About Author
Mohd Riyaz Beg
Institute of Chemical Technology, Mumbai
Email: mohdriyazbeg@gmail.com
With the recent increase in awareness about safety and healthcare amid coronavirus outbreak custodians of healthcare (regulatory bodies) around the globe started improving and advancing their regulations and policies to safeguard the health of their citizens. Following the trend Indian regulatory body who watch-over the safety of the cosmetics in the country – CDSCO (Central Drug Standard Control Organization) come up with new Cosmetics Rules. The Cosmetics Rules have been recently published through an official gazette notification dated 15th Dec, 2020 and are effective from the same date in the whole country.
The Cosmetics Rules, 2020 for the first time introduce the concept of a ‘new cosmetic’ which has been defined to mean ‘a cosmetic that contains a novel ingredient that has not been used anywhere in the world or is not recognized for use in cosmetics in any national or international literature’. These new rules streamline the process of import registration of cosmetics. Along with the rules the CDSCO, apex regulatory authority has issued frequently asked questions (FAQs) and guidance document for grant or retention of registration certificate (RC) or license for import or manufacture of cosmetics in the country. These new rules mandate importers/ manufactures of a ‘new cosmetics’ to make an application and seek an approval from the Central Licensing Authority (CLA) before such a ‘new cosmetics’ could be imported or manufactured in India. Such an application should be accompanied with requisite data on safety and effectiveness. Further, the manufacturer must comply with IS 4011:2018 standards for testing the safety of the ‘new cosmetic’.
The structure of Cosmetics Rules 2020 structured in different chapters. The Chapter Lays down the provisions for Licensing Authorities, Delegation of powers of Licensing Authorities, Government Analyst and its functions, Powers, duties and functions of Inspectors specially Authorised to inspect manufacture and sale of cosmetics etc. Also, the Chapter Lays down the provisions for Application for grant of license or loan license to manufacture cosmetics for sale or for distribution, Manufacture at more than one premises, Conditions of license or loan licence for manufacture of cosmetics, Grant or refusal of license, etc. The provisions for Prohibition of sale or distribution, Prohibition against altering inscription on containers, labels or wrappers of cosmetic, Prohibition against false or misleading claims, Standards of cosmetics, etc are also drafted in it along with the provisions for Application for grant of approval for testing cosmetics, Inspection before grant of approval, Procedure of approving authority, Validity of licence, General conditions after approval etc.
To counter the ambiguity Cosmetics Rules 2020 produced several FAQs like:
1. From which date Cosmetic Rules 2020 shall come into force?
Ans. Cosmetic Rules 2020 have been modified vide notification No. G.S.R. 763 (E) Dated 15-12-2020 and the same have come into force from the date of its notification i.e., 15-12-2020.
2. What is the status of existing cosmetic manufacturing license issued on form 32?
Ans. As per Rule 72 (Savings) the existing license shall be deemed to be valid for all purposes, still its expiry or for the period of 18 months from the date of these rules are notified, whichever is later.
3. What is the revised license fee under Cosmetic Rules 2020?
Ans. Fee is given in 3rd Schedule of the New Cosmetic Rules 2020.
– Fee for the grant of license is Rs. 10,000/- (Free: ten items of each category)
– Retention fee Rs. 10,000/-
– Item fee: Rs. 500/- per item
– Late Fee per month Rs. 2% of the license fee.
– Fee for additional category Rs. 10,000/-
– Fee for duplicate license Rs. 500/-
– Fee for the approval of testing laboratory Rs. 1,000/-
4. Is inspection required for the grant of cosmetic license?
Ans. As per Cosmetic Rules 2020, no inspection is required before granting the license, but State Licensing Authority (SLA) may verify the documents and fee submitted by the firm as per rule 23 of the Cosmetic Rules 2020.
5. On which form, cosmetic license is granted as per Cosmetic Rules 2020?
Ans. From Cos-8.
6. What are the new procedure for getting new cosmetic license as per Cosmetic Rules 2020?
Ans. Procedure given in Rule 23 as under: Firm has to apply new cosmetic manufacturing license through online portal on form Cos-5.
– The required fee is mentioned in 3rd schedule of Cosmetics Rules 2020.
– List of documents to be submitted as per 2nd schedule part-2 Cosmetics Rules 2020.
– SLA will issue the license on form Cos-8. If the application was not incomplete then SLA may reject the file and inform the applicant within 45 days.
– After obtaining license, firm has to upload the license on website of CDSCO within 30 days.
– SLA within 30 days of grant of license will inspect the premises and verify the information as given by the firm in self-certificate in form Cos-7.
7. For how much time manufacturing record is to be retained?
Ans. Manufacturing record to be retained for a period of 3 years from the date of expiry of the product.
