Pharma News
Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.
-
-

Subsidiary of Sun Pharma, Taro Pharmaceutical will acquire Canada's Aquinox Pharmaceutical for USD 8.2 million (approx Rs 61.35 crore).
-
-
-
-
Caption : In a test of antiviral effectiveness against the virus that causes COVID-19, an extract from edible seaweeds substantially outperformed remdesivir, the current standard antiviral used to combat the disease.
-
-
-
Scientists investigating the evolution of the virus that causes Covid19 say that its mutation seems to be directed by human proteins that degrade it, but natural selection of the virus enables it to bounce back. The findings could help in the design of vaccines against the virus.
-
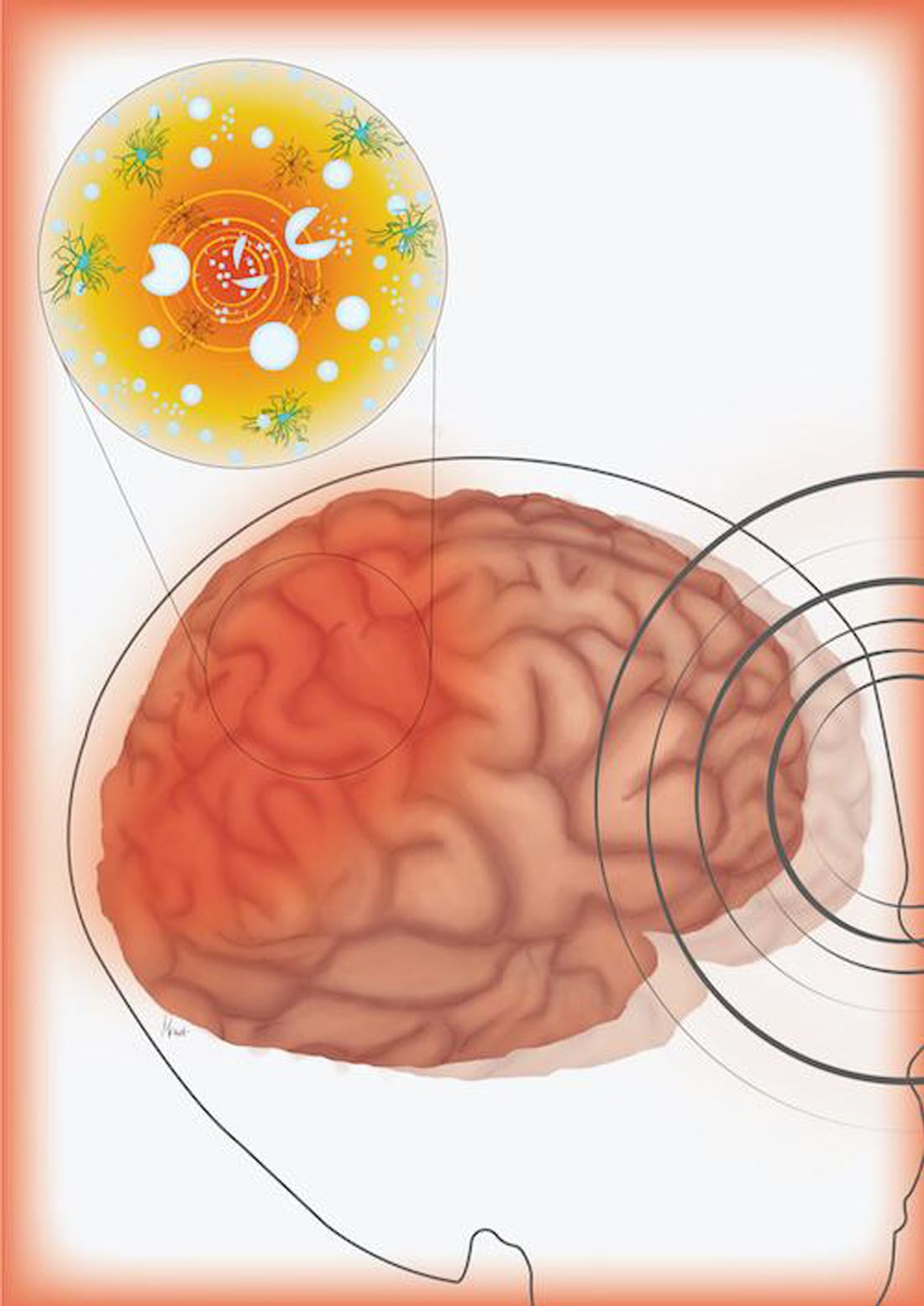
Caption : This illustration shows how head trauma can create microbubbles in the skull that collapse and damage nearby brain cells. Credit : Illustration by Mica Post