About Authors:
Umakant Sahu1,2, Nrusingha Charan Panda2, B.V.V Ravikumar2, Anjan Kumar2
1Drugs testing laboratory avam anusandhan Kendra, First Floor, Govt. Ayurvedic College Hospital Building, Ayurvedic College Campus, G.E. Road Raipur, Chhattisgarh- 492010
2Department of Pharmaceutical Chemistry, Roland Institute of Pharmaceutical Sciences, Berhampur, Orissa-760010, India
uksahu28@gmail.com
{ DOWNLOAD AS PDF }
Abstract
Chalcones considered as the precursor of flavonoids and isoflavonoids abundantly found in plants, and have also been shown to display a diverse array of pharmacological activities. This review is complementary to earlier reviews and covers more recent report of antimicrobial activity of chalcones as well as its important role as an intermediate for the synthesis of different synthetic molecules. The enone linkage presents in basic structure play crucial role for the activity of chalcone and chalcone derivatives. The members of the chalcone and flavonoid family have attracted a great deal of interest due to it has showed diffent activity as antibacterial, antifungal, anti-inflammatory, antihyperglycemic anti HIV and anticancer pharmacological activity. Recently a molecule of chalcone has been reported as a novel class of HIV-1 integrase inhibitors. The purpose of this review is to focus on some important pharmacological activity of the chalcone and its derivatives.
REFERENCE ID: PHARMATUTOR-ART-2071
1. Introduction
Chalcones are open chain flavonoids whose basic structure includes two aromatic rings bound by a three carbon α, β-saturated carbonyl group. They are usually obtained from natural product with extractive techniques, or by several homogenous[1]. Chalcones are polyhydroxylated in the aryl ring and phenolic group present in basic structure shows redical quenching properties. Chalcones rich plant extracts or chalcone compounds are also raised great interest as drugs or food preservatives due to its different pharmacological properties[2]. Chalcone are important intermediates in the synthesis of many pharmaceuticals and utilization of heterogeneous catalyst for the production of chalcones can also been used but there is the some article which state that the use of activated carbon as catalyst in the combination with ultrasounds, because their extended surface area, micro porous structure and high degree of surface activity[3]. Infectious disease caused by bacterial, fungi, viruses and parasites are still a major threat to public health, despite tremendous progress in medicinal chemistry[4] and importance of chalcone lies in the wide range of biological and chemical properties they possess, for this reason, they have been the mainly aimed at determining their structural and chemical reactivity[5]. Chalcones or 1, 3 diphenyl-2-propene -1-one have been found to posses various biological activities like antibacterial, antifungal, antimalarial, mutagenic, anticancer, anticytotoxic antileismenial antiprotozal, tyrosinase inhibitors, lipid peroxide inhibitors, immunosuppressive agent[6]. The solvatochromic hydrazones anions also can be derived from the chalcones, dinitrophenylhyrazones posses an acidic NH, capable of generating anions with an enhanced electron donating ability. Conjugation of this nitrogen anion with an acceptor group should lead to potentially interesting solvatochromic dyes[7]. Phytochemical studies on bioactive constituents of Vietnamese plants have examined the hitherto non investigated species vitex leptobotry, the ariel part of this plant besides series of known chalcones and ecdysteroids the three new chalcone[8]. Recently, licochalconeA, isolated from chinise liquorice root has been reported highly effective in an in vitro screen against chloroquine sensitive and chloroquine resistance isolated of plasmodium falciparum[9]. Due to the rapid development of bacterial and fungal strained resistant to the antibacterial or antifungal agent poses a significant threat to global health because of that an urgent need to discover and develop new antimicrobial agents[10].
The fungal threat will continue to increase as shown by occurrence of aspergillosis in severe acute respiratory syndrome (SARS) and by the inclusion of coccidioides humitis as a potent agent of bioterrorism .By the continuous efforts we have isolated furanoflavonoids from plant Pongamia pinnata fruits are mainly karanjin and pongamol[11]. The object of the review article is to summarize the discovery and developments of new chemical moiety containing basic chalcone structure. The aerial part of plant Crotalari Orixensis reported chalcone shows antimalarial activity [12].
2. Pharmacological activity
2.1 Antibacterial activity
During the past decades the environment consciousness compelled the chemist to make a new twist on an old theme. Solid supported reagentshave made a landmark and made significant contribution to preserve the green enviroment by reducing the waste effluent with the development of microwave ovensreaction is dry media. Tetrazole, thiadiazole, quinoline, indole and triazole derivatives are well known for their significant biological activities. Thiadiazepine are not only known for their potent antimicrobial activities but they are also excellent charge generating agents. They are also as intermediate for preparation of substituted caprolatum useful for the treatment of HIV diseases. Microwave accelerated solid state approach for their rapid assembly of substituted 1,2,4 triazolo [ 3,4 –b ]-1,2,4 thiadiazepines the antibacterial activity against five bacterial strains .
