About Authors:
Mayure Vijay kumar*, V. Sravanthi Yadav, C.P.Meher
Department of Pharmacology,
Maheshwara College of Pharmacy, Chitkul (v), Isnapur “X” Road,
Patancheru, Hyderabad-502307
*mayurevijaykumar@gmail.com
ABSTRACT:
A prodrug is a pharmacological substance that is administered in an inactive (or less than fully active) form, and is subsequently converted to an active pharmacological agent (drug) through normal metabolic processes (bioactivation). A prodrug serves as a type of precursor to the intended drug. Prodrugs can be used to improve how the intended drug is absorbed, distributed, metabolized and excreted (ADME). Prodrugs are often designed to improve oral bioavailability in cases where the intended drug is poorly absorbed through the gastrointestinal tract. A prodrug may also be used to improve how selectively the intended drug interacts with cells or processes that are not its intended target. This reduces the adverse or unintended effects of the intended drug, especially important in treatments like chemotherapy, which can have severe unintended and undesirable side effects. This review explains about the prodrug.
REFERENCE ID: PHARMATUTOR-ART-1658
INTRODUCTION:
Many therapeutic drugs have undesired properties that may become pharmacological, pharmaceutical, or pharmacokinetic barriers in clinical drug application. Among the various approaches to minimize the undesirable drug properties while retaining the desirable therapeutic activity, the chemical approach using drug derivatization offers perhaps the highest flexibility and has been demonstrated as an important means of improving drug efficacy. The prodrug approach, a chemical approach using reversible derivatives, can be useful in the optimization of the clinical application of a drug. The prodrug approach gained attention as a technique for improving drug therapy in the early 1970s. Numerous prodrugs have been designated and developed since then to overcome pharmaceutical and pharmacokinetic barriers in clinical drug application, such as low oral drug absorption, lack of site specificity, chemical stability, toxicity and poor patient acceptance (bad taste, odour, pain at injection site, etc). A prodrug is a pharmacological substance (drug) that is administered in an inactive (or significantly less active) form. Once administered, the prodrug is metabolized in vivo into an active metabolite. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and excretion (ADME) optimization. Prodrugs are usually designed to improve oral bioavailability, with poor absorption from the GI tract usually being the limiting factor. Additionally; the use of a prodrug strategy increases the selectivity of the drug for its intended target. An e.g; of this can be seen in many chemotherapy treatments, in which the reduction of adverse effects is always of a paramount importance. Drugs used to target hypoxic cancer cells, through the use of Redox-activation, utilize the large quantities of reductase enzyme present in the hypoxic cell to convert the drug into its cytotoxic form, essentially activating it. As the prodrug has low cytotoxicity prior to this activation. There is a markedly lower chance of it “attacking” healthy, non-cancerous cells which reduces the side effects associated with these chemotherapeutic agents. In rational drug design, the knowledge of chemical properties likely to improve absorption and the major metabolic pathways in the body allows the modification of the structure of new chemical entities for improved bioavailability. Sometimes the use of a prodrug is unintentional, however, especially in the case of serendipitous drug discoveries, and the drug is only identified as a prodrug after extensive drug metabolism studies [25].
1) NEEDS OF PRODRUGS:
· Improve patient acceptability.
· Alter and improve absorption.
· Alter biodistribution.
· Alter metabolism
· Alter elimination.
· Drugs is not (sufficiently) bioavailable.
· Drug does not permeate the blood-brain barrier (dopamine, GABA).
· Drug has poor properties (solubility, taste).
· Drug has no (sufficient) chemical stability (active principles of acetylsalicyclic acid, isoniazid, omeprazole, clopidogrel).
· Drug has no (sufficient) organ or cell specificity(sulfamethoxazole, capecitabine, acyclovir).[25]
2) UNDESIRABLE PROPERTIES:
Physical properties:
· Poor aqueous solubility
· Low lipophilicity and chemical instability
Pharmacokinetic properties:
· Poor distribution
· Across biological membranes
· Good substrate for first pass metabolism
· Rapid absorption/excretion when long-term effect desired
3) CLASSIFICATION:
Prodrugs can be classified into two major types, based on how the body converts the prodrug into the final active drug form. Type I prodrugs are bioactivated intracellularly. Examples of these are anti-viral nucleoside analogs and lipid-lowering statins. Type II prodrugs are bioactivated extracellularly, especially in digestive fluids or in the body's circulation system. Examples of these are antibody-, gene- or virus-directed enzyme prodrugs [ADEP/GDEP/VDEP] used in chemotherapy or immunotherapy.