8. For how much time the testing record is to be retained?
Ans. The test record is to be retained for a period of 3 years from the date of manufacturing.
9. Is there any change in qualification of Technical Staff in Cosmetics Rules 2020?
Ans. No. It will be same as previous.
10. Within how much time the firm has to obtain license due to change in constitution?
Ans. Within Six months
11. Within how much time the firm has to obtain license due to change in Name of the firm?
Ans. Within Two months.
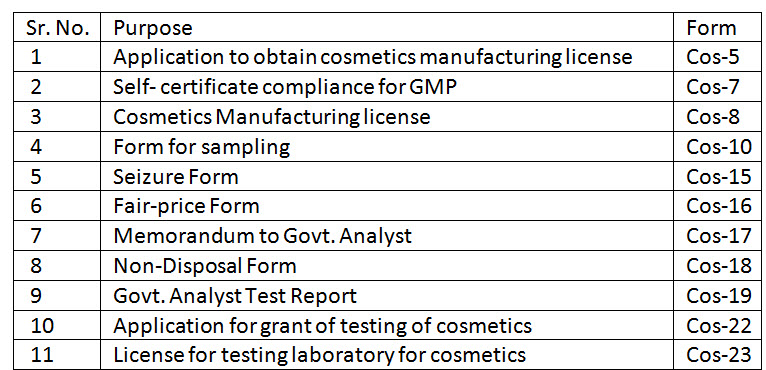
12. What are the Forms under Cosmetic Rules 2020?
Ans. List of such forms is as under:
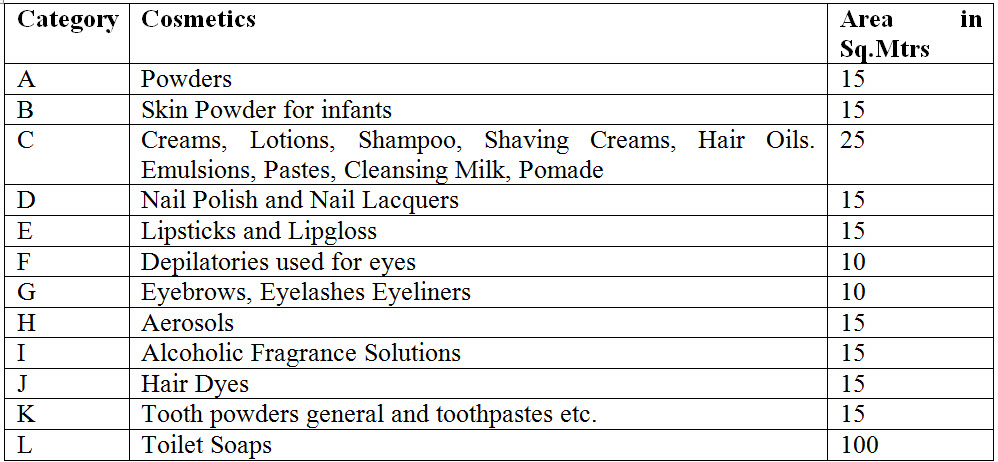
13. What are the different categories and area required to manufacture cosmetics under Cosmetic Rules 2020?
Ans. Details of categories and area required to manufacture cosmetics under Cosmetic Rules 2020 is as under.
14. Is definition of New cosmetic has been introduced in Cosmetic Rule 2020?
Ans. Yes, definition of New cosmetic has been given in Rule-3(f) of Cosmetics Rules 2020.
