About Author
1Tiwari H.S., 2Barfa Rajendra, 3Mehta Pooja, 4Alam Anzar, 5Panda B.P., 6Yadav Minu 7Mangal Gopesh, 8Sharma Sanjeev
1. Medical advisor,
2. Research Officer,
3. Ayurveda Physician,
4. Quality Control, Multani Pharmaceuticals Ltd. New Delhi,
5. Associate Professor (Pharmaceutical Biotech) Department of Pharmacognosy & Phytochemistry School of Pharmaceutical Education & Research Jamia Hamdard,New Delhi.,
6. P.G. Scholar Panchkarma Dept. National Institute of Ayurved Jaipur,
7. Associate Professor Panchkarma Dept. National Institute of Ayurved Jaipur,
8. Vice Chancellor National Institute of Ayurved Deemed To be University (De Novo) Ministry of AYUSH, Govt. of India Jaipur
ABSTRACT
The immune system acts to protect the host from infectious agents like bacteria, virus, fungi etc. The immunity or body defence mechanism is divided into two types- natural & specific. This study was planned to investigate the immunomodulatory effect of Kuka Immunity Booster Tablets. Kuka Immunity Booster have many herbs like Tulsi, Makoy, Giloy, Mulethi, Vasa, Ashwagandha, Brahmi etc. which are used for immunomodulatory effects. Various research has been done in the past as well as are going on presently for immunity enhancing. Exigency of the present study is to incorporate the immunity modulatory herbs in a combination to see the results on healthy individuals with the help of immunity markers.
The research was carried out for 30 days with a dose of 1 tab twice a day in 10-18 yrs. old age group & 2 tab twice in 19 to 60 yrs. old age group. Criteria for evaluation are changes in the level of Th1 & Th2 cytokines. This study was an open labeled single armed study of 50 healthy subjects. Assessment of changes in rest blood investigations like IgG, IgM, IgA, CBC, IgE, ESR etc. at baseline to the end of the therapy. Also, assessment of the changes in the subjective parameters like Anorexia, Bala, physical health & immunity status at baseline & end of therapy. All the 50 subjects were given Kuka Immunity Booster Tablets.
Primary End-Point Assessment: The changes in levels of Th1 & Th2 cytokines (interferon γ & interleukin 4) were assessed at pre-treatment (Day 0) & at the end of treatment (Day 30). The subjects were enrolled & informed consent was taken. The subjects were instructed regarding the study procedure & were advised to take Kuka immunity Booster Tablets for 30 days.
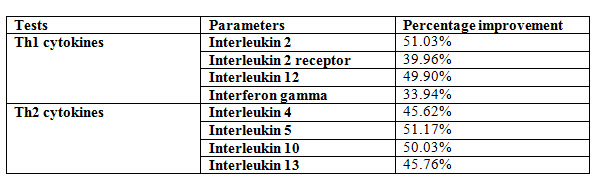
All the subjects were informed regarding the investigation that will be carried out during the period of the study. The primary data obtained shows an improvement of minimum of 33.94% to 51.17% from the baseline values of the primary outcome of Th1 & Th2 cytokines. Secondary End-points Assessment: There was improvement seen in Anorexia, Bala & physical health post completion of trial.
INTRODUCTION
Immunity is defined as the capacity of the body to resist pathogenic agents. The immune system acts to protect the host from infectious agents like bacteria, virus, fungi etc. The immunity or body’s defence mechanism is divided into two types- natural (innate) and specific (adaptive). Innate immunity is present before exposure to pathogens and is concerned with preventing the entry of the infectious agents into the body. It includes physical barriers, soluble factors, and phagocytic cells. Acquired immunity on the other hand is the resistance developed in the body against any specific foreign bodies like bacteria, viruses, toxins, vaccines, or transplanted tissues. The immune system responds to challenge with an increase in the activity of certain components that act in coordinated fashion to eliminate the source of the challenge. The various cells comprising immune system are-
i. Lymphocytes
ii. Monocytes and macrophages
iii. Mast cells and basophils
iv. Neutrophils
v. Eosinophils
Antibody- is a ‘Y’ shaped protein that is produced by B lymphocytes in response to the presence of an antigen. It is a γ globulin in nature and is also called as immunoglobulin (Ig). The five classes of circulating antibodies are immunoglobulins are IgG (forms 75%), IgA, IgM, IgE and IgD.
