About Authors:
Arvind Negi1*, Balraj Singh Gill2
1Centre for Chemical and Pharmaceutical Sciences, Central University of Punjab, Bathinda- 151001
2Centre for Biosciences, Central University of Punjab, Bathinda-151001
*arvindnegi2301@gmail.com
Abstract
Stereochemistry of clinical agents play a key role in their success to become drugs. Tautomerism is a structural isomerism, playing a key role in the orientation of organic compounds and also found significant in distinctive base pairing in nucleic acids. Keto-enol form usually occurs and found prominently among different types of tautomers. The enol form ionizes in the physiological solution into enolate and alter the biological activity. So, how this enolate form brings modification in pharmacokinetics and pharmacodynamics of a drug is very important and quite interesting to know. As this form is ionic in nature so it increases the interaction with the concerning receptors, enzymes, ion channels or functional proteins. In this review we cover and compile success, role and the significance of the enolate form of clinical agents which succeed to become drugs!
REFERENCE ID: PHARMATUTOR-ART-2058
Introduction
Usually therapeutic molecule has some sort of structural peculiarity in terms of its stereo-geometric forms. Thestereo-geometric forms give rise to various geometric forms known as isomers. Isomers are mainly present in paired form and can be of different types: Cis-Trans, R-S, Syn-Anti, E-Z or tautomers[1]. These tautomers are the constitutional isomers that can be easily interconvert into their forms through a concerning phenomena, called tautomerization and this sort of isomerism is known as tautomerism[2].
Tautomerism is a kind of isomerization resulted from the intramolecular migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond. However, this isomerism is a special case of structural isomerism and also found significant in distinctive base pairing in nucleic acid[3].
In tautomerism, there is chemical equilibrium between the tautomers and the proportionality of tautomers depend on several factors preferentially solvent, temperature, and pH of the solution[4, 5]. Tautomerism can be of different types, elucidated in the following section.
Most common tautomeric pairs are:
Amide - imidic acid, e.g., Nitrile hydrolysis
Anomers of reducing sugars in solution interconvert through an intermediate open chain form.
Enamine - enamine, e.g., during pyridoxalphosphate-catalyzed enzymatic reactions and has biological significance in some diseases[6, 7].
Enamine - imine
Ketene - ynol, e.g., for ethenone, (triphenylphosphoranylidene) ethenone
Keto– enol, e.g., especially in ketones
Lactam - lactim, antimetabolites drugs (purine and pyrimidine analogs and penicillins)
Among tautomers, keto-enol form is most studied and common, which can be easily seen in a number of FDA approved drugs.The ionic form of enol is enolates possessing an alkene(-C=C-) with a hydroxyl group(-OH group) located on one of the carbon atom of the same alkene, see in fig.1.

