marcel Dekker INC 2001; Vol 20:P. 800-11
19. Metia PK, Bandyopadhyay AK. In vitro evaluation of novel mucoadhesive buccal tablet of oxytocin prepared with Diospyros peregrina fruit mucilages. Yakugaku Zasshi 2008;128:603-609.
20. Tanabe M, Watanabe M, Yanagi M, Nishizawa S, Chigono Y, Matsuda J, Yamaoka K, Inoue K. Controlled indomethacin release from mucoadhesive film in vitro and clinical evaluations. Yakugaku Zasshi 2008;128:1673-1679.
21. Malik DK, Baboota S, Ahuja A, Hasan S, Ali. Recent advances in protein and peptide drug delivery systems. J Curr Drug Deliv 2007;4:141-151.
22. Munasur VAP, Pillay DJ, Chetty, Govender T. Statistical optimization of the mucoadhesivity and characterization of multipolymeric propranolol matrices for buccal therapy. International Journal of Pharmaceutics 2006;323:43-51.
23. Thielmann1 F, Naderi1 M, Khutoryanskiy V, Khutoryanskaya O. Mucoadhesive hydrogel films based on blends of poly(acrylic acid) and methylcellulose. Available at: http://www.aapsj.org/abstracts/NBC_2007/NBC07-000679.PDF.
24. Andrew G P, Laverty T P and Jones D S. Mucoadhesive polymeric for controlled drug delivery. European Journal of Pharmaceutics and Biopharmaceutics, 71 (3), 2009, pp. 505-518.
25. N.K. Jain , Controlled and Novel drug delivery , CBS Publishers & Distributors, New Delhi, First edition 1997 (reprint in 2001)
26. Sudhakar Y, Knotsu K, Bandopadhyay AK. Bio adhesive drug delivery – A promising option for orally less efficient drugs. J Control Rel 2006;114:15-40.
27. Silvia Rossi, Giuseppina Sandri , Carla M. Caramella. Buccal drug delivery -A challenge already won. Drug Spring 2005;2:59-65.
28. Rajashree Mashru, Vijay Sutariya, Mayur Sankalia, Jolly Sankalia. Transbuccal delivery of lamotrigine across procine buccal mucosa in vitro determination of routes of buccal transport. J Pharm Pharmaceut Sci 2005;8:54-62.
29. J. Van Roey, M. Haxaire, M. Kamya, I. Lwanga and E. Katabira, Comparative efficacy of topical therapy with a slow-release mucoadhesive buccal tablet containing miconazole nitrate versus systemic therapy with ketoconazole in HIV-positive patients with oropharyngeal candidiasis, J. Acquir. Immune Defic. Syndr. 35 (2004), pp. 144–150.
30. Repka, M.A.; Repka, S.L.; McGinity, J.M. Bioadhesive Hot-Melt Extruded Film for Topical and Mucosal Adhesion Applications and Drug Delivery and Process for Preparation Thereof. US Patent 6,375,963, 2002.
31. M.J. Rathbone, B.K. Drummond and I.G. Tucker, The oral cavity as a site for systemic drug delivery, Adv. Drug Deliv. Rev. 13 (1994), pp. 1–22.
32. S.C. Miller and M.D. Donovan, Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits, Int. J. Pharm. 12 (1982), pp. 147–152.
33. C.F. Wong, K.H. Yuen and K.K. Peh, Formulation and evaluation of controlled release Eudragit buccal patches, Int. J. Pharm. 178 (1999), pp. 11–22.
34. J.L. Chen and G.N. Cyr, Compositions producing adhesion through hydration. In: R.S. Manly, Editor, Adhesion in Biological Systems, Academic Press, New York (1970), pp. 163–180.
35. D. Tiwari, D. Goldman, R. Sause and P.L. Madan, Evaluation of polyoxyethylene homopolymers for buccal bioadhesive drug delivery device formulations, AAPS PharmSci 1 (1999), p. E13.
36. Y. Huang, W. Leobandung, A. Foss and N.A. Peppas, Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces, J. Control. Release 65 (2000), pp. 63–71.
37. J.-M. Gu, J.R. Robinson and S.-H.S. Leung, Binding of acrylic polymers to mucin/epithelial surfaces: structure–property relationships, Crit. Rev. Ther. Drug Carr. Syst. 5 (1998), pp. 21–67.