Lymphocytes- is the master of human immune system and are functionally divided into two subtypes. The lymphocytes that undergo maturation and differentiation in the bone marrow (B cells) and thymus (T cells). It acquires certain genetic and immune surface characters which determine their type and function; this is based on cluster of differentiation (CD) molecule on their surface .
B lymphocytes- These cells are involved in humoral immunity by inciting antibody response. On coming in contact with antigen, B cells are activated to proliferate and transform into plasmacytoid lymphocytes and then into plasma cells. Depending upon the maturation stage of B cells, specific CD molecules appear on the cell surface which can be identified by CD markers. Common B cell markers are: CD19, 20, 21, 23. These cells also possess B cell receptors for surface immunoglobin (IgM and IgG) and Fc receptors for attaching to antibody molecules . T cell help is provided to B cells by a subset of T helper cells, TH 2 by elaborated interleukins (IL-4, IL-5, IL-10, IL-13) .
T lymphocytes- These cells are implicated in inciting cell-mediated immunity and delayed type of hypersensitivity. Pan T cell markers are CD3, CD7 and CD2. Depending upon functional activity, T cells have two major subtypes: T helper (or CD4+) cells and T suppressor (or CD8+) cells.
T helper cells- Abbreviated as TH cells, these cells promote and enhance the immune reaction and are also termed as T-regulatory cells. They carry CD4 molecule on their surface and hence are also called CD4+ cells. CD4+ cells in circulation are about twice the number of CD8+ cells (CD4+/CD8 ratio 2:1) .
CD4+ cells are predominantly involved in cell-mediated reactions to viral infections, tissue transplant reactions and tumor lysis. CD8+ are particularly involved in destroying cells infected with viruses, foreign cells and tumor cells. These are directly cytotoxic to the antigen .
Natural killer (NK) cells- These are large granular lymphocytes and are part of natural or innate immunity. These cells recognize antibody coated target cells and bring about killing of the target directly. This process is termed as antibody-dependent cell-mediated cytotoxicity. These cells carry surface molecules of CD 2, CD16 and CD 56 but negative for T cell marker CD 3.
MATERIALS AND METHODS
In order to evaluate the effect of Kuka immunity booster tablets, healthy subjects aged between 10 to 60 years were enrolled into the study.
The subject between 10-18 years of age were advised to take one Kuka immunity booster tablet twice a day before meal with 150-180ml of lukewarm water for a period of 30 days.
The subjects between 19-60 years of age were advised to take two Kuka immunity booster tablets twice a day before meal with 150-180ml of lukewarm water for a period of 30 days.
Adverse effects if any were noted down. The subjects were free to withdraw from study if they so desired. No other medication intended for same use as study medication was allowed for these subjects. Laboratory investigations were performed on Day 0 and at the end of the study (Day 30). Clinical parameters, physical health status and immunity health questionnaire were assessed at Day 0 and Day 30.
INCLUSION CRITERIA
• Apparently healthy subjects of either gender between the age group of 10 years to 60 years (both inclusive). Healthy individuals were considered as those who do not have any acute medical condition or chronic medical/surgical condition that requires either immediate or continuous medical monitoring and treatment.
• Subjects aged between 10 to 60 years of either sex not taking any medicine were included in the study.
• Subjects willing to give informed consent for the study. Consent for child was taken from his/her parents or guardian.
• Subject devoid of any medication during last one month.
EXCLUSION CRITERIA
• Individuals with immediate life-threatening diseases such as pre-existing cardiovascular, liver, or neoplastic diseases or who received any immunosuppressant, sedative, hypnotic or tranquilizer within 30 days prior to enrolment.
• Individuals with any psychiatric illness which may impair the ability to provide written ICF.
• Individuals participating in any other clinical trial.
• Pregnant or lactating females.
• Subjects suffering from different diseases/disorders and/or undergone surgery during last one year, received organ transplant, chronic smokers, underlying conditions which might affect immunity.
• Alcohol and drug abusers.
EFFICACY AND SAFETY VARIABLES
• All the subjects underwent a set of blood test before the starting of trial (Day 0) and after completion of trial (Day 30).
• Clinical parameters- Assessment were done by the evaluation of Anorexia, Bala, Physical health and Immunity Status.
• Hematological and Biochemical tests- At Day 0 and Day 30.
• CBC, CRP, ESR, LFT, RFT, Serum Iron, IgG, IgM, IgE, IgA, total proteins.
