
Tracing, managing, and monitoring of COVID patients has become the centerfold solution of heading towards a COVID free society said by Mr. Prem Sharma, CEO, Day To Day Health.
Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.
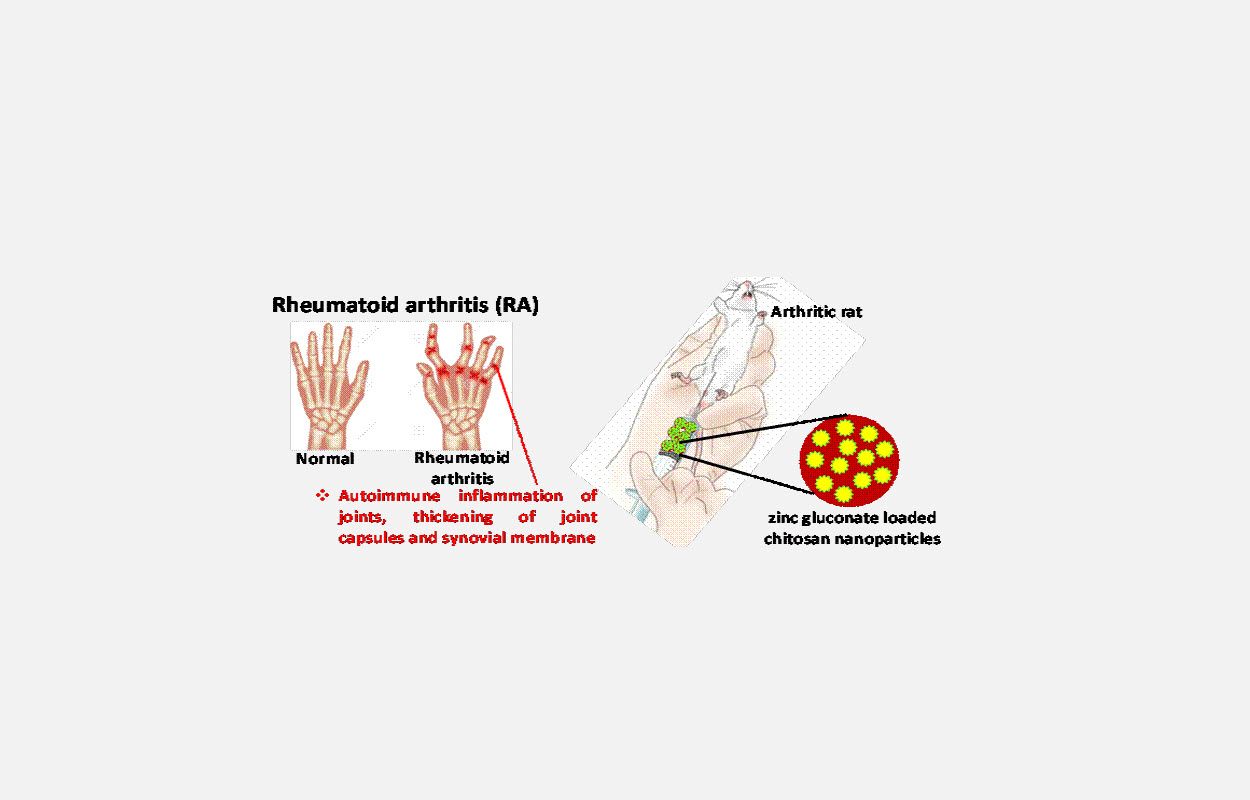
Scientists from the Institute of Nano Science & Technology (INST), Mohali, an autonomous institute of the Department of Science and Technology Government of India, have formulated nanoparticles with chitosan and loaded these nanoparticles with zinc gluconate for reducing the severity of rheumatoid arthritis.

The Drug Controller General of India (DCGI) has directed regulators to take steps to curb black marketing of remdesivir following a complaint black marketing and overpricing of antiviral drug by certain unscrupulous persons.

In continuation of our COVID-19 response efforts to support communities, Bayer remains privileged to extend our assistance to the Government of Maharashtra by providing its centre at Chittegaon to set-up a 100 bed Corona Care facility to treat affected patients.
With the announcement of COVAXIN by Bharat Biotech and ZyCov-D Vaccine by Zydus Cadila the proverbial silver line in the dark clouds of COVID19 appears at the horizon. Now the nod given by the Drug Controller General of India CDSCO (The Central Drugs Standard Control Organisation) for the conduct of the human trial for the vaccines, marks the beginning of the end.