Novel Immunotherapy Agent Safe, Shows Promise Against High-Risk Prostate Cancers
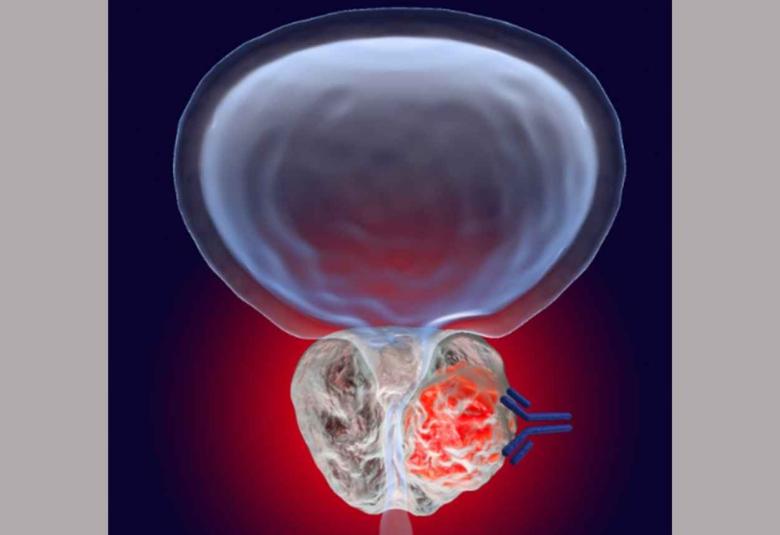
A new drug, a monoclonal antibody known as enoblituzumab, is safe in men with aggressive prostate cancer and may induce clinical activity against cancer throughout the body, according to a phase 2 study led by investigators at the Johns Hopkins Kimmel Cancer Center and its Bloomberg~Kimmel Institute for Cancer Immunotherapy. If confirmed in additional studies, enoblituzumab could become the first promising antibody-based immunotherapy agent against prostate cancer.