• Other immunological parameters such as Th1 and Th2 cytokines (interferon γ and interleukin 4), T-helper cells, T-cytotoxic cells and NK-cells.
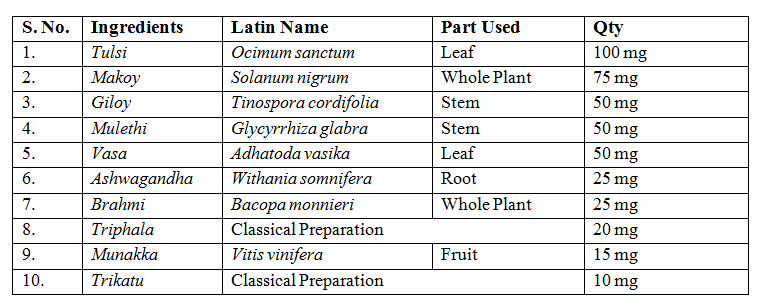
TEST PRODUCT
Kuka immunity booster tablets
TEST PRODUCT AND DOSAGE
Kuka immunity booster tablets- prepared by Multani Pharmaceuticals Ltd.,
Dose- Age- 10-18 years- 1 tablet twice daily before meal with one cup (150-180ml) of lukewarm water.
19-60yrs- 2 tablets twice daily before meal with one cup (150-180ml) of lukewarm water.
Before meal for 30 days.
Dosage form: Tablet
ASSESSMENT OF CLINICAL AND SUBJECTIVE PARAMETERS
The clinical parameters were assessed at pre-treatment (Day 0) and post-treatment (Day 30). Out of a total of 50 subjects, only 44 subjects completed the trial. The clinical assessment is recorded for the 44 subjects. The anorexia was absent at Day 0 in 30 subjects (68.18%), which increased to absence in 39 subjects (88.63% of the total subjects) post treatment.
The general physical health was fair in 20 subjects, good in 12 subjects before treatment, which improved to fair in 16 subjects and good in 20 subjects post treatment.
The general health grade changed on a 9-grade scale in 7 subjects before treatment to 11 subjects post treatments.
|
Clinical parameters analysis |
|||
|
Parameter |
Response |
Pre-treatment (Day 0) |
Post treatment (Day 30) |
|
Anorexia |
Absent (0) |
30 (68.18) |
39 (88.63) |
|
Loss of appetite without alteration in eating habits (1) |
9 (20.45) |
5 (11.36) |
|
|
Oral intake altered without significant weight loss (2) |
5 (11.36) |
0 (0) |
|
|
Associated with significant weight loss or malnutrition (3) |
0 (0) |
0 (0) |
|
|
Life threatening consequences: urgent intervention indicated (4) |
0 (0) |
0 (0) |
|
|
Life threatening consequences leading to death (5) |
0 (0) |
0 (0) |
|
|
Bala |
Can perform excessive work without fatigue (0) |
14 (31.81) |
14 (31.81) |
|
Can carry out routine activity without fatigue (1) |
22 (50.00) |
24 (54.54) |
|
|
Perform routine activity with fatigue (2) |
8 (18.18) |
6 (13.63) |
|
|
Difficulty to perform or cannot perform routine activity (3) |
0 (0) |
0 (0) |
|
|
How do you feel about your physical health?
