Q.4. (a) Discuss the theory of Mass spectroscopy
Ans.4.(a) Theory: Mass spectrometry is the most accurate method for determining the molecular mass of the compound and its elemental composition. In this technique, molecules are bombarded with a beam of energetic electrons. The molecules are ionized and broken up into many fragments, some of which are positive ions. Each kind of ion has a particular ratio of mass to charge, i.e. m/e ratio (value). For most ions, the charge is one and thus, m/e ratio is simply the molecular mass of the ion.
[adsense:336x280:8701650588]
A parent ion results when one electron is removed from the parent molecule of the substance
M+e --------------> M+ + 2e
The m/e value of the parent ion is equal to the molecular mass of the compound. In a few cases, the parent ion peak may be the base peak and can be easily recognized. In most of the cases, parent ion peak is not the base peak and is often of very small abundance. Many elements occur naturally as isotopes; out of these the lightest one greatly predominates. The mass spectrometer is designed to perform three basic functions.
These are:
(i). To vaporize compounds of varying volatility.
(ii). To produce ions from the neutral compounds in the vapour phase.
(iii). To separate ions according to their mass over charge ratio and to record them. The plot of m/e values taken along abscissa and their relative intensities along the ordinate is called the mass spectrum.
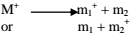
Neutral particles, produced in the process of fragmentation (whether neutral molecules or radicals) cannot be detected in the mass spectrometer.
The mass spectrum of neopentane is shown as follows:










