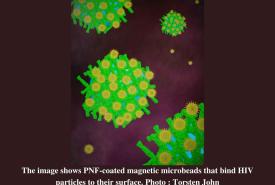
Mayo Clinic scientists pioneer immunotherapy technique for autoimmune diseases
Mayo Clinic scientists have developed an immunotherapy strategy that potentially lays the groundwork for treating a spectrum of autoimmune diseases.
The new technique, detailed in a preclinical study published in Nature Biomedical Engineering, involves combining chimeric antigen receptors (CAR) with mesenchymal stromal cells (MSC), resulting in engineered stem cells known as CAR-MSCs.