Q.4.(a) Basic fragmentation types, rules and factors influencing it.
Ans.4.(a) There are 3 basic types of fragmentation:
[adsense:336x280:8701650588]
1) Cleavage of σ bond
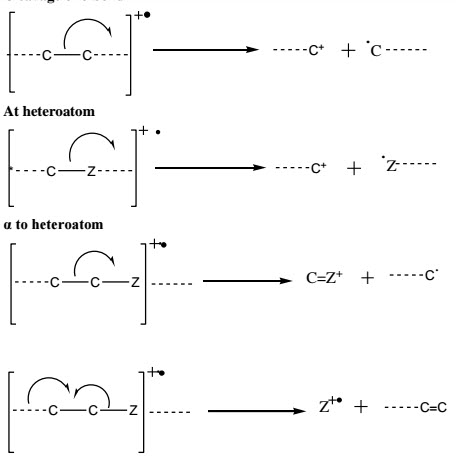
2)Cleavage of 2σ bond (rearrangements)
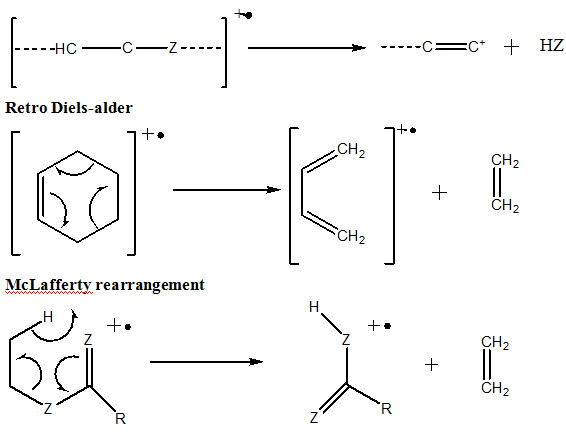
3) Cleavage of Complex rearrangements
Fragmentation rules:
1. The relative height of the molecular ion peak is greatest for the straight chain compound and decreases.
2. The relative height of the molecular ion peak usually decreases with increasing molecular weight in a homologous series. Fatty esters appear to be an exception.
3.Cleavage is favoured at alkyl-substituted carbon atoms; the more substituted, the more likely is cleavage. This is a consequence of the increased stability of a tertiary carbocation over a secondary, which in turn is more stable than a primary.
Cation stability order

Generally, the largest substitute at a branch is eliminated most readily as a radical, presumably because a long-chain radical can achieve somestabilityby delocalization of the lone electron.
4. Double bond, cyclic structure, and especially aromatic (or heteroaromatic) rings stabilize the molecular ion and thus increase the probability of its appearance.
5. Double bonds favour allylic cleavage and give the resonance-stabilized allylic carbocation. This rule does not hold for simple alkenes because of the ready migration of the double bond, but it does hold for cycloalkenes.
6. Saturation rings tends to lose alkyl side chains at α bond. This is merely a special case of branching (rule 3). The positive charge tends to stay with the ring fragment
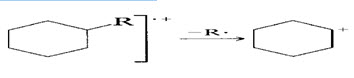
Unsaturated ring can undergo a retro Diels- Alder reaction.

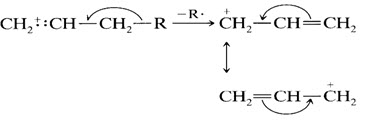
7. In alkyl-substituted aromatic compounds, cleavage is very probable at the bond β to the ring, giving the resonance-stabilized benzyl ion or, more likely, the tropylium ion
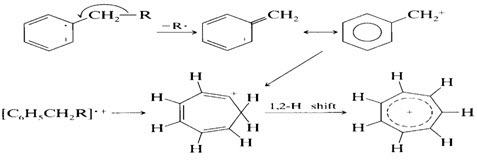
8. The C-C bonds next to a heteroatom are frequently cleaved, leaving the charge on the fragment containing the heteroatom whose nonbonding electrons provide resonance stabilization.
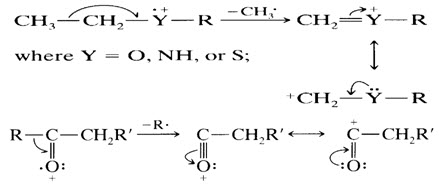
9. Cleavage is often associated with elimination of small, stable, neutral molecule, such as carbon monoxide, olefins, water, ammonia, hydrogen sulphide, hydrogen cyanide, mercaptans, ketene, or alcohols, often with rearrangement.
Subscribe to Pharmatutor Job Alerts by Email
Factors Affecting Fragmentation:
(i) Number of fragment peaks- The number of abundant ions and their distribution in the mass spectrum often gives good information about the type of the molecule. Thus, e.g., in the absence of an easily cleaved group, aromatic and saturated polycyclic molecules give abundant molecular ions. In the mass spectrum of naphthalene, C10H8 (M+•128), the molecular ion is of high stability, there being few fragment peaks of importance. On the contrary a spectrum which displays many fragment ions (increasing in abundance towards low m/z values) will suggest a largely aliphatic structure, eg, the mass spectrum of n-hexane.
A spectrum consisting of few prominent ions would show that the favoured decomposition pathways are only a few, to further indicate a small no. of labile bonds or stable products. This is seen by comparing the mass spectrum of n-butane with that of 2,3-butanedione. The mass spectrum of 2,3-butanedione C4H6O2 (M+•86) shows only two prominent peaks in addition to the molecular ion peaks. Thus the appearance of thespectrum is dominated by mass 43 (CH3CO) and 15 (CH3). The mass spectrum of n-butane C4H10 (M+•58) is more complex and in keeping with the fact that saturated hydrocarbon groups give series of ions separated by 14 mass units, corresponding to CH2.This spectrum displays major ions as masses 15 (CH3+), 29 (C2H5+), 43 (C3H7+).
(ii) Common fragment ions and structure- The consideration of one or two peaks in the mass spectrum may lead to useful results. If, e.g, the infrared spectrum shows the presence of a carbonyl group, the presence of an ion at m/z = 43 is often an evidence for the presence of a methyl ketone grouping, i.e., CH3CO. However, it is essential to determine the elemental composition of the ion at m/z = 43 by accurate mass measurement to support this assignment since C3H7 is also at m/z = 43.
The presence of an ion at m/z = 91 (C7H7+) is characteristic to show the presence of either a benzylic residue in a compound or a structural unit which can readily rearrange to give the (C7H7+) ion (tropylium ion). In fact, the presence of an ion at m/z = 91 is as dependable to point out an aromatic structure as the peaks in the 1H NMR spectra around δ 6.5-8.
Other characteristic ions include m/z = 31 (primary alcohols) 74 (methyl alkanoates) and 149 (phthalic acid and esters). As already said, some caution should be adopted in the use of these data.
(iii) Detection of characteristic m/z values due to functional groups- Distinction among isomeric alcohols- The fragmentation pathways characteristic of functional groups may provide useful information about the structure. If an unknown subject is suspected to be, e.g, an alcohol, then its mass spectrum can be examined for features of fragmentation pathways known for other alcohols. No doubt infrared spectrum will immediately reveal that the compound under study is an alcohol (strong O-H stretching around 3400 cm-1), however, mass spectrum will immediately not only confirm this but a distinction among isomeric alcohols, e.g., will be possible.