Escherichia coli, Bacillus subtilis, pseudomonas aergenosa, klebsilla aerogenosa and brodetella bronchiseptica were used to determined antibacterial activity as measured by cup plate agar diffusion method. Compound were dissolved in phosphate buffer at concentration of 50µg/mL, All compounds 1a, 1c, 1e and 2a were most potent and comparable to activity of norfloxacin. Only weak activity was observed with the other compound 1d, 2c, and 2d [13].
Compound containing tetrazole derivative gives good inhibition against bacillus subtilis and brodetella bronchiseptica respectively. Multi drug resistance gram positive bacterial including methicilline resistant staphylococcus aureus (MRSA), methicillin resistant staphylococcus epidermidis (MRSA) and vancomycin reisistant enterococcus (VRE) failed to address essential aspect of drug usage.
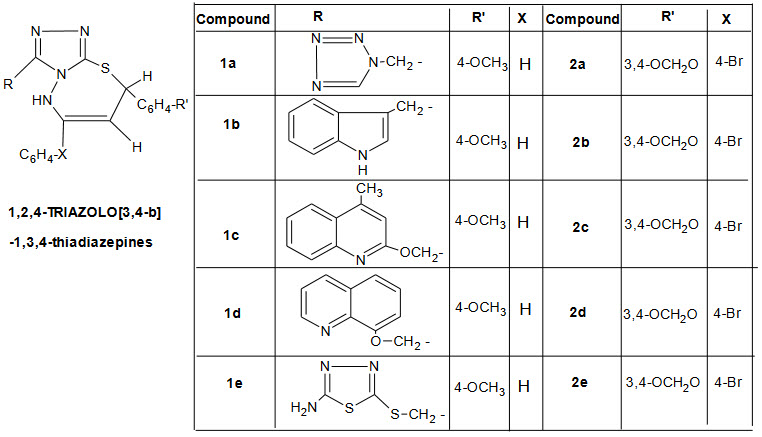
Piperazinyl linked ciprofloxacin dimer (an oxygenated chalcone isolated from the Chinese liquorice) are reported to be potent antibacterial agents against resistant strains a novel class of mixed D2 /DD4 receptor antagonist, dual calcium channel antagonist, antimalarial agents and potent antipsychotic agents. recently piperzine derivatives containing tetrazole nucleus have been reported as antifungal agents 3,4,5,6, and 7 indicated good activity against staphylococcus aureus 4 and 7 for E.coli remaining compounds are statically equivalent in antibacterial against their micro orgaism with moderate activity.
Most of the tested compound posses’ potent in vitro activity against P.vulgaris .K. pneumoniae. the MIC50 value is generally within the range of 2.22-100µg/ml against all the evaluated strains. Compound 5, 6, and 7 very high activity against s.aureus and 4, 5 and 7 show very high antibacterial activiy against E.coli[14].
Oxazolidinone exemplified by dup -721 are new class of synthetic antibacterial with activity against gram positive and anaerobic bacteria including resistant pathogens.
Chalcone and oxazolidinones by preparing 2 hybride molecules (Type A and Type B) having the feature of both oxazolidinone and chalcone in an effort to discover potent antibacterials.
The type A hyride molecule(carbonyl group away from oxazolidinone ring) 8-13 were designed in such way that the aromatic ring would have a substituted starting from the string electron donating to the strong electron withdrawing nature they exhibits only trace or no activity against all the tested organism.
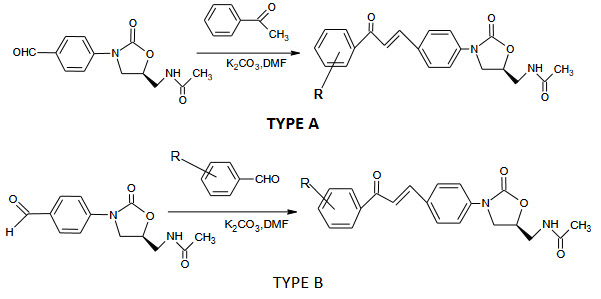
Type B hybride molecule (carbonyl group close to oxazolidinone ring) 14-18 possessing various substituents on the aromatic ring were also inactive. Moderate activity was observed for compound 19 and 20 when the aromatic ring of the chalcone was replaced with heterocycles. Introduction of fluorine atom in the aromatic ring could enhance the activity indeed these heterocyclic substituted hybride molecules 21 and 22 showed better antibacterial activity than the non-fluorine analogues.
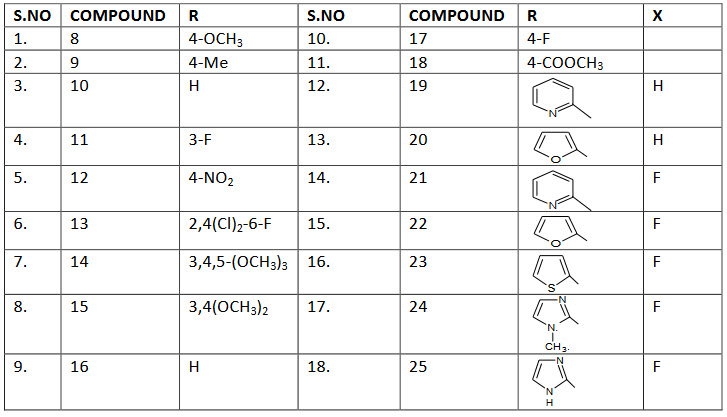
But thee introduction of thiophene and imidazole heterocyclic resulted in compound 23-25 that were inferior in activity. Compound 21 possing a pyridine ring was found to be the best molecules showing acceptable antibacterial activity (MIC 4µg/ml) against methicillin resistant series of staphylococcus aureus strains.