Both major types can be further categorized into Subtypes, based on factors such as (Type I) whether the intracellular bioactivation location is also the site of therapeutic action, or (Type 2) whether or not bio activation occurs in the body's gastrointestinal fluids or its circulation system.
4) MECHANISMS OF PRODRUGS:
· Metabolizing enzymes (most often)
· Chemical means [(hydrolysis, decarboxylation)] (less common)
5) TYPES OF PRODRUGS:
Major types of prodrugs (carrier-linked):
· Carboxylic acid and alcohols
· Amines
· Azo linkages
· Carbonyl compounds
Hard drugs:
· Compounds designed to contain the structural,
· Characteristic necessary for pharmacological activity,
· But in a form is not susceptible to,
· Metabolic or chemical transformation [26].
Characteristics of hard drugs:
· Production of toxic metabolites is avoided
· Increased efficiency of action
· Less readily eliminated[26]
Soft drugs:
Active compounds that after exerting their desired pharmacological effect are designed to undergo metabolic inactivation to give a nontoxic product.[26]
Subtypes
Type IA prodrugs include many antimicrobial and chemotherapy agents (e.g., 5-flurouracil).
Type IB Agents rely on metabolic enzymes, especially in hepatic cells, to bioactivate the prodrugs intracellularly to active drugs.
Type II Prodrugs are bioactivated extracelluarly, either in the milieu of GI fluids (Type IIA), within the systemic circulation and/or other extracellular fluid compartments (Type IIB), or near therapeutic target tissues/cells (Type IIC), relying on common enzymes such as esterases and phosphatases or target directed enzymes. Importantly, prodrugs can belong to multiple subtypes (i.e., Mixed-Type). A Mixed-Type prodrug is one that is bioactivated at multiple sites, either in parallel or sequential steps. For example, a prodrug, which is bioactivated concurrently in both target cells and metabolic tissues, could be designated as a “Type IA/IB” prodrug (e.g., HMG Co-A reductase inhibitors and some chemotherapy agents; note the symbol “ / ” applied here). When a prodrug is bioactivated sequentially, for example initially in GI fluids then systemically within the target cells, it is designated as a “Type IIA-IA” prodrug (e.g., tenofovir disoproxil fumarate; note the symbol “ - ” applied here). Many ADEPs, VDEPs, GDEPs and futuristic nanoparticle- or nanocarrier-linked drug moieties can understandably be Sequential Mixed-Type prodrugs. To differentiate these two Subtypes, the symbol dash “ - ” is used to designate and to indicate sequential steps of bioactivation, and is meant to distinguish from the symbol slash “ / ” used for the Parallel Mixed-Type prodrugs.[1]
Table 1: Classification of prodrugs [2]
|
Type |
Bioactivation site |
Subtype |
Tissue location of bioactivation |
Examples |
|
Type I |
Intracellular |
Type IA |
Therapeutic target tissues/cells |
Acyclovir, 5- fluorouracil, cyclophosphamide, diethylstilbestrol diphosphate L-dopa, 6-mercaptopurine, mitomycin C, zidovudine
|
|
Type I |
Intracellular |
Type IB |
Metabolic tissues (liver, GI mucosal cell,lung etc.) |
Carbamazepine, captopril, carisoprodol, heroin, molsidomine, paliperidone, phenacetin, primidone, psilocybin, sulindac, fursultiamine
|
|
Type II |
Extracellular |
Type IIA |
GI fluids |
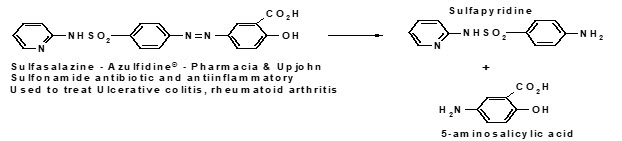
Lisdexamfetamine, loperamide oxide, oxyphenisatin, sulfasalazine
|
|
Type II |
Extracellular |
Type IIB |
Systemic circulation and Other Extracellular Fluid Compartments |
Acetylsalicylate, bacampicillin, bambuterol, chloramphenicol succinate, dihydropyridine pralidoxime, dipivefrin, fosphenytoin |
|
Type II |
Extracellular |
Type IIC |
Therapeutic Target Tissues/Cells |
ADEPTs, GDEPs, VDEPs |
6) EXAMPLES:
1. 6-Monoacetylmorphine: (6-MAM) is a heroin metabolite which converts into active morphine in vivo.