|
Very dissatisfied (-3) |
1 (2.27) |
0 (0) |
|
Somewhat dissatisfied (-2) |
7 (15.90) |
4 (9.09) |
|
|
Little dissatisfied (-1) |
7 (15.90) |
7 (15.90) |
|
|
Neutral (0) |
11 (25.00) |
11 (25.00) |
|
|
Little satisfied (1) |
5 (11.36) |
9 (20.45) |
|
|
Somewhat satisfied (2) |
11 (25.00) |
11 (25.00) |
|
|
Very satisfied (3) |
2 (4.54) |
2 (4.54) |
|
|
In general, would you say your physical health is:
|
Poor |
3 (6.81) |
1 (2.27) |
|
Fair |
20 (45.45) |
16 (36.36) |
|
|
Good |
12 (27.27) |
20 (45.45) |
|
|
Very Good |
4 (9.09) |
4 (9.09) |
|
|
Excellent |
1 (2.27) |
1 (2.27) |
|
|
Immune status questionnaire (ISQ) score
|
0 |
0 (0) |
0 (0) |
|
1 |
0 (0) |
0 (0) |
|
|
2 |
0 (0) |
0 (0) |
|
|
3 |
2 (4.54) |
2 (4.54) |
|
|
4 |
2 (4.54) |
2 (4.54) |
|
|
5 |
3 (6.81) |
3 (6.81) |
|
|
6 |
5 (11.36) |
5 (11.36) |
|
|
7 |
1 (2.27) |
1 (2.27) |
|
|
8 |
6 (13.63) |
6 (13.63) |
|
|
9 |
14 (31.81) |
14 (31.81) |
|
|
10 |
11 (25.00) |
11 (25.00) |
|
|
General Health grade
|
0 |
0 (0) |
0 (0) |
|
1 |
0 (0) |
0 (0) |
|
|
2 |
0 (0) |
0 (0) |
|
|
3 |
0 (0) |
0 (0) |
|
|
4 |
0 (0) |
0 (0) |
|
|
5 |
6 (13.63) |
1 (2.27) |
|
|
6 |
5 (11.36) |
4 (9.09) |
|
|
7 |
13 (29.54) |
13 (29.54) |
|
|
8 |
11 (25.00) |
12 (27.27) |
|
|
9 |
7 (15.90) |
11 (25.00) |
|
|
10 |
2 (4.54) |
3 (6.81) |
|
|
Immune functioning grade
|
0 |
0 (0) |
0 (0) |
|
1 |
0 (0) |
0 (0) |
|
|
2 |
0 (0) |
0 (0) |
|
|
3 |
0 (0) |
0 (0) |
|
|
4 |
0 (0) |
0 (0) |
|
|
5 |
2 (4.54) |
1 (2.27) |
|
|
6 |
6 (13.63) |
5 (11.36) |
|
|
7 |
7 (15.90) |
7 (15.90) |
|
|
8 |
19 (43.18) |
18 (40.90) |
|
|
9 |
9 (20.45) |
12 (27.27) |
|
|
10 |
1 (2.27) |
1 (2.27) |
|
|
Do you have reduced immune functioning at this moment?
|
Yes |
7 (15.90) |
7 (15.90) |
|
No |
37 (84.09) |
37 (84.09) |
|
|
Do you have a chronic disease?
|
Yes |
0 |
0 |
|
No |
44 (100) |
44 (100) |
|
|
Tests |
Parameter |
Mean ± SD (BT) |
Mean ± SD (AT) |
|
CBC |
ESR |
16.91 ± 11.00 |
15.91 ± 7.53 |
|
Haemoglobin |
12.86 ± 1.77 |
12.95 ±1.68 |
|
|
TLC |
7.16 ± 2.04 |
7.19 ± 2.18 |
|
|
TEC |
4.50 ± 0.56 |
4.54 ± 0.59 |
|
|
Platelet count |
279.89 ± 89.56 |
284.20 ± 82.19 |
|
|
Haematocrit |
38.08 ± 4.68 |
38.48 ± 4.54 |
|
|
MCV |
85.44 ± 10.67 |
85.68 ± 10.17 |
|
|
MPV |
10.25 ± 1.72 |
10.23 ± 1.54 |
|
|
MCH |
28.89 ± 4.16 |
28.87 ± 3.97 |
|
|
MCHC |
33.73 ± 1.08 |
33.62 ± 1.13 |
|
|
RDWCV |
12.18 ± 1.59 |
12.46 ± 2.30 |
|
|
Neutrophils (40-80%) |
55.24 ± 9.03 |
55.97 ± 10.84 |
|
|
Lymphocytes (20-40%) |
33.06 ± 7.52 |
32.00 ± 8.89 |
|
|
Monocytes (2-10%) |
7.32 ± 1.77 |
7.30 ± 1.91 |
|
|
Eosinophils (1-6%) |
2.92 ± 1.90 |
3.38 ± 2.75 |
|
|
Basophils (0-1%) |
0.93 ± 0.32 |
0.95 ± 0.43 |
|
|
CRP |
CRP (0.0-5.0mg/L) |
1.95 ± 1.24 |
6.64 ± 9.28 |
|
Serum Iron |
Serum Iron |
70.21 ± 37.60 |
74.29 ± 36.86 |
|
LFT
|
Total Serum Bilirubin |
0.61 ± 0.29 |
0.63 ± 0.25 |
|
Direct Serum Bilirubin |
0.30 ± 0.15 |
0.28 ± 0.13 |
|
|
Indirect Serum Bilirubin |
0.32 ± 0.19 |
0.36 ± 0.17 |
|
|
Total Serum Protein |
7.65 ± 0.41 |
7.56 ± 0.47 |
|
|
Serum Albumin |
4.80 ± 0.41 |
4.53 ± 0.43 |
|
|
Serum Globulin |
2.85 ± 0.40 |
1.74 ± 1.56 |
|
|
A/G Ratio |
1.73 ± 0.36 |
1.56 ± 0.40 |
|
|
SGOT |
29.76 ± 18.49 |
28.63 ± 15.07 |
|
|
SGPT |
25.50 ± 20.41 |
25.11 ± 18.64 |
|
|
Alkaline Phosphatase |
114.50 ± 44.96 |
108.84 ± 43.40 |
|
|
RFT
|
Serum Urea |
19.35 ± 4.88 |
19.84 ± 5.19 |
|
Serum Creatinine |
0.91 ± 0.18 |
1.04 ± 0.28 |
|
|
IgG |
IgG |
1526.43 ± 280.08 |
1471.21 ± 260.08 |
|
IgM |
IgM |
1.75 ± 0.43 |
1.32 ± 0.43 |
|
IgA |
IgA |
11.94 ± 57.81 |
9.10 ± 42.69 |
|
IgE |
IgE |
493.64 ± 457.51 |
370.81 ± 311.25 |
|
C3 protein |
C3 protein |
1.01 ± 0.28 |
1.37 ± 0.19 |
|
C4 protein |
C4 protein |
0.21 ± 0.07 |
0.30 ± 0.04 |
|
Th1 cytokines
|
Interleukin 2 |
1.01 ± 0.34 |
1.52 ± 0.31 |
|
Interleukin 2 receptor |
268.62 ± 104.24 |
375.98 ± 104.41 |
|
|
Interleukin 12 |
0.81 ± 0.28 |
1.22 ± 0.25 |
|
|
Interferon gamma |
2.27 ± 0.73 |
3.04 ± 0.46 |
|
|
Th2 cytokines
|
Interleukin 4 |
1.04 ± 0.43 |
1.52 ± 0.32 |
|
Interleukin 5 |
0.84 ± 0.24 |
1.27 ± 0.20 |
|
|
Interleukin 10 |
1.23 ± 0.22 |
1.84 ± 0.26 |
|
|
Interleukin 13 |
1.00 ± 0.27 |
1.46 ± 0.28 |
|
|
Absolute Lymphocyte count |
Absolute Lymphocyte count |
1752.59 ± 1368.09 |
1238.89 ± 284.38 |
|
T lymphocytes
|
CD3 (Total T cells) |
66.42 ± 6.52 |
57.35 ± 8.42 |
|
Absolute CD3 |
1412.81 ± 1512.28 |
1020.44 ± 153.63 |
|
|
CD4 (Helper T cells) |
39.74 ± 10.78 |
47.26 ± 5.65 |
|
|
Absolute CD4 |
902.56 ± 289.21 |
1027.30 ± 230.13 |
|
|
CD8 (Suppressor T cells) |
5.93 ± 2.19 |
3.23 ± 1.36 |
|
|
Absolute CD8 |
37.60 ± 12.60 |
21.81 ± 9.54 |
|
|
CD4/CD8 ratio |
1.54 ± 0.29 |
1.20 ± 0.24 |
|
|
NK cells
|
CD3-/ CD (16+56) |
14.95 ± 3.59 |
24.33 ± 28.89 |
|
Absolute CD3-/ CD (16+56) |
178.88 ± 58.30 |
276.88 ± 55.30 |
But the primary data obtained shows an improvement between a minimum of 33.94% to 51.17 % from the baseline values of the primary outcome of Th1 and Th2 cytokines.
OBSERVATION AND RESULT
Effect of Kuka immunity booster tablets Immunoglobulin Analysed parameters are IgG; IgM; IgA; and IgE before & after 30 days treatment in 44 subjects.

Effect of Kuka immunity booster tablets on Immunoglobulin-
It is seen that the Kuka immunity booster tablets significantly change the IgM & IgA. However, there is non-significant difference in IgG & IgE level in the human subject after 30 days treatment. This indicate that the Kuka immunity booster tablet is not enhancing the IgG & IgE level unless the person is infected. However, this will be reconfirmed from the experiment when Kuka immunity booter along with the infection with specific pathogen.