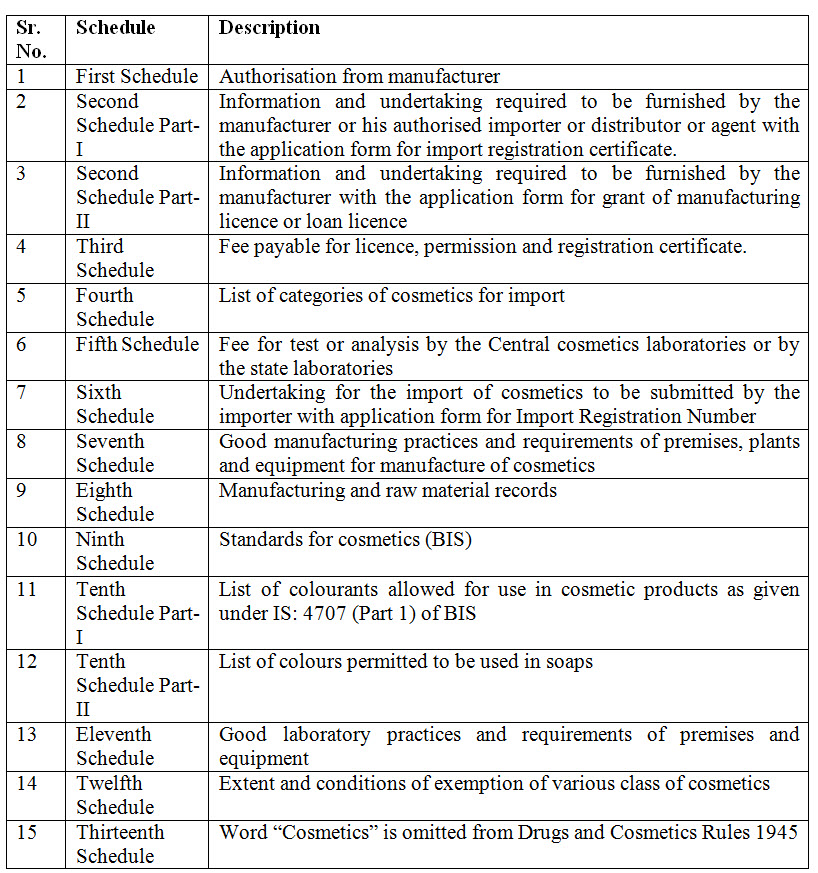
15. Which are new schedules introduced in Cosmetics Rules 2020?
Ans. Following schedules are introduced:
Quality and ingredients
Apart from the above-mentioned amendments, it has now become mandatory for manufacturers of cosmetics and make-up products in India to declare all ingredients, even those with concentration of less than 1%, to help buyers make more informed choices. Thus far, there was no Central law mandating them to declare the full composition of their skin and make-up products though there is such a rule for drugs and licences were granted without getting detailed information.
In order to ensure greater quality control of cosmetic products being manufactured, imported and used in India, the government has also decided to set up the first Central Cosmetics Laboratory and appoint inspectors across states.
Till now, there have been very few instances of crackdown on makers of spurious cosmetics. Under the new cosmetics rules, 2020 notified by the Union ministry of health and family welfare recently following consultations with the drug technical advisory board, hair dyes containing paraphenylenediamine or other dyes, colours and pigments should be labelled with specified warnings. Special provisions have been introduced on fluoride content in toothpaste as well.
It is more than 1000 ppm and the fluoride content shall be mentioned on the tube and carton. According to the new norms, no cosmetic, the manufacture, sale or distribution of which is prohibited in the country of origin, can be imported.
Also on the import ban list are cosmetics containing hexachlorophene a chemical preservative known for harmful effects on humans. Besides, cosmetics tested on animals cannot be imported. Also, inspectors will be required to inspect at least once in three years all premises licensed to manufacture cosmetics to ensure that the provisions of the Drugs and Cosmetic Act, 1940 and its rules are being fully met.
Import and Registration of Cosmetics:
• Most of the process of registration and import of cosmetics remains the same under the New Rules, albeit under new forms, and with significantly reduced statutory fees, which comes as a significant incentive for industry.
• The New Rules also streamline applications – the importer can make a single license application and seek a single registration certificate for the import of one or more cosmetics manufactured by the same manufacturer in a single manufacturing unit, which also encompasses multiple factories functioning conjointly.
• The New Rules provide that the import registration certificate must remain valid in perpetuity, subject to payment of retention fee before a period of five (5) years from the date of issue.
• The New Rules also prohibit import of cosmetics in the following cases:
i. The manufacture, sale or distribution of the cosmetic in question is prohibited in the country of origin;
ii. The “Use Before or Use by date” is less than six (6) months from the date of import;
iii. The cosmetic contains hexachlorophene; and
iv. The cosmetic has been tested on animals after November 12, 2014.
Manufacture of Cosmetics for Sale or Distribution
Similar to import and registration of cosmetics, the process for obtaining a manufacturing, sale and distribution license is largely similar to that under the DCR, albeit with new forms and with reduced statutory fees. The New Rules require that a self-declaration be furnished by the applicant confirming compliance with good manufacturing practices and other prescribed requirements. In case of manufacturing at more than one premises, separate licenses should be obtained for each premise. Similar to the import registration certificate, the manufacturing license/loan license must also remain valid in perpetuity, subject to payment of retention fee before a period of five (5) years from the date of issue.
Conclusion
With the increasing regulatory vigilance and concern towards the public health and social wellbeing it is now more become important to amend such rules and act which safeguard the health and welfare of the masses. These new cosmetics rules 2020 on one hand streamline the process of registration and import and on the other hand they guide a whole new horizon for manufacturing, sales or distribution. These rules will eventually prove to be effective in regulation of the cosmetics in the country and will serve the purpose of improvement in quality and safety of the cosmetics.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