2. Carisoprodol: Is metabolized into meprobamate. Until 2012, carisoprodol was not a controlled substance in the United States, but meprobamate was classified as a potentially addictive controlled substance that can produce dangerous and painful withdrawal symptoms upon discontinuation of the drug.
3. Enalapril: Is bioactivated by esterase to the active enalaprilat.
4. Valaciclovir: Is bioactivated by esterase to the active aciclovir.
5. Fosamprenavir: Is hydrolysed to the active amprenavir.
6. Levodopa: Is bioactivated by DOPA decarboxylase to the active dopamine.
7. Chloramphenicol: Succinate ester is used as an intravenous prodrug of chloramphenicol, because pure chloramphenicol does not dissolve in water.
8. Psilocybin: Is Dephosphorylated to the active psilocin.
9. Heroin: Is deacetylated by esterase to the active morphine.
10. Molsidomine: Is metabolized into SIN-1 which decomposes into the active compound nitric oxide.
11. Paliperidone: Is an atypical antipsychotic for schizophrenia. It is the active metabolite of risperidone.
12. Prednisone: A synthetic cortico-steroid drug, is bioactivated by the liver into the active drug prednisolone, which is also a steroid.
13. Primidone: Is metabolized by cytochrome P450 enzymes into phenobarbital, which is major, and phenylethylmalonamide, which is minor.
14. Dipivefrine: Given topically as an anti-glaucoma drug, is bioactivated to epinephrine.
15. Lisdexamfetamine: Is metabolized in the small intestine to produce dextroamphetamine at a controlled (slow) rate for the treatment of attention-deficit hyperactivity disorder
16. Diethylpropion: Is a diet pill that does not become active as a monoamine releaser or reuptake inhibitor until it has been N-dealkylated to ethylpropion.
17. Fesoterodine: Is an antimuscarinic that is bioactivated to 5-hydroxymethyl tolterodine, the principle active metabolite of tolterodine.
18. Tenofovir disoproxil fumarate: Is an anti-HIV drug (NtRTI class) that is bioactivated to tenofovir (PMPA).
19. Ximelagatran: Is dealkylated and dehydroxylated to the active melagatran.
7) ADVANTAGES FOR PRODRUGS:
· Reduce pain associated with administration.
· Alter absorption (increased).
· Alter distribution.
· Alter metabolism.
· Alter elimination.
· Elimination of unpleasant taste associated with drug.
· Decreased toxicity.
· Increased chemical stability.
· Prolonged or shortened duration of action[26].
ADVANTAGES AND DISADVANTAGES OF PRODRUGS BY CHEMICAL MEANS:
· Chemical transformation does not depend on the presence or relative amounts of metabolizing enzymes; less interpatient variability.
· These compounds are generally chemically unstable-problems with storage or shelf-life.[26]
8) CARRIER LINKED PRODRUGS:
Drugs which have been attached through a metabolically labile linkage to another molecule (promoiety).
Prodrug linkage and Enzyme involved in hydrolyzing the link[28].
|
Prodrug Linkage |
Hydrolyzing enzyme |
|
1. Ester |
|
|
Short medium chain |
Cholinesterase |
|
Aliphatic |
Ester hydrolase, Lipase, cholesterol Esterase, Acetyl Cholinesterase, Aldehyde oxidase. |
|
2. Long chain aliphatic carbonate |
Pancreatic lipase, pancreatin, lipase, carboxypeptidase. |
|
3. Phos[hate, organic |
Acid phosphatise, alkaline phosphatise. |
|
s4. Sulphate, organic |
Steroidal sulfatase. |
|
5. Amide |
Amidase |
|
6. Amino acid |
Proteolytic enzyme, Trypsin, carboxypeptidase A and B. |
|
7. Azo |
Azo reductase |
|
8. Carbamate |
Carbamidase |
|
9. Phosphamide |
Phosphoramidase |
|
10. β-Glucuronide |
Β-Glucuronidase |
Promoiety involved in carrier-linked prodrugs:
Kicked off; The promoiety is not necessary for activity but may impart some desirable property to the drugs, such as increased lipid, water solubility, or site-directed delivery e.g; chloramphenicol.[26]
9) BIOPRECURSOR PRODRUGS:
Drugs contain no promoiety, but rather rely on metabolism to introduce the functionally necessary to create an active species.