Converting the acetamide groups of 21 to the corresponding thiocarbamate. the resultant molecules 26 exhibited the in vitro activity in the range of 0.25-2µg/mL is many fold superior in activity when compared to its acetamide analogues.15
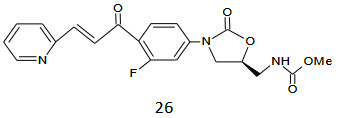
Bioisosteric replacement of the 4’-hydroxy groups making more acidic and exchanging the hydroxyl group with carbocylic acid or a bioisoster of these groups was investigated. we have prepared a number of acidic chalcone analogues in order to find optimal bioissoster with regard to antibacterial potency, having an acidic group in molecules markedly increase aqueous solubility of the compound s compared to the parent phenol compound. The antibacterial activity of the synthesized chalcones, expressed in terms of antibacterial minimum inhibitory concentration (MIC) against the gram positive bacterium staphylococcus aureus,
Two type of bioisoster replacement have been investigated:
(A) Changing the acidity of the phenol group by fluoro substitution in ortho position to a phenol group of the A –ring.
A phenol group having two neighboring fluorine atoms has been described to the almost as acidic as a carboxylic acid .In order the investigate the antibacterial effect of lowering the pKa value of the phenol group the 3,4 –difluoro-4-hydroxy analogue 27 and the 3;-fluoro-4’-hydrocy analogue 28 were prepared. The data clearly demonstrate that an increase in acidity of the phenol group cause a dramatic loss of activity.
(B) Exchanging the phenol group with a carboxylic acid or the tetrazole bioisoster of carboxylic acid.

The second approach by exchanging the hydroxyl group with a carboxylic group was much more successful .the 4’-carboxy analogues 29 of the parent compound and the 4’-tetrazole bioisoster 30 were prepared and it was shown that the carboxylic acid analogue 29 was almost equipotent to the phenol analogue. In addition 29 was much more soluble at physiological pH(0.6mg/ml) than 1-(4’-hydroxy) -3-(2,4-dichloro) 2-propene 1-one (0.001mg/ml). on the other hand 6 was shown to be almost in active (MIC ;300µM) proving that the 4’-tetrazole group is in this case not a true bioisoster of the carboxylic acid.
|
Compound |
R2 |
Compound |
R2 |
|
31 |
3-CF3 |
39 |
2-Cl |
|
32 |
3-Br |
40 |
2-OBu |
|
33 |
3-Cl |
41 |
3,5-DI-CF3 |
|
34 |
3-NO2 |
42 |
3,5-DI-Br |
|
35 |
3-OH |
43 |
3,5-DI-Cl |
|
36 |
3-OPh |
44 |
3,5-DI-CH3 |
|
37 |
2-CF3 |
45 |
3,5-DI-F |
|
38 |
2-Br |
46 |
3,5-DI-OCH3 |
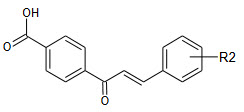
4’-hydroxyl group can be exchanged by a carboxylic acid without loss activity at the same time increase the solubility.
B ring was monosubstituted only 2-, 3-, or 4- positions were relevant due to the symmetry of the ring .The three positions in the B-ring have different influence on the antibacterial activity of the compound prepared. Introduction of substituent in the 4-position resulted in compound that were either inactive or marginally more potent than the nonsubstituted analogues. Compound having lipophilic -bromo,-chloro, or –trifluoromethyl group showed antibacterial activity whereas compound with more polar hydroxyl and methoxy groups were inactive.
Compound with substitution of 3- position of the B –ring(31-36) seem to be more potent. As seen for the 4-position is modulating the activity. The most potent compound has the lipophilic and electron withdrawing trifluormethyl group in the 3-position. In order to determine if the electronic properties is essential for the activity, compound 31 having the polar and electron withdrawing nitro –substituents in the 3-position was prepared. The was almost inactive indicating that lipophilic properties rather than electronic properties were responsible for the high activity of 14. The 2-position appears to be marginal importance for the activity as all the 2-substitued compounds (37-40) are either inactive or low potent.