38. H. Park and J.R. Robinson, Mechanisms of mucoadhesion of poly(acrylic acid) hydrogels, Pharm. Res. 4 (1987), pp. 457–464.
39. N.A. Peppas and P.A. Buri, Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues, J. Control. Release 2 (1985), pp. 257–275.
40. P.J. Flory, Principle of Polymer Chemistry, Cornell University Press, Ithaca, New York (1953), p. 541.
41. C.-M. Lehr, J.A. Bouwstra, E.H. Schacht and H.E. Junginger, In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers, Int. J. Pharm. 78 (1992), pp. 43–48.
42. D. Solomonidou, K. Cremer, M. Krumme and J. Kreuter, Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films, J. Biomater. Sci., Polym. Ed. 12 (2001), pp. 1191–1205.
43. M.J. Rathbone, B.K. Drummond and I.G. Tucker, The oral cavity as a site for systemic drug delivery, Adv. Drug Deliv. Rev. 13 (1994), pp. 1–22.
44. C.-M. Lehr, F.G.J. Poelma, H.E. Junginger and J.J. Tukker, An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop, Int. J. Pharm. 70 (1991), pp. 235–240.
45. J.F. Forstner, Intestinal mucins in health and disease, Digestion 17 (1978), pp. 234–263.
46. N.F.H. Ho, C.L. Barsuhn, P.S. Burton and H.P. Merkle, (D) Routes of delivery: case studies. (3) Mechanistic insights to buccal delivery of proteinaceous substances, Adv. Drug Deliv. Rev. 8 (1992), pp. 197–235.
47. Sudhakar Y, Knotsu K, Bandopadhyay AK. Bio adhesive drug delivery – A promising option for orally less efficient drugs. J Control Rel 2006;114:15-40.
48. Chaudary KP, Girish KK, Rao DG. Spectrophotometric determination of nitrendipine in pharmaceutical dosage forms. Indian Drug 1998; 35:645-7
49. Subrahmanyam CVS. “Text Book of Physical Pharmaceutics”. 2nd ed. New Delhi : Vallabh Prakashan 2001; 253-261.
50. Gilbert S. Banker and Neil R.Anderson Lachman, L, Liberman, H.A. and Knaig, J.L Eds. “The Theory and practice of Industrial Pharmacy”, (3rd edn),Varghese Publishing House, Bombay 1990; 293-345.
51. Vishnu M. Patel, Bhupendra G Prajapati, Harsha V Patel, Karshanbhi M patel. Mucoadhesive bilayer tablets of propranolol hydrochloride. AAPS PharmSciTech 2007; 8(3):E1- E6
52. Patel V.M, Prajapati B.G, Patel M.M, Design and characterization of chitosan containing mucoadhesive buccal patches of Propranolol hydrochloride, Acta Pharm., 57 (2007) 61– 72.
53. Thimmasetty J, Pandey G.S, Sathesh Babu P.R Design and in vivo evaluation of carvedilol buccal mucoadhesive patches, Pak. J. Pharm. Sci., 21(3) (2008) 241-248.
54. United States Pharmacopeia, XXIV NF 19,United State Pharmacopeia Convention, Rockville, (2000) 2388-2389
55. Ramana MV, Nagada C, Himaja M. Design and evaluation of mucoadhesive buccal drug delivery systems containing metoprolol tartrate. Ind J Pharm Sci 2007; 69(4):515-18.
56. Wani M.S, Dehghan M.H, Yadav V.B Mahendrakumar C, Polshettiwar S.A, Design and evaluation of terbutaline sulphate buccal patch, Res. J. Pharm. Tech., 2(1) (2009) 86-90. Boraie N.A, Nafee N.A, Ismail F.A, Mortada L.M, Mucoadhesive delivery systems. I. evaluation of mucoadhesive polymers for buccal tablet formulation, Drug Dev. Ind. Pharm., 30(9) (2004) 985–993. DOI: 10.1081/DDC-200037245
57. Jafar A, Ali N, Djavad F, Massoud A, Mahammad RSS. Development and evaluation of buccoadhesive propranolol hydrochloride tablet formulation: effect of fillers. Il Farmaco 2004; 59:155-161.
