Possible underlying cause of dementia detected
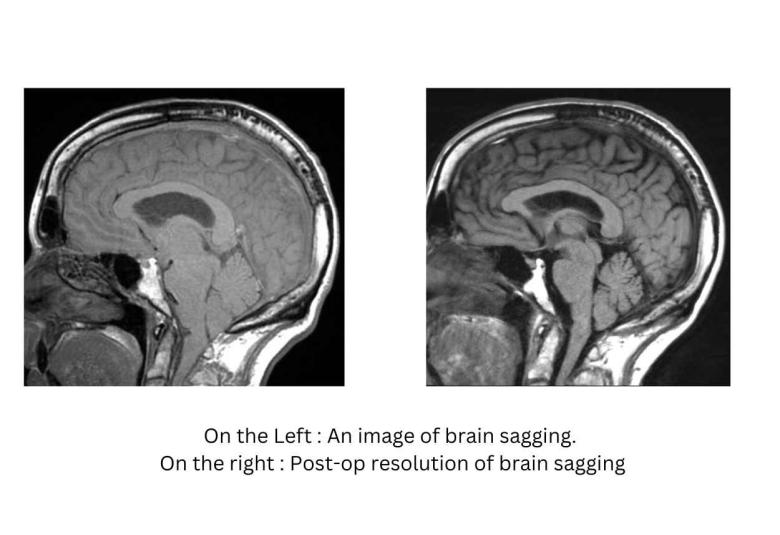
A new Cedars-Sinai study suggests that some patients diagnosed with behavioral-variant frontotemporal dementia (bvFTD)—an incurable condition that robs patients of the ability to control their behavior and cope with daily living—may instead have a cerebrospinal fluid leak, which is often treatable.
Researchers say these findings, published in the peer-reviewed journal Alzheimer’s & Dementia: Translational Research and Clinical Interventions, may point the way to a cure.