Fig.1.Enolate formation: Keto form interconverted into enol form via proton migration, which is followed by the abstraction of a proton (deprotonation) to achieve the ionic form of enol, “enolate”.
This property of forming keto-enol form is exclusively attributed by the ketones, aldehyde and α-unsaturated alcohols. This type of tautomers often occurs in nature and even in the human body which is prominently manifested viaacid or base-assisted catalysis. Usually it has been observed that the 'keto' form of the compound is more stable, but in certain physiological conditions, the 'enol' form can also be the stable in nature.
Keto-Enol tautomerism:
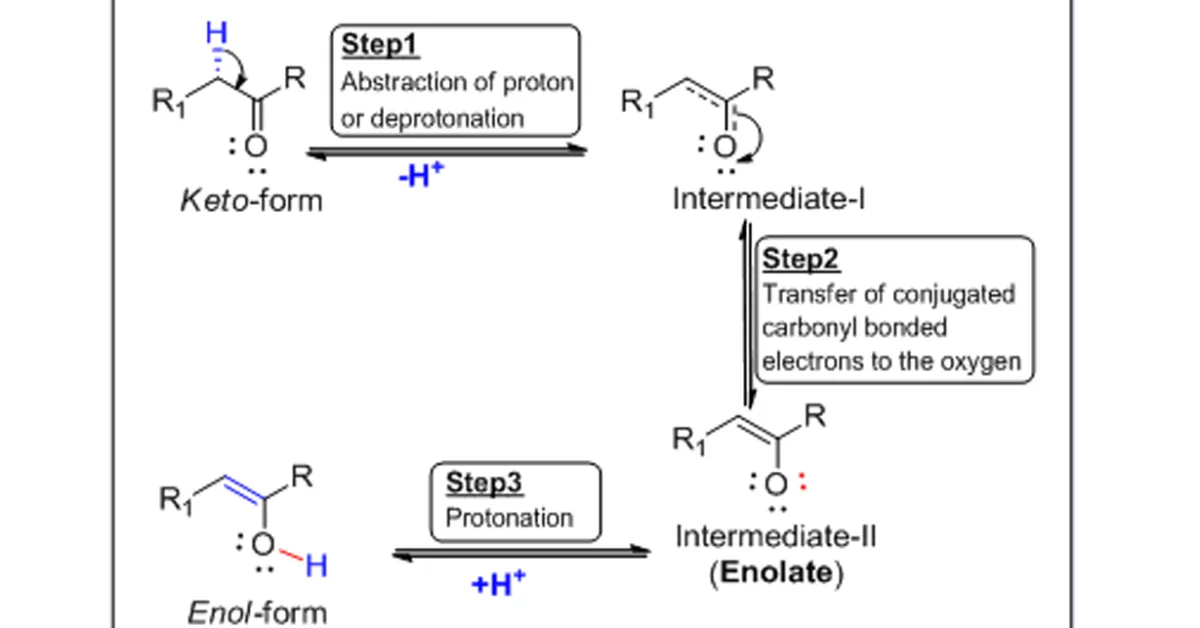
Keto-enoltautomerism refers to a chemical equilibrium betweenketo form (ketone or an aldehyde) and an enol (an alcohol with conjugated unsaturation). Simply it clearly indicates the interconversion relationship between “enol and keto forms are said to be tautomers [8]. The interconversion of the two forms involves the migration of a proton and the shifting of bonding electrons (see in fig.2.); hence, the isomerism qualifies as tautomerism and therefore mainly affected by the pH of the medium.
Mechanistically, a compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group(C=C-OH, linear representation of enol form). In general, the keto form predominates at equilibrium for most ketones. Furthermore, the deprotonated intermediate-II in the inter-conversion of the two forms, referred as an enolate anion[9], which is significant in carbonyl chemistry, see in fig. 2.

Fig.2.Mechanistic pathway of enolate formation.
keto-enoltautomerization governs thermodynamically but favored in keto form as compared to enol form. This can illustrated by classical example of vinyl alcohol and acetaldehyde [10].
What makes enolates so much interesting and how they are important in biological systems?
Enolates are the ionic product of enol form. In other words, it is deprotonated form having oxygen atom with un-bonded electron pair which finally makes it a strong nucleophile and having ability to bring necessary physiological reactions.
Enolate Implication in Medicinal chemistry
Enolate implication in Antiepileptic Drug
Antiepileptic drug act against convulsions, tremors,seizures and neuro-electrophysiological disorders where patient become motionless like a statue[11].
Hydratoin
Hydratoin is canonical class of antiepileptics which contain phenytoin, mephenytoin, ethotoin (See fig.2.).Out of these, phenytoinis effective against all types of partial and tonic-clonic seizures but failed against the absence seizures[12]. Its superior neuro-pharmacological role exerted antiseizure activity without causing general CNS depression. But intoxic doses, it aggravate the excitatory signs while lethal dosing causes a type of decerebrate rigidity[13].Its mechanism of action revealed a special mechanism which restrict the repetitive firing of action potentials, evoked by a sustained depolarization and mediated by a slowing rate of recovery of voltage-activated Na+ channels from inactivation[13].
Phenytoin is majorly (95%) metabolized by hepatic cytochrome enzymes. The principal metabolite is para-hydroxyphenol derivative which is inactive and its concentration cause saturation in further metabolism of the remaining drug. Whereas the intravenous use is restricted by its low aqueous solubility.Its water soluble prodrug, Fosphenytoin,achieve the higher status of success as it can rectify the limitation of intravenous use as it reaches in blood, it then rapidly converted into phenytoin by erythrocytes and phosphatases in liver.Fosphenytoinhave higher affinity for plasma proteins, primarily albumin and this binding is saturable in respect to displaced phenytoin from binding sites. Fosphenytoin is useful for adults with partial or generalized seizures when intravenous or intramuscular administration is indicated. Suggestively the water solubility or intravenous accessibility of these drugs can be attributed by ability of the hydratoin scaffold to form the enolate (see in fig.3.)[14-16].
Table.1.Generic names and different types of modification in the hydration scaffold.
|
Generic Name |
Substituent’s |
||
|
R1 |
R2 |
R3 |
|
|
Phenytoin,USP |
C6H5- |
C6H5- |
H |
|
Mephenytoin |
C6H5- |
C2H5- |
CH3- |
|
Ethotoin |
C6H5- |
H |
C2H5- |

