 About Author: Shishir Tripathi
About Author: Shishir Tripathi
M.Pharm-Pharmaceutics
Department of Pharmacy,
Barkatullha University,
Bhopal-462026, M.P., India
Abstract
According to the Migraine Research Foundation, migraine ranks in the top 20 of the world’s most disabling medical illnesses with more than 10 percent of the population, including children, suffering from the migraine. Sumatriptan Succinate is a choice of drug used for the treatment of migraine when received at oral dose its bioavailability is approximately 14%. Considering the low bioavailability after oral and intranasal administration, due to presystemic metabolism and incomplete absorption as well as additional inconveniences associated with parenteral and oral administration, the exploitation of an alternative route of sumatriptan delivery, such as transdermal administration (topical gel)—could be sort out mentioned problem and enhance its therapeutic benefit up to most possible extent. Topical delivery systems of Sumatriptan succinate gel were formulated using polymers like HPMC, Carbopol 934 and Pectin and penetration enhancers like eucalyptus oil, menthol and oleic acid. The gels were evaluated for various parameters such as homogeneity, grittiness, extrudability, in vitro drug release, viscosity, pH, spreadability and drug content. The release rate of the gel was found to obey koresmeyer peppas kinetics. It can be concluded that HPMC gel has properties similar to marketed preparation and eucalyptus oil acts as a good penetration enhancer in Sumatriptan gels as compare to menthol and oleic acid.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1107
Introduction
Pharmaceutical gels are semisolid preparations in which there is interaction (either physical or covalent) between colloidal particles of polymers within a liquid vehicle. The vehicle is continuous and interacts with the colloidal particles of polymers within the three-dimensional network that is formed by the bonds formed between adjacent particles. [1]
Migraine is an intense, often unbearable type of headache. It is a severe, throbbing vascular headache. This sort of headache is characterized by recurrent unilateral head pain combined with neurological & GI disturbances. [2] Migraine is a mysterious disorder characterized by pulsating headache, usually restricted to one side, which comes in attack lasting 4-48 hours and is often associated with nausea, vomiting, sensitivity to light and sound, vertigo, loose motions and other symptoms.
Sumatriptan Succinate serotonin receptor agonist majorly used for the treatment of migraine. [3] Its topical application may be a better alternative that also reduces the side effects associated with oral and parental therapy. As per patent filed by Ronald Aung-Din et al (2008) it has been reported that Sumatriptan could be efficiently used in the treatment of the migraine in the topical gel formulation. [4] The study confirmed that Sumatriptan gel is an effective and safe option for the management migraine. Topical preparations avoid GI irritation, prevent the metabolism of drug in the liver, increase the bioavailability of the drugs enhance the therapeutic effect of drug and provide its action directly at the site of action. Although gel formulation of Sumatriptan appears to be highly useful, but unfortunately there is lack of literature on the formulation development of Sumatriptan topical gels. Therefore main objective of the present work was to develop topical gel of Sumatriptan and by using penetration enhancers of natural, chemical and synthetic origin to study the formulation variables affecting the release of drug and it was found that eucalyptus oil; from natural source act as a best penetration enhancer. [5]
For a topical formulation to be effective, it must be initially penetrate the barriers of skin, only when the drug has entered the lower layers of the skin it can be absorbed by blood and available for the site of action, or penetrate deeper in areas where inflammation occurs. The stratum cornea provides the greatest resistance to penetration, and it is the rate-limiting step in percutaneous absorption of drug from formulation. The permeation of drugs through skin can be enhanced by physical methods such as mechanical disruption, electrical disruption, and chemical modification or by the use of chemical enhancers. Some compounds may increase skin permeability by increasing the partition coefficient of the drug into the skin. [6] Chemical penetration enhancers modify barrier properties of the stratum corneum and hence increase drug permeability across skin. Ideally, the effects of the penetration enhancer on the skin should be reversible, non-toxic, non-allergenic, compatible with drugs and excipients and non-irritating. However the synthetic permeation enhancers are associated with adverse effect of producing irritation and toxicity to the skin. [7] Hence natural penetration enhancers are increasingly been used as compatible permeation enhancers due to their better safety profile.
[adsense:468x15:2204050025]
Materials and Methods
Materials
Sumatriptan succinate was a gift sample from Nosch Laboratory Pvt. Ltd. Hyderabad. Propylene glycol, triethanolamine and ethanol were obtained from Central Drug House and all other chemicals are of analytical grade.
