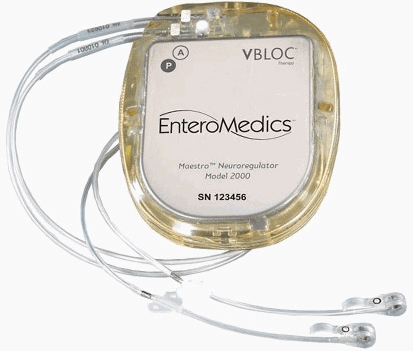
 The U.S. Food and Drug Administration today approved the Maestro Rechargeable System for certain obese adults, the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness.
The U.S. Food and Drug Administration today approved the Maestro Rechargeable System for certain obese adults, the first weight loss treatment device that targets the nerve pathway between the brain and the stomach that controls feelings of hunger and fullness.
The Maestro Rechargeable System, the first FDA-approved obesity device since 2007, is approved to treat patients aged 18 and older who have not been able to lose weight with a weight loss program, and who have a body mass index of 35 to 45 with at least one other obesity-related condition, such as type 2 diabetes.
[adsense:336x280:8701650588]