Fig.3. Hydratoin and its enolate form.
Enolates Implication as Hypnotics and Sedatives
Phenobarbital
Phenobarbital is acidic in nature as compare to the water therefore it protonates water and itself gets ionize in enolate form[17]. This enolate form(see Fig.4.) increases aqueous solubility and affect its dissolution rate which lastly improvise its bioavailability[18].

Fig.4.Enolate form of Phenobarbital.
Enolate Implication as Drug metabolite
Amphetamines and Heterocyclic amines
Structural features especially the α-substitution in the primary amine metabolize by N-hydroxylation. But it is observed that amphetamines and heterocyclic amines also undergo N-hydroxylation to N-hydroxyamphetamine[17]in in-vitro screening[19](see in fig.5).

Fig.5. Metabolism of N-Benzylamphetamine.
While, phenmetrazine acts as a sympathomimetic and have relative activities like dopamine and norepinephrine. After its oral administration, 70% of the drug is excreted within 24 hours and approximately 19% of that is excreted as the unmetabolised form and the rest as various metabolites. This metabolism is preferentially governed by the enolate formation and there is possibility of the other metabolites to form the enolates, see in fig.6[20, 21].

Fig.6.Metabolism of Phenmetrazine
Secondary Aromatic amines
In some special cases, secondary aromatic amines metabolism produce toxicity whereN-hydroxy derivative forms and oxidize ferrous form of haemoglobin to its higher oxidative form(ferricform)[17]. This oxidized ferric state of haemoglobin(called methemoglobin or ferrihaemoglobin) can no longer possess the ability to transport the oxygen and lastly leading to serious complications like hypoxia or anemia (see fig.7. depicting such type of metabolism)[22, 23].

Fig.7.Metabolism of secondary amines.
Enolate Implication as Diuretic
Amiloride
The metabolism assisted conversion of amiloride into its water soluble enolate form which excrete predominantlyvia renal route(see in fig.8.)[24]This means that the enolate form encompass the solubility profile and also attribute to its diuretics action[25, 26].

Fig.8.Amiloride and its enolate form.
Enolates Implication in Congestive heart Failure
Amrinone and Milrinone
The most valuable use of enolate form can be seen in amrinone and milrinone, which are especially prescribed in short-term support of the circulation in advanced heart failure. Usually they are present in salt form in parental dosage, but when dissolved with water for injection, they rapidly get converted into their enolate form, which increases their solubility and absorption(see in fig.9 and 10). Structurally both the drugs are bi-pyridine derivatives and relatively selective inhibitor of phosphodiesterase-3 isoform. These drugs cause direct stimulation of myocardial contractility, acceleration of myocardial relaxation, balanced arterial and venous dilation with a consequent fall in systemic and pulmonary vascular resistances, left and right heart filling pressures. All these effects together increase the cardiac output, stimulates the myocardial contractility and the decrease in left ventricular after load.

Fig.9.Enolate form ofAmrinone.

Fig.10.Milrinone and its enolate form.
Enolate Implication as Anti Hyperlipidemics
Atorvastatin
Atorvastatin has a long half-life (T1/2) which gives it an edge over the other drugs of the same class.Moreover, it ismarketed in combination with theCa2+-channel blocker (amlodipine), for patients with hypertension or angina[27]andalso directed in case of hypercholesterolemia[28, 29]. The story behind its long T1/2, hides in its structure peculiarity where its amide bond get ionize in the physiological solution and improvise bioavailable fraction and therapeutic efficacy (see fig. 11).

Fig.11. Atorvastatin and its enolate form.
Enolates Implication as Anti arrthymic Drug
Disopyramide
Disopyramide has a specialty in its mode of action where it exertsits electrophysiological effects quitesimilar like quinidine, but also pose different adverse effects. It is directed in maintaining the sinus rhythm in patients with atrial flutter or atrial fibrillation or to prevent recurrenceof ventricular tachycardia or ventricular fibrillation[17, 30].These actions are mainly governed by its racemate form. Thein-vitro electrophysiological actions of its S-(+)-disopyramide form are similar to those of quinidine whereasR-(–)-enantiomer produces similar Na+ channel blocking effects but are shortly terminated[31, 32].
Unlike quinidine, racemic disopyramide exert prominent anticholinergic actions and therefore cause unpleasant side effects.Its rate of excretion depends on the concentration ratio between unchanged versus changed drug concentration, which is mainly manifested by the amide-enolate form (fig.12).