Methods
Preparation of gel
Sumatriptan succinate gel formulations were prepared using hydroxypropylmethyl cellulose, carbopol 934 and pectin as gelling agent. Gelling agent was dispersed in a small quantity of distilled water and then remaining distilled water was mixed with the mixture of ethanol and propylene glycol then weighed amount of drug was dissolved in solvent system with continuous stirring with the help of mechanical stirrer and drug solution was added slowly in the gel base. pH of the vehicle was brought near to skin by using triethanolamine. The final weight of the gel was adjusted to 50 gm with remaining solvent system. Entrapped air bubbles were removed by keeping the gels overnight at room temperature. Table 1 shows the composition of the Sumatriptan succinate gels.
Evaluations of Gel
Measurement of pH: [8]
The pH of various gel formulations was determined by using digital pH meter. One gram of gel was dissolved in 100 ml distilled water and stored for two hours. The measurement of pH of each formulation was done in triplicate and average values were calculated.
Viscosity study: [9]
The measurement of viscosity of the prepared gel was done by Brookfield Viscometer. Brookfield DV – II + Pro Viscometer. Model D220. Serial no. 6527715. Made in USA.
Homogeneity: [10]
All developed gels were tested for homogeneity by visual inspection after the gels have been set in the container. They were tested for their appearance and presence of any aggregates.
Spreabability:[11] [12]
It was determined by spreadibility apparatus. About 1g of gel was added to the pan and the time was noted for upper slide (movable) to separate completely from the fixed slides. Spreadability is expressed in terms of time in seconds taken by two slides to slip off from gel and placed in between the slides under the direction of certain load. It is calculated by using the formula:
S = M . L / T
Where M = wt. tied to upper slide, L = length of glass slides
T = time taken to separate the slides
Extrudability study: [13]
The formulations were filled in the collapsible tubes after the gels were set in the collapsible tube. The extrudability of the formulation was determined in terms of weight in grams required to extrude a 0.5 cm ribbon of gel in 10 second.
Grittiness: [14]
All the formulations were evaluated microscopically for the presence of particles no remarkable particulate matter was seen under light microscope. Hence obviously the gel preparation fulfils the requirement of freedom from particular matter and from grittiness as desired for any topical preparation.
Drug content: [15]
A specific quantity (100mg) of developed gel and marketed gel were taken and dissolved in 100ml of phosphate buffer of pH 7.4. The volumetric flask containing gel solution was shaken for 2hr on mechanical shaker in order to get complete solubility of drug. This solution was filtered and estimated spectrophotometrically at 227.0 nm using phosphate buffer (pH 7.4) as blank.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
In vitro diffusion studies:
The diffusion studies of the prepared gels were performed in modified diffusion cell to studying the dissolution release of gels. Gel sample (0.5g) was taken and the diffusion studies were carried out at 37 ± 1° using 200 ml of phosphate buffer (pH 7.4) as the dissolution medium. 5 ml of each sample was withdrawn and was replaced with equal volume of fresh dissolution medium in the diffusion cell. Then the samples were analyzed for the drug content by using phosphate buffer as blank by UV spectroscopy at 227 nm.
Result and Discussion
Topical gel of Sumatriptan succinate was prepared by using different polymers (synthetic, semi synthetic and natural) such as HPMC, Corbopol and Pectin. It was found that Carbopol and pectin were precipitated during the preparation of gel and HPMC didn’t show any sign of unstability or any variation in their consistency during formulation. The prepared Gel was evaluated for their suitability as a perfect formulation as compare to marketed formulation.
Prepared HPMC gel was transparent, good in appearance and consistency. The evaluation studies of all the formulations were performed by standard methods.
The pH, grittiness, homogeneity, viscosity, spreadibility, extrudability and in vitro diffusion studies of the formulation were performed and they all were found to be satisfactory. We also compared gel with standard marketed gel and the result obtained was similar to marketed gel preparation which was shown in table no. 3 (a) and 3 (b).
Comparative studies were performed between different polymers and penetration enhancers and it revealed that gel containing HPMC as a gelling agent were shown satisfactory results as compare to gel formulations containing different gelling agents such as carbopol and pectin. The results of the in vitro release study from HPMC gel by using different concentration of different penetration enhancers across the biological membrane was performed and 5% eucalyptus oil was found to be better penetration enhancer as compare to menthol and oleic acid because its penetrability was best among the other enhancers and it showed good release rate also.