Bioprecursor activation:
· Oxidation (most common)
· Reduction
· Phosphorylation (widely exploited in the development of antiviral agents)
Example: NSAID sulindac is inactive as the sulfoxide and must be reduced metabolically to the active sulphide
Metabolic sulindac: Sulindac is administrated orally, absorbed in the small intestine and subsequently reduced to the active species. Administration of the inactive form reduces GI irritation [26].
10) ABILITIES TO PREPARE PRODRUGS REQUIRE:
Either a hydroxyl groups or carboxyl groups present in the molecule [26].
11) MUTUAL PRODRUGS:
A slight variation on the carrier-linked prodrug in which the carrier (promoiety) also has to be therapeutic
Example: Estramustine (used in the treatment of prostate cancer)
Steroid portion directs drug to prostate, then hydrolysis occurs to give the normustard and CO2. The normustard then acts as an alkylating agent, exerting a cytotoxic effect. The estradiol also has an antiandrogenic effect on the prostate and slows cancer cell growth [26].
12) PRODRUG NOTION:
Prodrug or pro agent is the masked form of active drug, introduced by Albert and et.al, in 1950s, to increase the efficiency of drugs and to decrease its associated toxicity. Though given many names like latentiated drugs (since the concept was discussed in late 1950s), bio reversible derivatives, congeners, prodrugs suits a lot and is commonly accepted term[5]. Appropriate exploit of prodrugs was only since the late 19th century, which mainly focused to improve underisable properties of drugs [6]. Prodrugs can be defined as pharmacologically inert chemical derivatives that can be transformed in vivo to the active drug molecules, enzymatically or non enzymatically, to wield therapeutic effect. [7][8]
13) PERSONA OF AN IMPERATIVE PRODRUG:
An ideal prodrug should have the following characteristics so as to be released in the pharma market.
a. Readily transported to the site of action.
b. Rapid transformation in to active form.
c. Once the prodrug is selectively generated at the site of action, the tissue must retain the active drug without further degradation.
d. Release non-toxic metabolic fragments after its transformation with rapid elimination.[9][10]
14) PRODRUG THERAPY:
The prodrug design includes an infixed position that can be altered to improve in membrane permeability, solubility or absorption, distribution, metabolism, and elimination (ADME) properties. Different prodrug used for cancer therapies and other related therapies include:
1. Antibody-directed enzyme prodrug therapy.
2. Gene-directed enzyme prodrug therapy.
3. Virus-directed enzyme prodrug therapy.
4. Polymer-directed enzyme-activated prodrug therapy.
5. Lectin-Directed enzyme-Activated prodrug therapy.
6. Clostridial-directed enzyme prodrug therapy.
1. Antibody-directed enzyme prodrug therapy (ADEPT):
In this targeted therapy, tumor-specific antibody-enzyme conjugate is administered followed by an non-toxic prodrug after a specific time interval [11]. The antibody-enzyme conjugate will help in selective binding of enzyme to the tumor cell and then the prodrug converts in to active drug (toxic) by this targeted enzyme, thus only affecting the tumor and leaving back the normal cells. This idea of using antibodies to carry a specific enzyme to the tumor was explored by philpott et.al [12]. The first clinical trial of this is done using benzoic acid mustard L-glutamate, which is cleaved by carboxypeptides G2 to give L-glutamic acid and the more toxic nitrogen mustard derivatives of benzoic acid [13].
2. Gene-directed enzyme prodrug therapy (GDEPT):
GDEPT, also known as suicide gene therapy is a similar targeted technique where the gene for foreign enzyme is delivered (mostly by liposomal or polymer mediated gene delivery) [29-35] to the tumor cell which further activates the non toxic prodrug at the site of action [14]. Here the genes are controlled at transcription site by using specific promoter that becomes active in target cells transcribing to mRNA which further translates in to protein. GDEPT genes are isolated from different bacteria, yeast, plants and viruses. The best studied system which is under the clinical trial is herpes virus thymidine kinase (TK), used with the cold-sore drug ganciclovir (GCV), which is converted to a DNA synthesis inhibitor that is only active in dividing cells[15]. Many genetic modifications and mutations can be done to bring up this technique in effortless way. In certain cases, it is alleged that a single dose of this GDEPT gene will aid in bringing change in the tumor count.