Effect of Kuka immunity booster tablets on Cytokinin parameter (IL2; IL -2 receptor; IL12; gamma interferon; IL-4; IL-5; IL-10; IL-13) before & after 30 days treatment in 44 subjects.
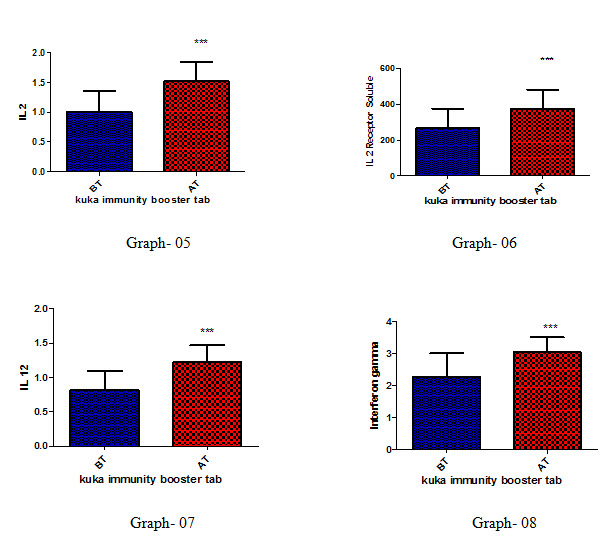
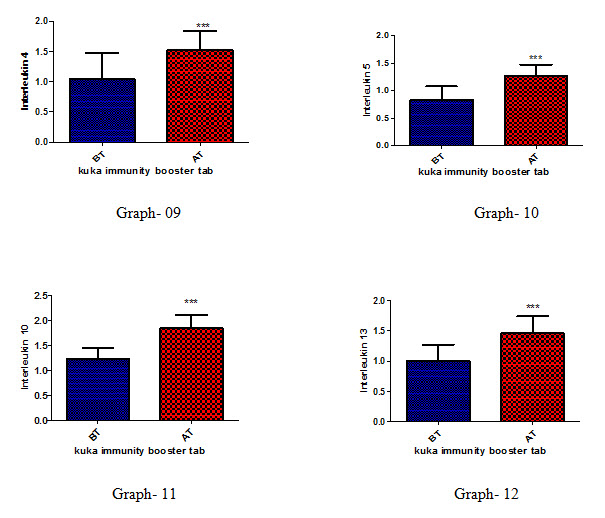
Effect of Kuka immunity booster tablets on Cytokinin- It is seen that the Kuka immunity booster tablets significantly increase the Cytokinin parameters such as IL2; IL -2 receptor; IL12; gamma interferon; IL-4; IL-5; IL-10; IL-13 level in the human subject after 30 days treatment. This indicate that Kuka Immunity booster tablet is enhancing the IL12 & gamma interferon level in the human by enhancing the activity of Activated T Cell. Increased level of IL2, Gamma interferon indicate higher functioning & activation of Th1 cells. Increased level of IL4, IL5 & IL13 indicate higher functioning & activation of Th 2 cells. Further there is increase in level of IL10 which indicate higher functioning & activation of T-reg (T regulatory) cells.
Effect of Kuka immunity booster tablets on Immune cell parameter (absolute lymphocyte count; CD3 (Total T cell); absolute CD3 cell; CD 4 T helper cell; CD 8 Suppressor T cell) before & after 30 days treatment in 44 subjects.
