ISF College of Pharmacy (An Autonomous College), organized six day from April 27 to May 2, 2020, online short term Training Program sponsored by ALL India council of technical education (AICTE).
Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.
ISF College of Pharmacy (An Autonomous College), organized six day from April 27 to May 2, 2020, online short term Training Program sponsored by ALL India council of technical education (AICTE).

Selvita S.A. one of the largest preclinical contract research organizations in Europe, has signed a grant agreement with the National Centre for Research and Development for the development of a cell-based phenotypic platform based on high content imaging system integrated with artificial intelligence data analysis for multiple therapeutic areas, including neuroinflammatory and fibrosis drug discovery (HiScAI – High Content Screening Artificial Intelligence).
In a major step to make India self-reliant in COVID-19 testing, Mylab Discovery Solutions, a Pune based molecular diagnostics has collaborated with Kiran Mazumdar Shaw’s Syngene, a leading global contract research organization, to cater to India’s demand for RT-PCR COVID-19 testing kits.

U.S. Food and Drug Administration issued a Drug Safety Communication regarding known side effects of hydroxychloroquine and chloroquine, including serious and potentially life-threatening heart rhythm problems, that have been reported with their use for the treatment or prevention of COVID-19, for which they are not approved by the FDA. These risks, which are in the drug labels for their approved uses, may be mitigated when health care professionals closely screen and supervise these patients such as in a hospital setting or a clinical trial, as indicated in the Emergency Use Authorization (EUA) for these drugs to treat COVID-19.
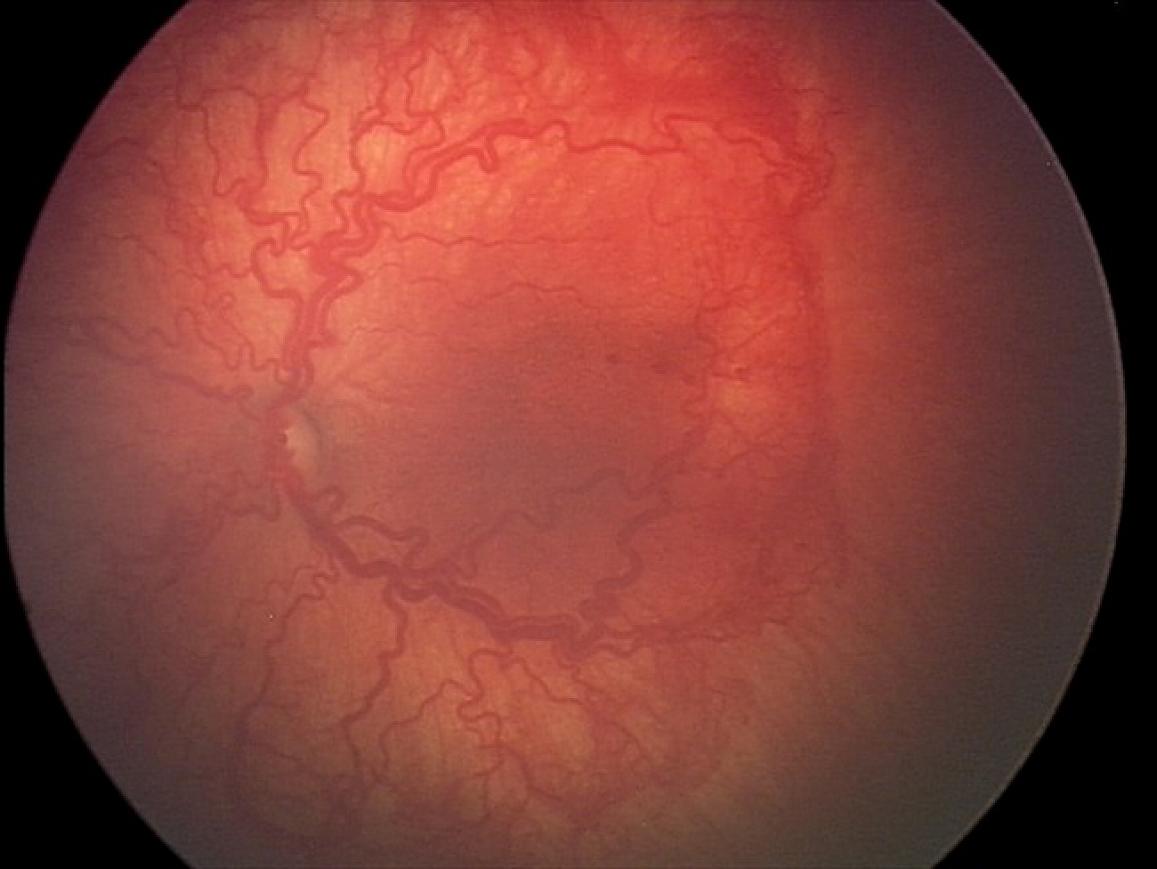
Results from a clinical trial published in 2011 confirmed the benefit of using Avastin over laser therapy for treating the most severe cases of ROP, which occur in a region of the retina known as posterior zone 1, as shown. David Wallace, M.D.
Babies born prematurely who require treatment to prevent blindness from retinopathy of prematurity (ROP) could be treated with a dose of Avastin (bevacizumab) that is a fraction of the dose commonly used for ROP currently. Results from the dose-finding study were published April 23 in JAMA Ophthalmology. The study was conducted by the Pediatric Eye Disease Investigator Group (PEDIG) and supported by the National Eye Institute (NEI), part of the National Institutes of Health.
Preterm babies are at high risk of abnormal blood vessel growth in the retina, the light-sensitive tissue in the back of the eye. These abnormal blood vessels are fragile and prone to leaking. If left untreated, vessel growth can lead to scarring and retinal detachment, the main cause of ROP-related vision loss. ROP is one of the leading causes of blindness in children.
Established ROP treatments include laser therapy and cryotherapy. Both interventions work by causing the abnormal blood vessels to stop growing before they can cause scarring and retinal detachment.
Avastin is one of several available drugs that inhibit abnormal blood vessel growth by suppressing the overproduction of a signal protein called vascular endothelial growth factor (VEGF).
The U.S. Food and Drug Administration approved Avastin in 2004 as a cancer therapy. Since then, ophthalmologists have used it off-label to inhibit abnormal blood vessel growth in ROP, as well as in other ocular disorders. Results from a clinical trial published in 2011 confirmed the benefit of using Avastin over laser therapy for treating the most severe cases of ROP, which occur in a region of the retina known as posterior zone 1.
They plan to follow children over time to compare the long-term effects of each strategy on vision and organ development. Previous studies suggest that babies treated with Avastin versus laser may be less likely to become myopic and require glasses for nearsightedness as they grow older.
The study involved 59 preterm infants with type 1 ROP, the most severe form. Each infant had one eye treated by a single injection containing 0.016 mg, 0.008 mg, 0.004 mg, or 0.002 mg of Avastin. If the other eye required treatment, it received twice the concentration (one dose level higher). By comparison, currently used doses of Avastin for ROP range from 0.25 mg to 0.625 mg.
Treatment was considered a short-term success if ROP improved by day four after therapy, and if there was no recurrence or need for additional treatment within four weeks. Such success was achieved in all eyes treated with the 0.016 mg and 0.008 mg doses, and in 9 of 10 eyes receiving 0.004 mg, but only in 17 of 23 eyes receiving 0.002 mg, resulting in the conclusion that 0.004 mg may be the lowest effective dose.