Fig.12. Disopyramide and its enolate form.
Enolates Implicationas Anti-Hypertensive Drug
Hydralazine
Hydralazine causes direct relaxation of arteriolar smooth muscle via altering the intracellular Ca2+ concentrations. Most of hydralazine’s pharmacological effects are confined to the cardiovascular physiology[33]. Low systemic bioavailability is the major shortcoming of this drug besides ofits well absorption from the gastrointestinal tract.
Firstly its benzylic oxidation brings a product which is quite susceptible for acetylation and also possess the ability of tautomerism and to form enolate isomer. Thereafter, HydralazinegetsN-acetylated in the bowel and/or the hepatic portal system[34]. This acetylatedcompound is inactive in nature. However, hydralazine rapidly combines with circulating α-keto acids to form hydrazones, andthe major metabolite recovered from the plasma is hydralazine pyruvic acid hydrazine [35]. Thismetabolite has a longer T1/2 than hydralazine but does not appear to be very active. The rate of acetylationis an important determinant of hydralazine bioavailability. Althoughits T1/2 in plasma is ~1 hour, but the duration of the hypotensive effect of hydralazine can long last as12 hours. There is no clear explanation for this discrepancy, suggestively it can be concurred by its ability to form the tautomeric enolate form (for depicting its metabolism pathway, see in fig.13)[36].

Fig.13.Metabolism of Hydralazine hydrochloride.
Enolates Implication as Anti-Hyperglycemic Drug
Repaglinide
Diabetes categorizes as third disease which cause fatality in the world-wide. But present circumstances are so critical that it causepatient more prone towards the cancer[37]. Moreover there are many drugs for the treatment of diabetes. Repaglinide is one of them which is an oral insulin secretagogue of the meglitinide class[38]. Like sulfonylureas, it stimulates insulin release by closing ATP-dependent K+-channelsin pancreatic cells. It is absorbed rapidly from gastrointestinal tract and attainspeak blood levels within 1 hour after oral administration. These features permit multiple preprandial uses as compared with the classical once or twice-daily dosing of sulfonylureas[17]. It is metabolizedmainlyin liver to inactive derivatives and should be used cautiously in patients with hepaticand renal insufficiency. The major shortcoming of this drug is hypoglycemia[39]. Its pharmacokinetic parameters are altered by its enolate form which can be easily achieve higher level in physiological solution and affect its metabolism and excretion (see in fig.14).

Fig.14. Repaglinide and it tautomeric form.
Enolates Implication as Antithyroid Drugs
Thiouracil
Initially, thiouracil discovered great successas anti-thyroid agent but its severe metabolic side effects and less selectivity, creates a dawn in the path of its success [17]. Moreover some reports highlightits structural feature which makes it more prone towards the isomerizationandunwanted adverse effects. Thiouracil presentin enol form shows tautomerism viaketo-form, see in fig.15.

Fig.15. Different forms of Thiouracil.
Propylthiouracil
propylthiouracilis another antithyroid drug havingshorter T1/2 and prescribed in severe hyperthyroid states, even a 500-mg dose of propylthiouracil must be dosed every 6-8 hours to yield complete thyroid inhibition[17]. The drug is concentrated in the thyroid and its metabolites mainly excreted in the urine. Propylthiouracil has ability to cross the placenta[40]and mammary epithelial cells and can often found in milk[41]. This awkward presence in these places disclosed the possible role of enolate formwhich increases its hydrophilicity and assist in cellular transportation to reach to placenta and mammary ducts (in fig.16).