3. Virus-Directed enzyme prodrug therapy (VDEPT):
In this pharmacologically oriented gene therapy, viral vector encoding the prodrug-activating enzyme are used followed by efficient treatment with prodrug to attain high levels of activated cytotoxic at the proposed site of action. VDEPT has three possible resource of toxoxity: vector, prodrug, and the vector/prodrug combination. It has been reported that, a VDEPT approach using the gene suicide system NTR/CB1954 (E.coli nitroreductase enzyme prodrug CB1954) combination appears promising for the treatment of cancer metastatic to the liver and the peritoneum [16]. Many such different viral, plasmid, retroviral, adenoviral vectors are used now a days for this target delivery process.
4. Polymer-Directed enzyme prodrug therapy (PDEPT):
A novel two step antitumor approach where the polymeric prodrug is administered, first promoting tumor targeting and later activating polymer-enzyme conjugate (PEG-L-asparginase).here the polymer enzyme conjugates are used as drug carrier, used as anticancer agent[17].this strategy is presently under phase I/II which includes conjugates like N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-doxorubicin (PK1, FCE28068). HPMA copolymer-paclitaxel(PNU 166945) etc. [18][19]. A recent technique involving the same technique is polymer-enzyme liposomes therapy (PELT) that can cause explosive release of drug from either polymeric prodrugs or liposomes within the tumor interstitium. Another novel technology, microsphere carrier systems made from biodegradable polymers, having bioadhesive property is coming in to picture dealing with the disease Myasthenia Gravis.
5. Lectin-Directed enzyme-Activated prodrug therapy (LEAPT):
LEAPT is a biparticle drug delivery system that exploits endogenous carbohydrate-to-lectin binding to localize glycosylated enzyme which determines site of drug release and a capped prodrug released by that enzyme[20] . Since this a new emerging technique, only little information has been shared regarding this and more have to be explored yet.
6. Clostridial-Directed enzyme prodrug therapy (CDEPT):
A fact of tumor- targeting properties of non-pathogenic strains of clostridia and spores which are incapable of germinating in healthy tissue made researchers to bring this novel technique called clostridial-directed enzyme prodrug therapy [21]. This came in to existence when Minton et.al declared that therapeutic proteins delivered using clostridial spores have been prodrug converting enzymes [22].specific reports on nitroreductase (NTR) class which converts the 4-nitrogroup of the prodrug CB1954(5-aziridinyl-2,4-dinitrobenzamide) to toxic 4-hydroxylamine (4HX) derivative (apoptosis-including agent) bought evidence to bring up this efficient therapy to treat many cancers[23].
15) APPLICATIONS OF PRODRUGS [28]:
1) PHARMACEUTICAL APPLICATIONS:
a) Improvement of taste:
One of the reasons for poor patient compliance particularly in case of children, is the bitterness, acidity or causticity of the drug.
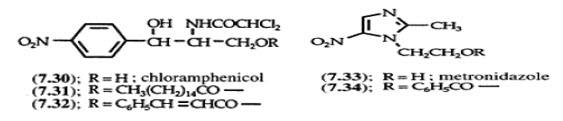
Two approaches can be “utilized to overcome the bad taste of drug”. Is reduction of drug solubility in saliva and is to lower the affinity of drug towards taste receptors. E.G. Parent drug. Chloramphenical palmitate ester is to increase patient acceptance.The antibacterial drug Clibdamycin is bitter and not well tolerated by children. Clindamycin palmitate is not bitter.
b) Improvement of odour:
The odour of a compound depends upon its vapour pressure. A liquid with high vapour pressure will mercaptan which is a foul smelling liquid, it is useful in the treatment of leprosy.
c) Change of physical form for preparation of solid dosage forms
d) Reduction of GI irritation
e) Reduction of pain on injection:
Intramuscular injection are particularly painful when the drug precipitates or penetrates in to the surroundings cells or when the solution is strongly acidic, alkaline or alcoholic, E.g; the lower aqueous solubility of clindamycin Hcl, the alkaline solution of phenytoin are responsible for pain on injection.
f) Enhancement of drug solubility and dissolution rate (hydrophilicity of drug)
· Anti inflammatory corticosteroids in the form of dosodium phosphate.
· Phosphate esters of steroidal anti-inflammatory agents phosphatases more rapidly available ACTIVE DRUG.
· Water soluble derivative of phenytoin

g) Enhancement of chemical stability of drug
· To prevent the breakdown on prolonged storage
· To prevent the rapid degradation on oral administration
h) Prodrug to eliminate Formulation problems:
Formaldehyde is a gas with a pungent odour that is used as a disinfectant. Too toxic for direct uses. It is a stable solid that decomposes in aqueous acid. The pH of urine in the bladder is about 4.8, so methenamide is used as a urinary tract antiseptic. Has to be enteric coated to prevent hydrolysis in the stomach.
i) Prodrug for improved absorption through skin:
· Corticosteroids -inflammation, allergic, pruritic skin conditions flucinolone acetonide.