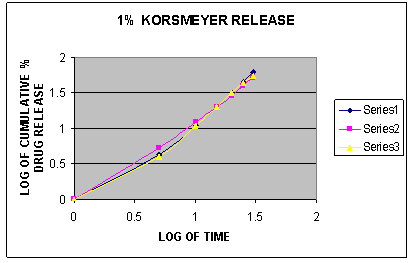
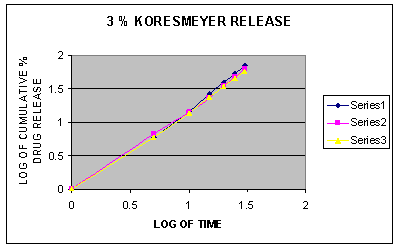
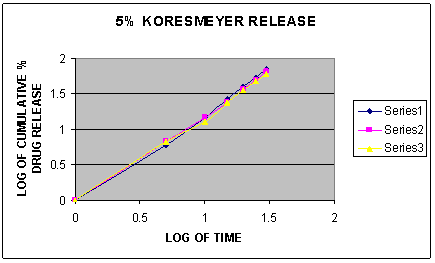
To establish the order and mechanism of drug release, dissolution data of all the formulations were fitted to four different kinetic models named as zero order model, first order model, higuchi model and koresmeyer peppas model. [16] The model for best fit was predicted from the value of r2. The value which was closer to 1 was selected as the best fit model for the drug release. [17] The in vitro drug release was found to follow Korsmeyer-Peppas kinetics as correlation coefficient r2 =0.9983 which was closer to 1.
The aim of this project was to formulate the topical gel using different polymers and penetration enhancers which may deliver the drug better than conventional dosage form of Sumatriptan on site of action and avoid the first pass metabolism as well as other gastric side effects of drug. Above mentioned aim was fulfilled by formulation of different formulations having combination of different gelling agent as well as penetration enhancers. An Optimized formulation was formulated showed good, spreadibility, viscosity, extrutability, consistency, appearance, penetrability and in vitro release. In the present study results showed that natural penetration enhancers may also used satisfactory as compare to chemical ones. It is also considerable that by developing such sort of promising formulations may open the doors for drugs having bioavailability problems and are not able to give efficient therapeutic effect which is a soul for the development of perfect, potent and therapeutic efficient drug delivery system.
Acknowledgements
The author is deeply thankful to Nosch Laboratory Pvt Ltd, Hyderabad, India for providing gift sample of Sumatriptan Succinate. The authors are grateful to Department of pharmacy, Barkatullah University Bhopal for providing facilities to carry out the research work.
References
1. Jones David, Fast Track, Pharmaceutics dosage form and design” Pharmaceutical disperse systems: ointments, pastes, lotions, gels and related formulations, edition 1st, London, Pharmaceutical Press, 2008, 88-99.
2. Ballington.A, Don, Laughlin.M Marry,”Pharmacology”, 3rd edition, 138-9.
3. Tripathi KD, Essentials of Medical Pharmacology, edition 5th, New Delhi, Jaypee Brothers Medical Publishers (P) Ltd, 2003, 145-155.
4. Ronald Aung-Din, Transdermal Migraine Therapy, United States Patent Application Publication, Pub No. US 2008/0090894 A1, Pub Date: Apr. 17, 2008, Pg No.1-12.
5. V. R. Sinha and Maninder Pal Kaur, Permeation Enhancers for Transdermal Drug Delivery, Drug Develop Ind Pharm, 26(11), 2000, 1131-1140.
6. Loganathan V, Jaswanth A, Sulaiman A, Rajaseskaran A, Manimaran S, Kumar SB. The effects of polymers and permeation enhancers on release of flurbiprofen from gel formulation. Indian J. Pharm. Sci., 2001; 200-204.
7. Michniak B, Player MR, Chapman J, Sowell JW. In vitro evaluation of a series of azone analogs as dermal penetration enhancers. Int. J. Pharm. 1993; 85–93.
8. Kashyap Nagariya, et al, Formulation Development and Characterization of Aceclofenac Gel Using Poloxamer 407, Journal of Chemical and Pharmaceutical Research, 2010, vol 2, 357.