The activity of the compound correlated with the lipohilcity of the substituents in ring B. the lipophilic compound(41,42) were very potent and as the sustituentsn become more polar the activity gradually decreased (43-46). having two substituents in the ring B gave a synergistic increase in activity resulting in very potent carboxy chalcones (25,26:MIC 2µM).the in vitro activity launched equipotent to ciprofloxacin and recently launched antibiotics linezolid.16
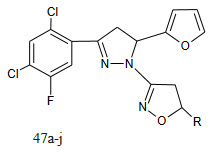
The chalcones, on cyclization with hydroxylamine hydrochloride in alkaline medium give the corresponding isoxazoline derivatives.
|
S.NO |
Comp. |
R |
|
Comp. |
R |
|
1 |
47a |
3,4,5-Trimethoxy phenyl |
6 |
47f |
4-Fluorophenyl |
|
2 |
47b |
3-Phenoxyphenyl |
7 |
47g |
2-Hydroxy-4 methoxyphenyl |
|
3 |
47c |
4-Methoxyphenyl |
8 |
47h |
2-Nitrophenyl |
|
4 |
47d |
2-Chlorophenyl |
9 |
47i |
4-N,N-Dimethylaminophenyl |
|
5 |
47e |
4-Hydroxyphenyl |
10 |
47j |
2-Thienyl |
The target molecules were tested for antibacterial activity against the variety of test organisms Escherichia coli, Pseudomonas aeruginosa (gram-negative bacteria) and Staphylococcus aureus, Salmonella paratyphi A (gram-positive bacteria). The screening results indicate that compounds 47a and 47f show promising activity and compounds 47b and 47e poor activity against E. coli. Compounds 47a and 47fshow good activity and compounds 47b and 47e low activity against P. aeruginosa.Compounds 47a and 47i show high activity and compounds 47b and 47e low activity against S. aureus. Compounds 47a and 47j show high activity and compounds 47b and 47c low activity against S. paratyphi A.
The result indicates that the presence of methoxy-, chloro-, and fluorogroups enhanced the antibacterial activity. However, no specific structure–activity relationship could be established.17
Antifungal activity
Antifungal profile against aspergillus flavus are also described ‘all the synthesized compound was screened for their in vitro antibacterial activity13
Modern medicine has greatly increased the number of immunocompromised patients.chemotherapy parentral nutrition, transplantation, surgry and the use of broad spectrum antimicrobial agent, added to the occurrence of the acquired immunodeficiency syndrome.
Some phenolic synthetic chalcones posses moderate antifungal properties .Two phenolic chalcones isolated from myriad serrate did not display any activity against Candida albicans.
Mechanism of action of most active chalcones was assayed for their capacity of inhibiting in vitro β (1, 3)-glucan and chitin synthase enzyme that catalyzed the biosynthesis of β (1, 3) glucan and chitin polymer of fungal cell wall.
Strong antifungal activity were obtained for the compound of series against dermatophytes
In particular compound 48-50, 52-57 and 58 showed strong antifungal activity comparable to those of amphotericin B and ketoconazole.
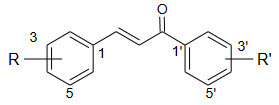
|
S.NO |
Comp |
R |
R’ |
S.NO |
Comp |
R |
R’ |
|
1 |
48 |
H |
H |
11 |
58 |
2,4-Cl2 |
2’,4’-Cl2 |
|
2 |
49 |
4-NO2 |
H |
12 |
59 |
4-CH3 |
4’-CH3 |
|
3 |
50 |
2-NO2 |
H |
13 |
60 |
3,4-(CH3O)2 |
3’,4’-(CH3O)2 |
|
4 |
51 |
2,4-Cl2 |
H |
14 |
61 |
3,4,5-(CH3O)2 |
3’,4’,5’-(CH3O)2 |
|
5 |
52 |
3-CH3O |
H |
15 |
62 |
4-CH3O |
4’-CH3 |
|
6 |
53 |
4-CH3O |
H |
16 |
63 |
2,3-(CH3O)2 |
4’-Br |
|
7 |
54 |
4-CH3 |
H |
17 |
64 |
4-N(CH3O)2 |
3’,4’-(CH3O)2 |
|
8 |
55 |
3,4-(CH3O)2 |
H |
18 |
65 |
3-CH3O |
4’-Br |
|
9 |
56 |
2,4-(CH3O)2 |
H |
19 |
66 |
AMP |
|
|
10 |
57 |
2,3-(CH3O)2 |
H |
20 |
67 |
KET |
|
It can be state that all the tested dermatophytes were inhibited at 50µg/ml and some of them at lower concentration. the most sensitive’s species was epidermatophyton floccasum.
Regarding the influence of substituents on ring A:
a) Electron donating groups tended to weaken the antifungal activity.
b) Electron withdrawing groups in Para position increases the potency of nevertheless when NO2 or Cl group is in position -2 decrease of activity is observed suggested that the presence of these group in ortho position of the B ring could introduce important steric effect(effect that result from the size e of the substitution and repulsion between them)
Regarding the correlation of the antifungal activity of substituted chalcones with polarity of their molecules.
When an OCH3 added to C-2 of the compound the activity is did not change significantly.