58. Rao YM, Vishnu YV, Chandrasekhar K, Ramesh G. Development of Mucoadhesive patches for buccal administration of Carvedilol. Current Drug Delivery 2007; 4:27-39.
59. Brahmankar DM, Jaiswal SB. biopharmaceutics and pharmacokinetics a treatise. 1st ed. Delhi 2003: 230-272
60. Shidhaye S.S, Saindane N.S, Sutar S,Kadam VMucoadhesive bilayered patchesfor administration of sumatriptan, AAPSPharmSciTech,9(3) (2009) 909- 916. DOI:10.1208/S12249-008-9125-X.
61. Vansi Vishnu Yamsani , Ramesh Gannu,Chandrashkar Kolli, Development and in vitro evaluation of buccoadhesive carvedilol tablets, Acta Pharm. 57 (2007) 185-197.
62. B. K. Satishbabu and B. P. Srinivasan, Preparation and Evaluation of buccoadhesive Films of Atenolol, Indian Journal of Pharmaceutical Sciences,marchapril 2008,page no:175-179.
63. Park K, Park H. Test methods of bioadhesion, Bioadhesive drug delivery systems. In: Lenaerts V, Gurney R, editors. Florida, Boca Raton: CRC Press; 1990.
64. McCarron PA, Donnelly RF, Zawislak A, Woolfson AD, Price JH, McClelland R. Evaluation of a Water-soluble Bioadhesive patch for photodynamic therapy of vulval lesions. Int J Pharm. 2005;293:11–23. [PubMed]
65. McCarron PA, Donnelly RF, Zawislak A, Woolfson AD. Design and evaluation of a water-soluble bioadhesive patch formulation for cutaneous delivery of 5-aminolevulinic acid to superficial neoplastic lesions. Eur J Pharm Sci. 2006;27:268–79. [PubMed]
66. Donnelly RF, McCarron PA, Zawislak AA, Woolfson AD. Design and physicochemical characterisation of a bioadhesive patch for dose-controlled topical delivery of imiquimod. Int J Pharm. 2006;307:318–25. [PubMed]
67. Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–5. [PubMed]
68. Rossi S, Bonferoni MC, Lippoli G, Bertoni M, Ferrari F, Caramella C, et al. Influence of mucin type on polymer-mucin rheological interactions. Biometerials. 1995;16:1073–9.
69. Hagerstrom H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci. 2000;9:301–9. [PubMed]
70. Rossi S, Ferrari F, Bonferoni MC, Caramella C. Characterization of chitosan hydrochloride--mucin rheological interaction: Influence of polymer concentration and polymer: Mucin weight ratio. Eur J Pharm Sci. 2001;12:479–85. [PubMed]
71. Hagerstrom H, Edsman K. Limitations of the rheological mucoadhesion method: The effect of the choice of conditions and the rheological synergism parameter Eur J Pharm Sci. 2003;18:349–57.
72. Ch’ng HS, Park H, Kelly P, Robinson JR. Bioadhesive polymers as platforms for oral controlled drug delivery.II Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers. J Pharm Sci. 1985;74:399–05. [PubMed]
73. Davis SS. The design and evaluation of controlled release systems for the gastro-intestinal tract. J Control Release. 1985;2:27–38.
74. Christian K, Karin A, Melanie G, Christian W, Paul D, Andreas BS. in vivo determination of the time and location of mucoadhesive drug delivery systems disintegration in the gastrointestinal tract. Magn Reson Imaging. 2008;26:638–43. [PubMed]
75. Michael J.R., Sevda S., Current status and the future of buccal drug delivery systems Indiran Pather , Expert Opin. Drug Deliv. 5(5), 2008, 531-542.
76. http://seekingalpha.com/article/71819-generex-biotechnology-oral-lyn-buccal-insulin-takes-center-stage, accessed on 27thMarch 2010.
77. Russell D.G.R., Conway B.R., Batchelor H.K., Proc Pharma Sci World Congress, 2004, 251
78. Lee V.H.L., Yamamoto A., Penetration and enzymatic barriers to peptide and protein absorption. Adv. Drug Del. Rev. 4(2), 1989, 171-207.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