9. Madan Jyotsana, Formulation and Evaluation of Aloe Vera Topical Gels, International Journal of Pharmaceutical Sciences, 2010, 551-555.
10. Setty C Mallikarjuna et al, Development of valdecoxib topical gels:Effect of formulation variables on the release of Valdecoxib, International Journal of Pharmacy and Pharmaceutical Sciences, 2010, Volume 2, Suppl 1, 70-75
11. Alka Garg, et al, Spreading of Semisolid Formulations: An Update. Pharmaceutical Technology, 2002, 84-105.
12. Gupta V* et al, “Formulation and Evaluation of Naproxen Gel Containing Tulsi Oil as Penetration Enhancer, International Journal of Pharmaceutical and Clinical Research; 1(3) 2009: 153-155.
13. Shivhare U.D, et al, Formulation Development and Evaluation of Diclofenac Sodium Gel Using Water Soluble Polyacrylamide Polymer, Digest Journal of Nanomaterials and Biostructures Vol. 4, No.2, 2009, p. 285 – 290.
14. Kaur Loveleen Preet et al, Development and Evaluation of Topical Gel of Minoxidil from Different Polymer Bases in Application of Alopecia, International Journal of Pharmacy and Pharmaceutical Sciences, 2010, Vol 2, 43-47.
15. P Ravi Prakash, et al, Formulation, Evaluation and Antiinflammatory Activity of Topical Etoricoxib Gel, Asian Journal of Pharmaceutical and Clinical Research, April?June 2010, Vol 3 Issue 2, ISSN 0974?2441, 126.
16. M.A. Kalam et al, Release kinetics of modified pharmaceutical dosage forms: A Review, Continental J. Pharmaceutical Sciences, 2007, 30 – 35.
17. Verma Surender et al, Development and evaluation of montelukast sodium colon targeted matrix tablets based on pulsatile approach for nocturnal asthma, International Journal of Pharmaceutical Sciences Review and Research, 2011, Volume 8, 129-137.
Table: 1 Different Concentration of polymers used in formulation of Gel.
|
Formulation code |
Concentration |
Formulation code |
Concentration |
Formulation code |
Concentration |
|
C1 C2 C3 C4 |
0.5% 1.0% 1.5% 2.0% |
H1 H2 H3 H4 |
0.5% 1.0% 1.5% 2.0% |
P1 P2 P3 P4 |
0.5% 1.0% 1.5% 2.0 |
C = Carbopol, H = HPMC, P = Pectin
Table no. 2 Composition of Sumatriptan succinate gel
|
INGREDIENTS |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
HPMC OLEIC ACID MENTHOL EUCALYPTUS OIL PROPYLENE GLYCOL ETHANOL DISTTILLED WATER DRUG TRIETHANOLAMINE |
1.5% 1% ----- ----- 1% 3% 6% 1% QS
|
1.5% 3% ----- ----- 1% 3% 6% 1% QS |
1.5% 5% ----- ----- 1% 3% 6% 1% QS |
1.5% ----- 1% ----- 1% 3% 6% 1% QS |
1.5% ----- 3% ----- 1% 3% 6% 1% QS |
1.5% ----- 5% ----- 1% 3% 6% 1% QS |
1.5% ----- ----- 1% 1% 3% 6% 1% QS |
1.5% ----- ----- 3% 1% 3% 6% 1% QS |
1.5% ----- ----- 5% 1% 3% 6% 1% Q |
Table: 3 (a) Evaluation parameters of prepared gel formulations
|
PARAMETERS |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
|
VISCOSITY (cps)
SPREADIBILITY
HOMOGENITY
pH
GRITTINESS
DRUG CONTENT (%) |
2828.00 ± 4.93
18.3 ± 0.18
++
6.76 ±0.18
-
93.70 ± 1.55 |