We investigated the role of played by ring B in the antifungal activity of chalcone 1 by replacing phenyl ring with naphthalene and quinolines result in an almost complete loss of activity .The presence of an enone linkage would be structural requirement necessary but not by itself sufficient for antifungal activity.
The lack of activity of compound 58 possessing Cl group in the ortho position in both ring A and B suggested that the steric factor play crucial role in antifungal properties of substituted chalcone. 18
The chalcone were tested for their growth inhibitory activity against 33 strains belonging to S. cerevisiae (13 strains), H. polymorha (17 strains) and K.lactis (3 strains). The MIC was determined by the agar dilution method.
The most active chalcone were 68, 69, 70, 71, and 72 and rest of compound exhibits antifungal activity at very high concentrations (>40µg/ml).
The antifungal activity of substituted compound was compared with those of the parent chalcone 68, following correlation were observed:
|
S.no |
Comp. |
n |
R’4 |
R2 |
R3 |
R4 |
R5 |
|
1 |
68 |
1 |
H |
H |
H |
H |
H |
|
2 |
69 |
1 |
H |
H |
OH |
H |
H |
|
3 |
70 |
1 |
H |
H |
OH |
OCH3 |
H |
|
4 |
71 |
1 |
Cl |
H |
OH |
H |
H |
|
5 |
72 |
1 |
Cl |
H |
H |
OH |
H |
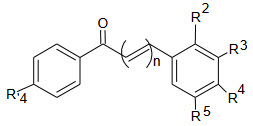
1) Introduction of Electron withdrawing group (Cl,CN and NO2 ) in Para position in ring B yielded the less active chalcones then parent chalcone 59
2) Introduction of Electron donating substituents (OH, CH3 and OCH3) in Para position of ring B produced inactive chalcone compound.
3) Presence of single hydrogen atom was effective at m-position in ring B introduction of single methoxy group at m-position of ring B led to active compound.
4) The combination of m-hydroxy group and p-methoxy group in ring B with the interchange of the position of the hydroxyl and methoxy group and when the hydroxyl group was placed in o-position and the methoxy group was in m-position.
5) Introduction of p’-chloro atom in ring A was beneficial only for the chalcones with single hydroxyl group at m-and p-position. The m-position was more favorable than the p-position. presence of m- and p-hydroxyl groups together led to the inactive chalcone
6) Elongation of the conjugated system by introduction of one additional double bond between the ketovinyl moiety and the ring b did not produce an active compound.
Based on the observation it could be concluded that the electronic effects of the p-substituents in ring B of chalcones are not crucial for displaying antifungal activity towards the tested fungi. this is contradictory to the anti fungal effects, which chalcones with EW an ED substituents in ring B have shown against several dermatophytes and the yeast C. albicans (59, 61,) beside in this study the position hydroxyl group in ring B was found important for the chalcone activity as opposed to some another antifungal studies .interestingly the favorable location for the hydroxyl group was the m-position in ring B. in contras , in our previous study we showed that o-position was the most favorable for the antifungal activity of hydroxyl chalcone against C .Albicans.19
Search for the compound and lead compound of plant origin we are investigating species from the Panamanian flora. One of these plants, piper dilatatum L.C. Rich (piperaceae) has been studied since it is used by the Kuna Indian of panama as a constituent of a mixture of plantsapplied as a tonic bath for various afflictions. Moreover the piperaceae family has been shown to be rich in various bioactive chemical classes, such as alkaloids, amides, lignans, flavonoids or lactones.Compound 73to 77displayed antifungal activitiesagainst the plant pathogenic fungus cladosporium cucumerinum in the biautographic test on TLC plates.[20]
Antimalarial Activity
Malaria is serious problems for recent era because of the number of antimalarial drug present has developed resistance to nearly all available drugs. No wonder that the antimalarial activity of chalcone has generated great interest. Michael addition of nucleophilic species to the double bond of the enone is probably the reason for the antimalarial activity.