The U.S. Food and Drug Administration has issued warning letters to two companies for illegally selling unapproved products containing cannabidiol (CBD) in ways that violate the Federal Food, Drug and Cosmetic Act (FD&C Act). This action is a continuation of the FDA’s efforts to pursue companies that illegally market CBD products with claims that they can treat medical conditions, including opioid addiction or as an alternative to opioids.

The U.S. Food and Drug Administration authorized the first diagnostic test with a home collection option for COVID-19. Specifically, the FDA re-issued the emergency use authorization (EUA) for the Laboratory Corporation of America (LabCorp) COVID-19 RT-PCR Test to permit testing of samples self-collected by patients at home using LabCorp’s Pixel by LabCorp COVID-19 Test home collection kit.

U.S. Food and Drug Administration granted accelerated approval to Trodelvy (sacituzumab govitecan-hziy) for the treatment of adult patients with triple-negative breast cancer that has spread to other parts of the body. Patients must have received at least two prior therapies before taking Trodelvy.
Jaipur, 22nd April 2020: Considering the ongoing lockdown due to COVID-19, Jaipur based IIHMR University has decided to conduct GD/PI online over virtual communication app, to complete the admission process. The step was taken to support state government decision on announcement of summer vacation in public and private universities till 31st May due to Coronavirus outbreak.

Johnson & Johnson announced a collaboration between the Janssen Pharmaceutical Companies of Johnson & Johnson and Emergent BioSolutions, Inc. to support the manufacturing of its lead investigational COVID-19 vaccine candidate. This is the first in a series of prospective global collaboration agreements designed to accelerate manufacturing of Johnson & Johnson’s COVID-19 vaccine candidate, and further the Company’s goal to supply more than one billion doses of the vaccine globally.