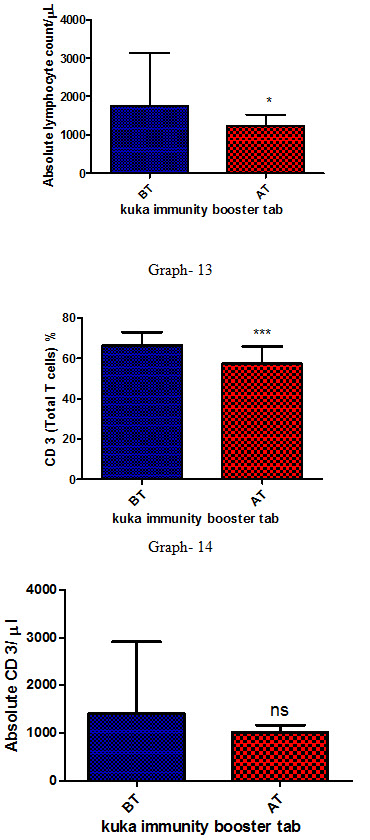
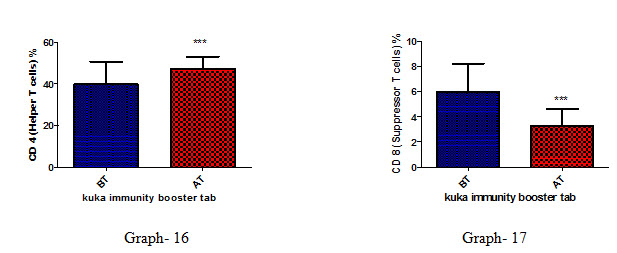
Effect of Kuka immunity booster tablets on Immune cell parameter is seen that the product significantly increases the CD4 T helper cell level & decrease in CD 8 Suppressor T cell in the human subject after 30 days treatment.
DISCUSSION
Normal function of immunity is for body defence, its failure or derangement in any way results in disease of the immune system which can be classified into four sub-groups. Immunodeficiency disorders are characterized by deficient or absent cellular & or humoral immune functions. It comprises of primary & secondary immunodeficiency disease including AIDS. Hypersensitivity reactions are characterized by hyper functions or inappropriate response of the immune system & include various mechanism of immunological tissue injury. Auto-immune disease occurs when the immune system fails to recognise ‘self’ from ‘non-self’. It includes various disease like rheumatoid arthritis, SLE etc. Possible immune disorders in which the immunological mechanism is suspected in their etiopathogenesis. Assessment was done on the basis of laboratory investigations which was done on Day 0 & at the end. Physical health status & immunity health questionnaire were assessed at Day 0 & Day 30. Lab Investigation include haematological & biochemical tests like CBC, CRP, ESR, LFT, RFT, Serum Iron, IgG, IgM, IgE, IgA, total proteins. Other immunological parameters such as Th1 & Th2 cytokines (interferon ϒ and interleukin 4), T-helper cells, T-cytotoxic cells and NK-cells. Primary data obtained shows an improvement between a minimum of 33.94% to 51.17% from the baseline values of the primary outcome of Th1 & Th2 cytokines.
Kuka immunity booster is a polyherbal Ayurvedic formulation which contains herbs that are effective in enhancing immunity-
Tulsi: Modern research has revealed that Tulsi has anti-bacterial, anti-viral and anti-fungal activity that includes activity against many pathogens responsible for human infections .
Giloy: Active compounds 11-hydroxymustakone, N-methyl–2-pyrrolidone, N-formylannonain, cordifolioside A, magnoflorine, tinocordiside and syringin has been reported to have potential immunomodulatory and cytotoxic effects .
Makoy: Till date Kakamachi has been screened for only its anti-nociceptive, anti-inflammatory, antipyretic, anticonvulsant, antioxidant and anti-hyperlipidemic, hepatoprotective, cytoprotective, antimicrobial, larvicidal, narcotic, diuretic, anti-angiogenic activities .
Mulethi: The immunomodulatory activity of an aqueous root extract of G. glabra was demonstrated in vitro to be linked to the presence of the phenolic compound glycyrrhizin reported the increased production of lymphocytes and macrophages from human granulocytes after contact with G. glabra root extract .
Vasa: Vasa, a major component of VA, is indicated in diseases such as Shwasa, Rajayakshma (tuberculosis), Raktapitta, Shotha (edema), and Jwara (fever). Vasicine and vasicinone, the bitter alkaloids available in the plant, has bronco-dilatory effect .
Triphala: Triphala research has found the formula to be potentially effective for several clinical uses such as appetite stimulation, reduction of hyperacidity, antioxidant, anti-inflammatory, immunomodulating, antibacterial and radioprotective effects, and prevention of dental caries .
Ashwagandha: Ashwagandha improves the body's defense against disease by improving the cell-mediated immunity. It also possesses potent antioxidant properties that help protect against cellular damage caused by free radicals .
Munakka: Munakka is useful for managing constipation due to its laxative property and helps to control acidity due to its cooling property. It is effective in dry cough and respiratory tract inflammation due to its cough suppressant and soothing properties .
Trikatu: Trikatu shows some important benefits like, it promotes the healthy digestion, improves all gastric functions, it increases food absorption. It reduces congestion in digestive tract. It is recommended for poor digestion and poor appetite. It is recommended for improving lung functions .