Quantitative structure-activity relationships of a series of chalcone derivatives (1,3-Diphenyl-2-propen-1-one) as anti-Plasmodium falciparumagents (antimalarial agents). The study investigated the factors that may be important in the inhibitory activity of chalcone derivatives on P. falciparum cysteine protease. The obtained models presented good capacity to explain the observed values of biological activity, high adjustment level, statistical significance and good predictivecapacity.[21] Recently, licochalconeA, isolated from chinise liquorice root has been reported highly effective in an in vitro screen against chloroquine sensitive and chloroquine resistance isolated of plasmodium falciparum.[22]
Antimalarials that are effective against chloroquine-resistant strains of P. falciparum, Cheng et al [23]had developed a methodology for the solid phase synthesis of chalcone analogues in reasonably high yields. On the basis of their structure activity relationship (SAR) and computer modeling data, they expected that the chalcone derivatives with hydroxyl functionality on one of the aromatic rings and with some other appropriate substitutions on the other ring will be even more potent as antimalarials. They found that the chalcone ‘1-(2-chloroquinolin-3-yl)-3-(3 hydroxyphenyl)prop-2-en-1-one’ (25) was synthesized in the highest percentage yield, 97%.As a part of the search for novel antimalarial agents from plants or via chemical synthesis, Lim et al [24] preparedtwenty derivatives of flavonoids and chalcones, four derivatives for each of flavones, flavanones, chalcones, dihydrochalcones, and 3'-chlorochalcones, and evaluated for in vitro antimalarial activity against P. falciparumstrain FCR-3 and cytotoxicity against FM3A cells (a mouse mammary tumor cell). The aim was toderive predictivestructure activity relationships to guide lead compound design. Among the chalcones tested, the most activecompound was 3-(3,4-dimethoxyphenyl)-1-(2-hydroxy-4-methoxy-phenyl)propan-1-one (26). Synthesis of a new series of chloroquine-chalconebased hybrids (8-22) and their antimalarial efficacy against both chloroquine-susceptible (3D7) and chloroquine-resistant (K1) strains of Plasmodium falciparum. Most of the compounds showed enhanced antimalarial activity as compared to chloroquinein chloroquine-resistant (K1) strain of Plasmodium falciparum.[27] Chalconederivatives and their antimalarial activity against asexual blood stages of Plasmodium falciparum .The most active compound was 1-(4-benzimidazol-1-yl-phenyl)-3-(2, 4-dimethoxy-phenyl)-propen-1-one with IC(50) of 1.1μg/mL, while that of the natural phytochemical, licochalcone A is 1.43μg/mL. The presence of methoxy groups at position 2 and 4 inchalconederivatives appeared to be favorable for antimalarial activity as compared to other methoxy-substituted chalcones. Furthermore, 3, 4, 5-trimethoxy groups onchalconederivative probably cause steric hindrance in binding to the active site of cysteine protease enzyme, explaining the relative lower inhibitory activity.[28]
Anti HIV Activity Tetrazole, thiadiazole, quinoline, indole and triazole derivatives arewell known for their significant biological activities. Thiadiazepine are not only known for their potent antimicrobial activities but they are also excellent charge generating agents. They are also as intermediate for preparation of substituted caprolatum useful for the treatment of HIV diseases.
Anti-diabetic activity
The anti-apoptotic effect of 2',4'-dihydroxy-6'-methoxy-3',5'-dimethylchalcone (DMC) from the buds of Cleistocalyx operculatus, on H2O2 induced MIN6 cells damage was investigated. Inhibiting beta-cell apoptosis through ameliorating oxidative mitochondrial dysfunction with specific natural products may have preventive or therapeutic potential.[27] Chalcones bearing electron donating or electron withdrawing substitutions were prepared and their glucose uptake activity was evaluated.Chalcones with chloro, bromo, iodo and hydroxy substitutions at position 2 on A-ring exhibited the highest activity with glucose medium concentration (210 to 236 mg/dl) compared to pioglitazone and rosiglitazone (230 and 263 mg/dl, respectively). Also chalcones with iodo substitution at position 3 on A-ring were comparably active.[28]Alzheimer's disease (AD) is the most common neurodegenerative disease to cause dementia in the elderly. Amyloid β (Aβ)-peptide induced oxidative stress causes the initiation and progression of AD. Recently, newchalconederivatives termed the Chana series were synthesized. Chana 1 showed high free radical scavenging activity (72.5%), as measured by a DPPH (1,1-diphenyl-2-picrylhydrazyl) assay. In this study, we investigated the effect of Chana 1 against Aβ-induced cytotoxicity and cognitive deficits. Additionally, we sought to estimate the lethal dose, 50% (LD50) of Chana 1 in mice using an acute oral toxicity test. We found that Chana 1 significantly protected against Aβ-induced neuronal cell death in PC12 cells. Oral administration of Chana 1 at a dose of 50 mg/kg body weight/day significantly improved Aβ-induced learning and memory impairment in mice, as measured in Y-maze and passive avoidance tests. In acute toxicity tests, the LD50 in mice was determined to be 520.44 mg/kg body weight.[29]
Anticancer activity
Prenylated chalcone xanthohumol associates with histones in breast cancer leads immune precipitation of cell lysates was confirmed using immune detection with a specific anti-histone H2A antibody.[30]Previous studies suggested chalcones as antineoplastic drug candidates. We synthesized a New chalcone derivative (E)-3-(4-methoxyphenyl)-2-methyl-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one with increased cytotoxic potency relative to its parent compound in cell culture at low nanomolar levels, inhibited prostate cancer (PCa) cell growth through cell cycle arrest and caspase-dependent apoptosis.[31]Recent studies report that chalcones exhibit cytotoxicity to human cancer cell and the form of cell death induced by these compounds is apoptosis.[32]A newly synthesized series of chalcone derivativescontaining pyrazole rings were synthesized and evaluated for their cytotoxic activities in vitro against human breast cancer cell lines MCF-7, HEPG-2, and HCT-116. [33]Some chalcones are able to inhibit tubulin polymerization, giving cytotoxicity and destruction of tumoral vascolature.[34]Xanthohumol (XN), a prenylated chalcone present in hops exhibits anti-inflammatory, antioxidant and anticancer activity. In the present study we show that XN inhibits the proliferation of mouse lymphoma cells and IL-2induced proliferation and cell cycle progression in mouse splenic T cells.[35]Chalcones, aromatic ketones and enones acting as the precursor for flavonoids such as Quercetin, are known for their anticancer effects. Although, parent chalcones consist of two aromatic rings joined by a three-carbon α,β-unsaturated carbonyl system, various synthetic compounds possessing heterocyclic rings like pyrazole, indole etc. are well known and proved to be effective anticancer agents. In addition to their use as anticancer agents in cancer cell lines, heterocyclic analogues are reported to be effective even against resistant cell lines.[36]Novel chalcone derivatives have been designed and synthesized, and their biological activities were also evaluated as potential inhibitors of tubulin. These compounds were assayed for growth-inhibitory activity against MCF-7 and A549 cell lines in vitro. Compound 3d showed the most potent antiproliferative activity against MCF-7 and A549 cell lines with IC(50) values of 0.03 and 0.95 μg/mL and exhibited the most potent tubulin inhibitory activity with IC(50) of 1.42 μg/mL.[37]
Antioxidant activity
The effects of nitric oxide (NO) on antioxidant activity and contents of phenolics and flavonoids in mushroom Russula griseocarnosa were investigated. The results showed that the antioxidant activities of the mushrooms fumigated with NO were significantly increased when compared to the controls. Moreover, NO fumigation significantly enhanced phenolic and flavonoid contents and stimulated the activities of phenylalanine ammonia -lyase and chalcone synthase.[38]Anewchalcone(4,2'-dihydroxy-3methoxy-5-brominechalcone; C) was confirmed to be highly potent in scavenging 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and OH free radicals in vitro. Tests of anti-free radical activity in response to oxidative stress in mice revealed that C could elevate glutathione peroxidase (GSH-PX) and super oxide dismutase (SOD) levels and lower malonaldehyde (MDA) level in a free-radical-injured scopolamine-induced Alzheimer's model. Further behavioral tests with the Morris water maze showed that C could antagonize the learning impairments in the Alzheimer's model, which suggests that C has a potential role in Alzheimer's diseases [39]
REFERENCES
1. Devia, A.C., Ferretti, F.H., Ponce, C.A., Tomas, F., Journal of Molecular Structure (Theochem). 1999, 493, 197-197.
2. Zdzislawa Nowakowska. European Jornal of Medicinal Chemistry. 2007, 42,125-137.
3. Carlos Javier Durán-Valle, Isabel M. Fonseca, Vanesa Calvino-Casilda, Maria Picallo, António José López-Peinado and Rosa Maria Martín-Aranda. Catalysis Today, 2005, 107-108, 500-506
4. Dimmock, J.R., Elias, D.W., Beazely, M.A., Kandepu, N.M.,. Current Medicinal Chemistry,1990,6 1125-1149.
5. Daniela Millan , Moises Dominguez ,Marcos Caroli Rezende, , Dyes and Pigments, 2008, 77 441-445.
6. TRINTH THI THUY, ANDREA PORZEL, HELMUT RIPPERGER, TRAN VAN SUNG, GUNTER ADAM, 1998, PII: S30031-9422000411-7.
7. Ram Vishnu J., Saxena Abhishek S. ,Srivastava Shalini, Chandra Subhash, Bioorganic and medicinal Chemistry letters 10, 2010, 2159-2161
8. Prem P. Yadav, Prasoon Gupta, A. K. Chaturvedi, P. K. Shukla, Rakesh Maurya. “Bioorganic and Medicinal Chemistry, 2005, 13, 1497-1505.
9. T.Narendra,Shweta,K.Tanvir,M.Srinivasa Rao,K.Sriwastava,S.K.Puri, Bioorganic and medicinal Chemistry letters 15, 2005, 2453-2455.
10. George, A., Kraus, Ganesh Kumar, Gregory Phillips, Kris Michalson, Maria Mangano Bioorganic and medicinal Chemistry letters 2008,18 , 2329-2332.
11. Xiaoling Liu; Mei-Lin Go. Bioorganic & medicinal chemistry 2007; 15(22):7021-34.
12. P.L.Majumder,S.LAHIRI,A.N.MUKHOTI, Phytochemistry ,VOL-40,NO-1,271-274.
13. Kidwai, M., Sapra, P., Misra, P., Saxena, R.K., Singh, M. Bioorganic & Medicinal Chemistry, 2001, 9, 217-220.
14. Tomar, V., Bhattacharjee, G., Kamaluddin, Ashok Kumar. Bioorganic and medicinal Chemistry letters 2007, 17, 5321-5324.
15. Selvakumar, N., Sunil Kumar, G., Malar Azhagan, A., Govinda Rajulu G., Shikha Sharma, European Journal of Medicinal Chemistry , 2007 ,42, 538-543
16. Simon Feldbaek Nielsen, Thomas Boesen, Mogens Larsen, Kristian Schønning, Hasse Kromann Bioorganic & Medicinal Chemistry, 2004,Volume: 12, Issue: 11, Pages: 3047-3054.