· The cornea has significant esterase activity.
j) Prodrug for increased water solubility:
The ester must be stable enough in water for a shelf life of more than 2 years, but must be hydrolyzed in vivo with a hail-life < 10 minutes. To avoid formulation of etoposide with detergent, PEG, and EtOH (used to increase water solubility), it has been converted to the phosphate prodrug.
2) PHARMACOKINETIC APPLICATIONS:
a) Enhancement of bioavailability (lipophilicity)
b) Prevention of presystemic metabolism
c) Prolongation of duration of action
d) Reduction of toxicity
e) Site-specific drug delivery (drug targeting)
Areas of improvement for prodrugs:
1. Site specificity
2. Protection of drug from biodegradation
3. Minimization of side effects
4. Site-specific drug delivery
After its absorption into systemic circulation the drug is distributed to the various parts of the body including the target site as well as the non target tissues such a distribution pattern has several.[24]
16) DESIGN OF PRODRUG:
DESIGN OF PRODRUG: [28]
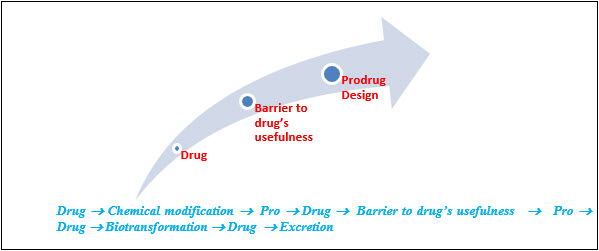
BARRIER’S TO PRODRUG: [28]
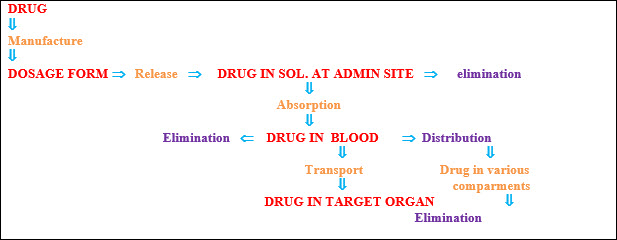
Steps in prodrug design:
Identification of drug delivery problem and identification of desired physicochemical properties-selection of transport moiety which will give prodrug desired transport properties and be readily cleaved in the desired biological compartment.
Design and Structure:
Currently, many prodrugs employ primarily hydroxyl, amine and carboxyl groups. Esters are found most commonly in commercial prodrug, such as the drug oseltamivir. More atypical groups have been investigated for use in prodrugs, such as thiols and imines. The bioconversion of the prodrugs to their active parent drugs is by way of enzyme activity of hydrolases. Prodrugs can be designed to bioconvert based upon the specific characteristic of the enzymes that catalyze the reaction, specifically substrate recognition.
Examples:
The prodrug Famvir has recently been developed in an attempt to prevent the spread of three different types of the herpes simplex virus: type 1 and type 2, and the varicella zoster virus. This drug contains two esters groups and requires two enzymes to bioconvert the molecule into its active form, which takes place predominantly in the liver.
Hepsera (Adefovir dipivoxil), another diester prodrug, is responsible for inhibiting nucleoside reverse transcriptase against the hepatitis B virus. Absorption of this drug occurs orally and at a rapid pace, with maximum absorption occurring after just ¾ of an hour.
Viread is a carbonate based prodrug, as opposed to the ester based prodrugs mentioned previously. It’s purpose is to inhibit the nucleoside transcriptase in the HIV virus and the hepatitis B virus. The carbonate group contained in this prodrug has been found to be more stable than the ester groups contained in the aforementioned prodrugs, while maximum absorption is reached more slowly. [3]
17) Active metabolite:
An active metabolite is an active form of a drug after it has been processed by the body. Sometimes drugs are formulated deliberately so they will break down inside the body to form the active drug, these are called prodrug. The reason for this may be because the drug is more stable during manufacture and storage as the prodrug form, or because the prodrug is better absorbed by the body or has superior pharmacokinectics. e.g.; lisdexamphetamine.