Brahmi: Its main pharmacological actions are antioxidant, anti-inflammatory, anticonvulsant, cardiotonic, bronchodilator, and peptic ulcer protection Various indications for use of Brahmi described in Ayurvedic medicine are memory improvement, epilepsy, insomnia, and anxiolytic .
The present report subjects taken Kuka immunity booster tablets have shown improvements in enhancing immune functions, physical health, and anorexia. The efficacy of Kuka immunity booster tablets can be due to the synergistic actions of the potent herbs present in the formulation. There were no adverse effects either reported or observed during the clinical study.
CONCLUSION : Kuka immunity booster tablets significantly change the Immunoglobulin IgM & IgA without change in IgG & IgE level. Kuka immunity booster tablet also significantly increases the Cytokinin parameters such as IL2; IL -2 receptor; IL12; gamma interferon indicates higher function of Th1 (T Helper 1 cell) & activated T cell activity; IL-4; IL-5; IL-13 level are increased because of higher function of Th2 (T Helper 2 cell); regulatory T Cell function is enhanced as indicated by increased IL10 level. This tablet also significantly increases the CD4 T helper cell level & decrease in CD 8 Suppressor T cell in the human subject in the human subject after 30 days treatment. However immune booster tablet along with infection with pathogen will give conclusive data on IgG &IgM for better understanding of immune function enhancement of Kuka immunity booster tablet. There were no adverse effects either reported or observed during the clinical study.
REFERENCES
1. K Sembulingam, Prema Sembulingam, Essentials of Medical physiology, Reprint edition 2011, Jaypee Brothers Medicla Publishers (P) Ltd., Chapter 17- Immunity, page 99-109.
2. Ibid
3. Liao W, Lin JX, Leonard WJ (October 2011). “IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation”. Current Opinion in Immunology. 23(5): 598-604. Doi:10.1016/j.coi.2011.08.003. PMC 3405730. PMID 21889323.
4. K Sembulingam, Prema Sembulingam, Essentials of Medical physiology, Reprint edition 2011, Jaypee Brothers Medicla Publishers (P) Ltd., Chapter 17- Immunity, page 99-109.
5.Ibid
6. María F. Ramírez , Johannes M. Huitink2 , Juan P. Cata, Perioperative Clinical Interventions That Modify the Immune Response in Cancer Patients, Open Journal of Anesthesiology, 2013, 3, 133-139.
7. Cohen MM. Tulsi - Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med. 2014;5(4):251-259. doi:10.4103/0975-9476.146554.
8. Saha S, Ghosh S. Tinospora cordifolia: One plant, many roles. Anc Sci Life. 2012;31(4):151-159. doi:10.4103/0257-7941.107344.
9. Chandrashekhar Jagtap, Rajeshree Patil, Prajapati PK. Brief review on therapeutic potentials of Kakamachi (Solanum nigrum Linn.). Ayurpharm Int J Ayur Alli Sci. 2013;2(2):22-32.
10. Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother Res. 2018;32(12):2323-2339. doi:10.1002/ptr.6178.
11. Paneliya AM, Patgiri B, Galib R, Prajapati PK. Efficacy of Vasa Avaleha and its granules on Tamaka Shwasa (bronchial asthma): Open-label randomized clinical study. Ayu. 2015;36(3):271-277. doi:10.4103/0974-8520.182760.
12. Peterson CT, Denniston K, Chopra D. Therapeutic Uses of Triphala in Ayurvedic Medicine. J Altern Complement Med. 2017;23(8):607-614. doi:10.1089/acm.2017.0083.
13. Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208-213. doi:10.4314/ajtcam.v8i5S.9.
14. Nassiri-Asl, Marjan & Hosseinzadeh, Hossein. (2016). Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update: Pharmacological Effects of Grape. Phytotherapy Research. 16. 10.1002/ptr.5644.
15. P.R. Malvankar and M.M. Abhyankar, Antimicrobial Activity of Water Extracts of Trikatu Churna and its Individual Ingredient, International Journal of Pharmaceutical Sciences And Research, 1087-1089.
16. Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer's Disease. Ann Neurosci. 2017 May;24(2):111-122. doi: 10.1159/000475900. Epub 2017 May 12. PMID: 28588366; PMCID: PMC5448442.
DOWNLOAD THIS ARTICLE AS PDF >>
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE