Technology Developed at UH Could Advance Treatment of Lymphoma
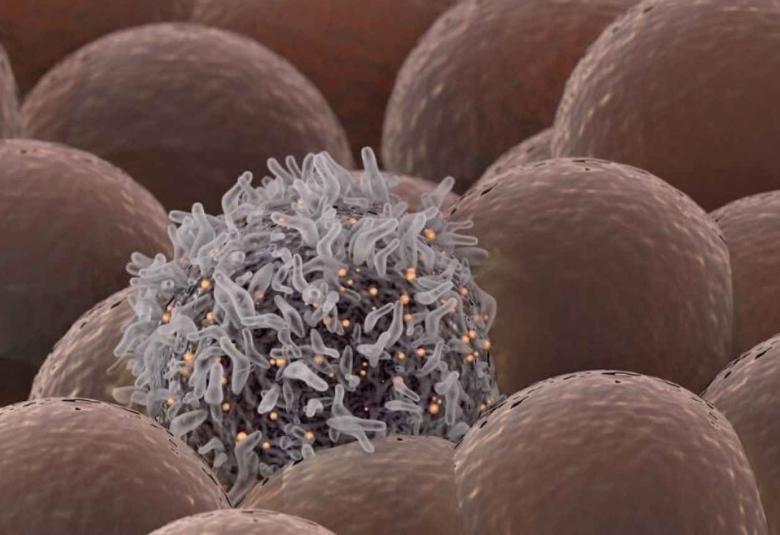
In the war against cancer, one of the most critical battles is waged on a cellular level as T cells from the immune system are altered in the lab to attack cancer cells. This form of immunotherapy, called chimeric antigen receptor (CAR) T-cell therapy, can be a life-saving treatment resulting in tumor control lasting ten years or longer.