2888.66 ± 80.15
17.90 ± 0.40
+++
6.76 ± 0.14
-
93.68 ± 1.17 |
3518.00 ± 33.29
16.51 ± 0.38
++
6.68 ± 0.02
-
92.10 ± 2.15 |
3656.66 ± 55.57
13.0 ± 0.48
+++
7.43 ± 0.32
-
92.34 ± 1.01 |
4026.66 ± 25.16
11.26 ± 0.36
++
7.26 ± 0.208
-
93.11 ± 2.2 |
4288.33 ± 34.03
10.85 ± 0.1
+++
7.4 ± 0.12
-
92.20 ± 1.14 |
2606.6 ± 30.55
17.53 ± 0.37
++
7.5 ± 0.26
-
91.78 ± 2.9 |
3714.33 ± 10.20
15.44 ± 0.12
++
7.4 ± 0.44
-
91.19 ± 3.0 |
4376.00 ± 25.16
11.63 ± 0.70
++
7.74 ± 0.028
-
93.76 ± 2.55 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table: no. 3 (b) Evaluation of marketed gel formulations
|
PARAMETERS |
Marketed formulation |
|
VISCOSITY (cps) SPREADIBILITY HOMOGENITY pH GRITTINESS DRUG CONTENT (%) |
4410 ± 25cps 11.94 ± 0.36 +++ (Excellent) 7.2 ± 0.5 - (No grittiness) 99.5 ± 6.5 |
Fig.1. Korsmeyer release plot of different formulations of gel containing 1% Eucalyptus oil, Menthol and Oleic acid
Diffusion profiles of Sumatriptan succinate from Hydroxypropylmethylcellulose gels
Fig. 2. Koresmeyer plot of different formulations of gel containing 3% Eucalyptus oil, Menthol and Oleic acid
Diffusion profiles of Sumatriptan succinate from Hydroxypropylmethylcellulose gels
Fig.3. Koresmeyer Release of different formulations of gel containing 5% Eucalyptus oil, Menthol and Oleic acid
Diffusion profiles of Sumatriptan succinate from Hydroxypropylmethylcellulose gels
Table no 4. Regression analysis drug releases from of all formulations
|
FORULATION CODE S.NO. |
ZERO ORDER RELEASE |
FIRST ORDER RELEASE |
HIGUCHI’S RELEASE |
KORSMEYER PEPPAS RELEASE |
|
Y & R2 |
Y & R2 |
Y & R2 |
Y & R2 |
|
|
F1
F2
F3
F4
F5
F6
F7
F8
F9 |
y = 1.8796x - 4.7275 R2 = 0.9773
y = 1.9781x - 3.6107 R2 = 0.9875
y = 2.0349x - 2.8857 R2 = 0.9778
y = 1.7192x - 3.3598 R2 = 0.9811
y = 2.0861x - 4.1879 R2 = 0.9787
y = 2.6068x - 4.5132 R2 = 0.9581
y = 2.0397x - 5.94 R2 = 0.9608
y = 2.375x - 5.445 R2 = 0.9786
y= 2.1462x - 4.3323 R2 = 0.9795 |
y = 0.0415x + 1.0734 R2 = 0.3592
y = 0.0414x + 1.0709 R2 = 0.359
y = 0.0412x + 1.0686 R2 = 0.3576
y = 0.0415x + 1.0738 R2 = 0.3591
y = 0.0415x + 1.0704 R2 = 0.3602
y = 0.0413x + 1.0661 R2 = 0.3596
y = 0.0411x + 1.0763 R2 = 0.3534
y = 0.0413x + 1.0696 R2 = 0.3583
y = 0.0413x + 1.0689 R2 = 0.3588 |
y = 9.8554x - 10.632 R2 = 0.8134
y = 10.502x - 10.275 R2 = 0.8427
y = 10.747x - 10.585 R2 = 0.837
y = 9.0885x - 9.0168 R2 = 0.8301
y = 11.007x - 10.98 R2 = 0.8249
y = 13.766x - 13.039 R2 = 0.8089
y = 10.581x - 11.952 R2 = 0.7827
y = 12.489x - 13.031 R2 = 0.8193
y = 11.326x - 11.327 R2 = 0.8259 |
y = 1.2023x - 0.095 R2 = 0.9826
y = 1.2004x - 0.0307 R2 = 0.998
y = 1.1998x - 0.0214 R2 = 0.9968
y = 0.8621x + 0.0383 R2 = 0.9962
y = 1.2037x - 0.0182 R2 = 0.9981
y = 1.2549x - 0.043 R2 = 0.9965
y = 0.8081x + 0.0952 R2 = 0.9822
y = 1.2549x - 0.043 R2 = 0.9965
y = 1.2138x - 0.0193 R2 = 0.9983 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