Metabolites of drug:
Another kind of active metabolite is when a drug is broken down by the body into a modified form which continues to produce effects in the body. Usually these effects are similar to those of the parent drug but weaker, although they can still be significant e.g.; 11-hydroxy-THC, morphine-6-glucuronide. Certain drugs , such as codeine and tramadol have metabolites that are stronger than the parent drug (morphine and o-desmethyltramadol)respectively and in these cases the metabolite may be responsible for much of the therapeutic action of the parent drug. Sometimes however metabolites may produce toxic effects and patients must be monitored carefully to ensure they do not build up in the body. This is an issue with some well known drugs as pethidine and dextropropoxyphene.[4]
18) PRODRUG TECHNOLOGIES:
TSRL’S proprietary prodrug technology enables the development of unique orally available prodrugs for individual therapeutic candidates. Prodrugs are optimized for oral bioavailability by utilizing intestinal carrier proteins, such as the dipeptide transporter (hPEPT1), as a shuttle. Drug candidates that are inherently not capable of crossing intestinal membranes to any appreciable extent can thereby reach oral bioavailability levels of 20-50%. This opens up a large market for the convenient oral delivery of potent therapeutics which currently have to be administered parenterally. This strategy also functions as an effective means of lifecycle extension through creation of novelchemical matter patents.
We use our understanding of intestinal physiology and associated molecular biology to enable identification of prodrug moieties that will uniquely benefit an individual drug candidate. Prodrug synthesis is based on well established chemical synthesis processes and projected costs of goods are low. Also since the drug candidates may already be marketed as a parenteral product, the development program of the prodrug may rely to some extent on existing toxicology information. Safety profiles can therefore be projected based on pharmacokinetic comparisons with the existing parenteral formulations, which will allow for a shorter and more focused toxicology strategy[27]
19) NANOCARRIERS USED FOR PRODRUG:
Nanocarriers are currently being widely used as prodrug vehicles because of their ability to enhance storage stability, modulate prodrug release and tumor-targeted delivery and protect against enzymatic attack. This combined approach of prodrugs and nanoparticles has a particular attraction for developing anticancer therapies. Polymeric nanoparticles and lipid nanoparticles are commonly used for prodrug encapsulation. Macromolecule prodrugs can spontaneously form self-assembled nanoparticles with no intervention of other additives. Expert opinion: more profiles involved in animal and clinical studies will encourage the future applicability of prodrug nanocarriers therapy. The possible toxicity associated with nanoparticles is a concern for development of prodrug delivery [29].
CONCLUSION:
Prodrug approach is setting a good example for the target drug delivery process which uses simple designing process considering the site of action, targeted cell/ tissue, type of administration etc. The strapping prop up for this is the large fraction of newly approved drugs that are prodrugs and similar inclination in the patent literature. Different categories of prodrug like pro-prodrugs, mutual drugs etc have been discussed each having their own specificities to be used as a patented drug. In the current appraisal, prodrug/enzyme related therapies are conversed giving importance mainly to the targeted drug delivery process considering different carrier molecules like antibiody, virus, gene, polymers etc, used to direct the drug towards the site of action. Nanocarriers used for prodrug delivery stratagem procured revolution in the present era of drug administration, proving its capability as enhanced nanomedicine.
REFERENCES:
1) Wu,K.M.: A New Classification of Prodrugs: Regulatory Perspectives Pharmaceuticals 2:77-81, 2009.
1) Adapted from Pharmaceuticals (2:77-81, 2009) and Toxicology (236:1-6, 2007).
2) Stella, VJ; Charman, WN; Naringrekar, VH (1985). "Prodrugs. Do they have advantages in clinical practice?". Drugs 29 (5): 455–73.
3) Imai, Teruko, and Masakiyo Hosokawa. “prodrug Approach usind carboxylesterases Activity: Catalytic properties and Gene Regulation of Carboxylesterase in Mammalian Tissue.
4) Muller CE(November 2009). “prodrug approaches for enhancing the bioavailability of drugs with low solubility”. Chemistry and biodiversity 6(11): 2071-83.
5) Harper NJ (1962) Drug Latentiation. Prodrug Res 4:221-294.
6) Kristiina MH, Raunio H, Rautio J (2011) Prodrugs-from serendipity to Rational Design. Pharmacological Reviews 63: 750-771.
7) Sinkula AA, Yalkowsky SH (1975) Rationale for Design of Biologically Reversible Drug Derivatives: prodrugs J pharm sci 63:181-210.
8) Stella VJ, charman WN, Naringrekar VH (1985) prodrugs. Do they have advantages in clinical practicle drugs 29: 455- 473.
9) Surya prakash Rao H (2003) capping Drugs: Development of prodrugs. Resonance 19-27.
