Stress Protein in Fibroblasts May Be a Good Target for Future Cancer Drugs, Study Finds
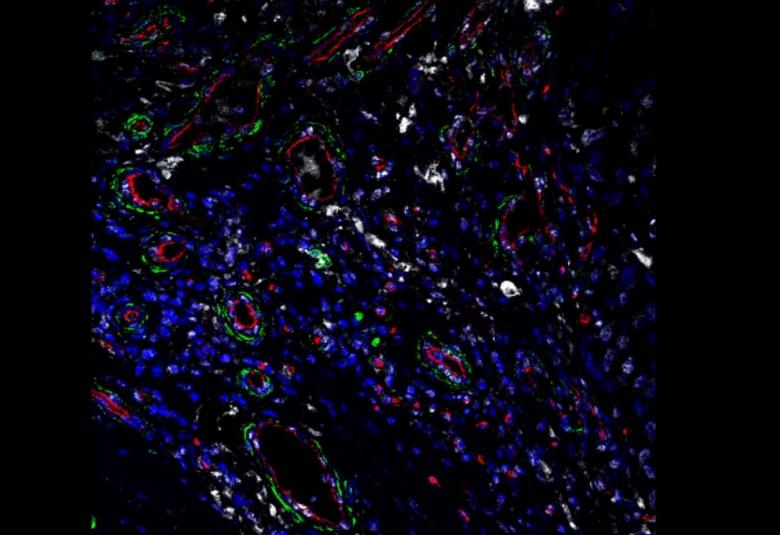
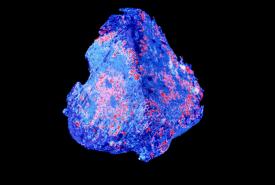
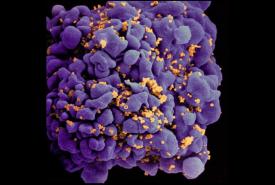
A stress protein that is overactive in many types of tumor cells also has a key role in tumor-supporting cells called fibroblasts, and may be a good target for future cancer treatments, suggests a study from researchers at the Perelman School of Medicine at the University of Pennsylvania.