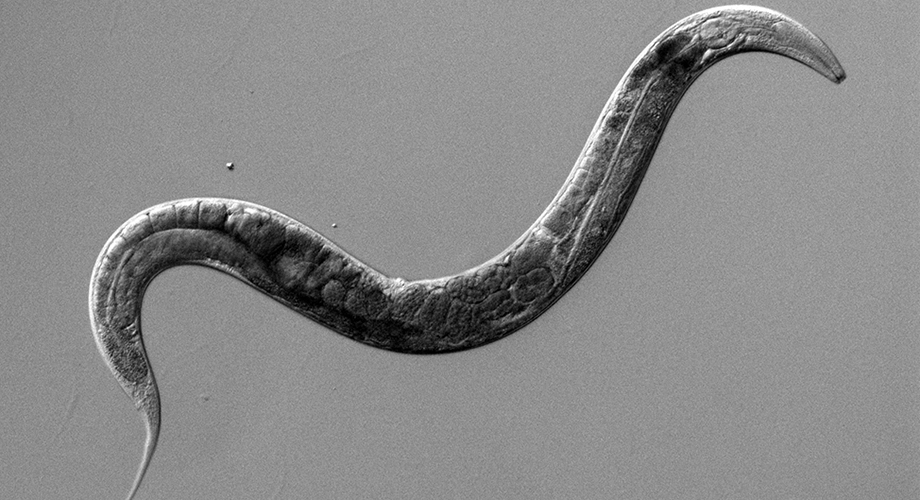
The nematode roundworm c. elegans. / Gill lab
In a discovery that may further the understanding of diabetes and human longevity, scientists at Scripps Research have found a new biological mechanism of insulin signaling. Their study, involving the roundworm C. elegans, reveals that a “decoy” receptor is at work in binding to insulin molecules and keeping them from sending signals for increased insulin production.