10) Tegeli VS, Throat YS, chougula GK, shivsharam US, Gajeli GB, et.al.(2010) Review on concepts and advances in prodrug tech. International journal of drug formulation and research 1: 32-57.
11) Bagshawe KD (2006) Antobody-Directed Enzyme prodrug Therapy (ADEPT) for cancer. Expert Rev anticancer ther 6: 1421-1431.
12) Bagshawe KD, Sharma SK, Springer Rogers GT (1994) Antibody directed enzyme Prodrug Therapy (ADEPT) A Review of some theoretical, Experimental and clinical Aspects. Annals of Oncology 5:879-891.
13) Mauger AB, Burke PJ, Somani HH, Friedlos F, Knox RJ (1994) Self-Immolative Prodrugs: Candidates for Antibody-Directed Enzyme Prodrug Therapy in conjunction with a Nitroreductase Enzyme. J Med Chem 3: 3452-3458.
14) Vaghasia N, Federman N (2011) Liposomes for Targeting Cancer: One Step Closer to the Holy Grail of cancer Therapeutics J Nanomedic Biotherapeu Discover 1: 105e.
15) Both GW (2009) Gene-directed Enzyme prodrug therapy for cancer: A Glimpse in to the future? Discov Med 8: 97-103.
16) Chung-Faye G, Palmer D, Anderson D, Clark J, Downes M,et.al (2001) Virusdirected Enzyme prodrug therapy with Nitroimidazole ReductaseA phase I and Pahrmacokinetic study of its prodrug clin cancer Res 7: 2662-2668.
17) Plassat V, Renoir JM, Autret G, Marsaud V, Menager C,et.al. (2011) Systemic Magnetic Tatgeting of Pure-Antiestrogen-Loaded Superparamagnetic Nanovesicles for Effective Therapy of Hormone- Dependent Breast cancers. J. Bioanal Biomed S2:1.
18) Duncan R, Gac-Breton S, Keane R, Musila R, Sat YN,et.al (2001) polymer-Drug Conjugates, PDEPT And PELT: Basic Principles for design and Transfer from the Laboratory to clinic.J. Control Release 6: 135-146.
19) Sun CZ, Lu CT, Zhao YZ, Guo P, Tian JL, et.al (2011) characterization of the Doxorubicin-Pluronic F68 conjugate Micelles and their Effect on Doxorubicin resistant Human Erythroleukemic cancer cells,J Nanomedic Nanotechnol 2:114.
20) Garnier P, Wang XT, Robinson MA, van kasteren s, Perkins AC,et.al(2010) Lectin-Directed Enzyme Activated prodrug Therapy (LEAPT): synthesis and evaluation of Rhamnose-capped prodrugs.J. Drug Target 18: 794-802.
21) Shie-Chau Liu, Ahn G, Mitomu K, Mary JD, Patterson AV,et.al (2008) Optimized clostridium-Directed Enzyme prodrug Therapy improves the Antitumor Activity of the Novel DNA cross-Linking Agent PR-104. Cancer Res 68: 7995-8003.
22) Minton NP, Mauchline ML, Lemmon MJ, Brehm JK, Fox M,et.al. (1995) Chemotherapeutic Tumour Targeting using Clostridial spores. FEMS Microbiol Rev17: 357-364.
23) Palmer DH, Milner AE, Kerr DJ, Young LS, (2003) Mechanism of cell Death induced by the Novel Enzyme-Prodrug combination, Nitroreductase/CB1954. And Identification of Synergism with 5-Fluorouracil.Br J Cancer 89: 944-950.
24) Foye’s Chemistry Pharmaceutical Dosage Forms and Drug Delivery Systems, Howard C, Ansel Oral Drug Delivery Tech. Okunuru Jithan The theory and Pratice of Industrial pharmacy, Leon Laxhman and Libermann.
25) Monclerjacken 10z.npage.deDL. on December 17, 1012.
26) Unit 10 prodrugs: www.freezingblue.com
27) Wikipedia’s page on prodrug technologies wikipedia.org/wiki prodrug technologies.
28) Povl Krogsgaard-Larsen, Tommy Liljefors and Ulf Madsen Textbook of Drug Design and Discovery Third ed. First published 2002 by Taylor and Francis 11 New Fetter Lane, London EC4P 4EE.
29) Nanoparticles as delivery carriers for anticancer prodrugs, Fang, j. Y.,Al-Suwayeh,S.A.Expert.Opin.Drug. Deliv (2012)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









