Stallergenes's Oralair - sublingual allergen extract gets approval from FDA
Recently, U.S. Food and Drug Administration approved Oralair, first sublingual allergen extract approved in the United States, to treat allergic rhinitis with or without conjunctivitis that is induced by certain grass pollens in age group of 10 to 65 years. Oralair contains a mixture of freeze-dried extracts from the pollens of five grasses, including Kentucky Blue Grass, Orchard, Perennial Rye, Sweet Vernal and Timothy.



 The U.S. Food and Drug Administration approved Otezla (apremilast), manufactured for Celgene Corporation, Summit, N.J., to treat adults with active psoriatic arthritis (PsA), which is a form of arthritis that affects some people with psoriasis. Most people develop psoriasis first and are later diagnosed with PsA. Joint pain, stiffness and swelling are the main signs and symptoms of PsA.
The U.S. Food and Drug Administration approved Otezla (apremilast), manufactured for Celgene Corporation, Summit, N.J., to treat adults with active psoriatic arthritis (PsA), which is a form of arthritis that affects some people with psoriasis. Most people develop psoriasis first and are later diagnosed with PsA. Joint pain, stiffness and swelling are the main signs and symptoms of PsA. The U.S. Food and Drug Administration approved the first implantable device for people 18 and older with severe or profound sensorineural hearing loss of high-frequency sounds in both ears, but who can still hear low-frequency sounds with or without a hearing aid. The Nucleus Hybrid L24 Cochlear Implant System may help those with this specific kind of hearing loss who do not benefit from conventional hearing aids.
The U.S. Food and Drug Administration approved the first implantable device for people 18 and older with severe or profound sensorineural hearing loss of high-frequency sounds in both ears, but who can still hear low-frequency sounds with or without a hearing aid. The Nucleus Hybrid L24 Cochlear Implant System may help those with this specific kind of hearing loss who do not benefit from conventional hearing aids.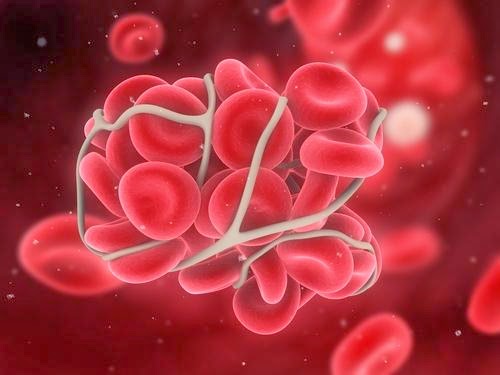 Bristol-Myers Squibb Company and Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) approved a Supplemental New Drug Application (sNDA) for Eliquis (apixaban) for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery.
Bristol-Myers Squibb Company and Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) approved a Supplemental New Drug Application (sNDA) for Eliquis (apixaban) for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients who have undergone hip or knee replacement surgery. The USFDA approved Chelsea Therapeutics's product Northera capsules (droxidopa), orphan-product, for the treatment of neurogenic orthostatic hypotension (NOH) which is rare, chronic and often debilitating drop in blood pressure upon standing that is associated with Parkinson's disease, multiple-system atrophy,
The USFDA approved Chelsea Therapeutics's product Northera capsules (droxidopa), orphan-product, for the treatment of neurogenic orthostatic hypotension (NOH) which is rare, chronic and often debilitating drop in blood pressure upon standing that is associated with Parkinson's disease, multiple-system atrophy, 