17. Xiaoling Liu, Mei-Lin Go. Bioorganic & Medicinal Chemistry,2007,15, 22, 7021-7034.
18. Shah, T.K. and Desai, V. Journal of. Serbian. Chemical. Society, 2007, 72 (5) 443–449.
19. LOPEZ Silvia N ., CASTELLI Maria V., ZACCHINO Susana A., DOMINGUEZ José N., LOBO Gricela CHARRIS-CHARRIS ,Jaime CORTES Juan C. G. RIBAS Juan C. , DEVIA Cristina , RODRIGUEZ Ana M., ENRIZ Ricardo D. Bioorganic and Medicinal chemistry, 2001, 9,1999-2013.
20. Lahtchev, K.L., Batovska, D.I., Parushev, ST.P., Ubiyvok, V.M., Sibirny, A.A. European Journal of Medicinal chemistry xx,2008,1-9.
21. Motta, L.F., Gaudio, A.C., Takahata, Y. Internet Electronic Journal of Molecular Design, 2006, 5, 555-569.
22. Narender, T., Shweta, K., Tanvir, M., Srinivasa Rao, Srivastava, K., Puri, S.K., Bioorganic and Medicinal Chemistry Letters, 2005, 15, 2453-2455.
23. Cheng, M.S., Shili, R., Kenyon, G. Chinese Chemical Letters,2000, 11, 851-854.
24. Lim, S.S., Kim, H.S., Lee, D.U. Bulletin of the Korean Chemical Society, 2007, 28, 2495-2497.
25. Achanta, G., Modzelewska, A., Feng, L., Khan, S.R., Huang, P., Molecular Pharmacology,2006, 70426-433.
26. Romagnoli, R., Baraldi, P.G., Carrion, M.D., Cara, C.L., Cruz-Lopez, O., Preti, D., Bioorganic and Medicinal Chemistry. 2008, 16, 5367-5376.
27. Sashidhara KV, Avula SR, Palnati GR, Singh SV, Srivastava K, Puri SK, Saxena JK.,Bioorg Med Chem Lett. 2012,Sep 1;22(17):5455-9.
28. Yadav N, Dixit SK, Bhattacharya A, Mishra LC, Sharma M, Awasthi SK, Bhasin VK.Chem Biol Drug Des. 2012 Aug;80(2):340-7.
29. Luo Y., Lu Y., Pharmazie. 2012 Sep;67(9):798-803.
30. Hsieh C.T., Hsieh T.J., El-Shazly M., Chuang D.W., Tsai Y.H., Yen C.T., Wu S.F., Wu Y.C., Chang F.R., Bioorg Med Chem Lett. 2012 Jun 15;22(12):3912-5.
31. Kwak J., Kim M.J., Choi K.C., Choi H.K., Jun W., Park H.J., Lee Y.H., Yoon H.G., Int J Mol Med. 2012 Jul;30(1):193-8
32. Wyns C., van Steendam K., Vanhoecke B., Deforce D., Bracke M., Heyerick A.,Mol Nutr Food Res. 2012 Nov;56(11):1688-96.
33. Zhang Y., Srinivasan B., Xing C., Lü J.,Anticancer Res. 2012 Sep;32(9):3689-98.
34. de Vasconcelos A., Campos V.F.,Nedel F., Seixas F.K., Dellagostin O.A., Smith K.R., de Pereira C.M., Stefanello F.M.,Collares T., Barschak A.G., Cell Biochem Funct. 2012 Sep 18
35. Mohamed M.F., Mohamed M.S., Shouman S.A., Fathi M.M., Abdelhamid I.A.,Appl Biochem Biotechnol. 2012 Sep 5.
36. Utsintong M., Massarotti A., Caldarelli A., Theeramunkong S., Med Chem. 2012 Aug 29.
37. Liu Y., Gao X., Deeb D., Arbab A.S., Dulchavsky S.A., Gautam S.C. J Exp Ther Oncol. 2012;10(1):1-8.
38. Sharma V., Kumar V., Kumar P., Med Chem. 2012,Jun 18
39. Zhang H., Liu J.J., Sun J., Yang X.H., Zhao T.T., Lu X., Gong H.B., Zhu H.L.,Bioorg Med Chem. 2012 May 15;20(10):3212-8.
40. Dong J, Zhang M, Lu L, Sun L, Xu M., Food Chem. 2012 Dec 1;135(3):1220-541. Pan Y, Chen Y, Yu X, Wang J, Zhang L, He Y, Zheng Y, Zheng J. ,Cell Physiol Biochem. 2012;29(5-6):949-58.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 25/11/2014; Accepted On: 03/12/2014; Published On: 15/01/2014 How to cite this article: U Sahu, NC Panda, BVV Ravikumar, A Kumar, Activity of chalcone and its derivatives - a Review, PharmaTutor, 2014, 2(1), 62-75 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