Fig.16. Propylthiouracil and its enolate form.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Conclusion
The implication of enolates in the medicinal chemistry brings new interface in rational drug discovery and drug designing. This form is an intermediaryand short lived, so need of proper assessment is required to track themode of mechanismof enolate forming drugs.Therefore the possibility of enolate formation during the drug action helpsto the researcher in understandingthe novel mechanism inside the physiology of the body. However, proper studies help in teasingthe pharmacokinetics of drugs in order to improve their ADMET profile.
Acknowledgment
I would like to give my special thanks to Dr. Raj Kumar for his support. Along with, I would like to thank Navgeet and all the other persons who supported me during the preparation of this manuscript.
References
1.Mislow K, Siegel J: Stereoisomerism and local chirality. Journal of the American Chemical Society1984, 106 (11): 3319-3328.
2.Elguero J, Marzin C, Katritzky AR, Linda P: The tautomerism of heterocycles. Academic Press New York: 1976.
3.Shukla MK, Leszczynski J: Tautomerism in nucleic acid bases and base pairs: a brief overview. Wiley Interdisciplinary Reviews: Computational Molecular Science2013.
4.Culbertson J: Factors affecting the rate of hydrolysis of ketimines. Journal of the American Chemical Society1951, 73 (10): 4818-4823.
5.Ball P, Nicholls C: Azo-hydrazone tautomerism of hydroxyazo compounds—a review. Dyes and Pigments1982, 3 (1): 5-26.
6.Negi A, Bhushan S, Gupta P, Garg P, Kumar R: Cystathionine-Lyase-Like Protein with Pyridoxal Binding Domain Characterized in Leishmania major by Comparative Sequence Analysis and Homology Modelling. 2013.
7.Metzler DE: Tautomerism in pyridoxal phosphate and in enzymatic catalysis. Advances in Enzymology and Related Areas of Molecular Biology1979, 50: 1-40.
8.Allen G, Dwek RA: An NMR study of keto-enol tautomerism in β-diketones. Journal of Chemical Society B1966: 161-163.
9.Iglesias E: Tautomerization of 2-acetylcyclohexanone. 1. Characterization of keto-enol/enolate equilibria and reaction rates in water. Journal of Organic Chemistry2003, 68 (7): 2680-2688.
10.Cederstav AK, Novak BM: Investigations into the chemistry of thermodynamically unstable species. The direct polymerization of vinyl alcohol, the enolic tautomer of acetaldehyde. Journal of the American Chemical Society1994, 116 (9): 4073-4074.
11.Rogawski MA, Löscher W: The neurobiology of antiepileptic drugs. Nature Reviews Neuroscience2004, 5 (7): 553-564.
12.Stewart IC, Bergman RG, Toste FD: Phosphine-catalyzed hydration and hydroalkoxylation of activated olefins: use of a strong nucleophile to generate a strong base. Journal of the American Chemical Society2003, 125 (29): 8696-8697.
13.Hardman JG, Limbird LE: Goodman & Gilman's the pharmacological basis of therapeutics. McGraw-Hill New York: 1996.
14.Bauer LA, Blouin RA: Age and phenytoin kinetics in adult epileptics. Clinical Pharmacology & Therapeutics1982, 31 (3): 301-304.
15.Leff R, Fischer L, Roberts R: Phenytoin metabolism in infants following intravenous and oral administration. Developmental pharmacology and therapeutics1986, 9 (4): 217.
16.Richens A, Dunlop A: Serum-phenytoin levels in management of epilepsy. The Lancet1975, 306 (7928): 247-248.
17.Block JH, Beale JM: Wilson and Gisvold's textbook of organic medicinal and pharmaceutical chemistry. Lippincott Williams & Wilkins: 2004.
18.Tabern D, Shelberg E: Physico-chemical properties and hypnotic action of substituted barbituric acids. Journal of the American Chemical Society1933, 55 (1): 328-332.
19.Vree T, Muskens A, Rossum Jv: Some physico?chemical properties of amphetamine and related drugs. Journal of Pharmacy and Pharmacology1969, 21 (11): 774-775.
20.Martin W, Sloan J, Sapira J, Jasinski D: Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clinical pharmacology and therapeutics1970, 12 (2): 245-258.
21.Perrone R, Carbonara G, Tortorella V: Chemical Studies on Drug Metabolism: Oxidation with Ruthenium Tetroxide of Some Medicinal Alicyclic N?Methylamines. Archiv der Pharmazie1984, 317 (1): 21-27.
22.Nohl H, Stolze K: The effects of xenobiotics on erythrocytes. General Pharmacology: The Vascular System1998, 31 (3): 343-347.
23.GEORGE P, Lyster R In A Survey of the Evidence for and against a Crevice Configuration for the Heme in Hemoglobin, Conference on Hemoglobin. Nat. Acad. Sei., National Research Council, Washington, DC, Publication, 1958; p 33.
24.L'allemain G, Franchi A, Cragoe E, Pouyssegur J: Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. Journal of Biological Chemistry1984, 259 (7): 4313.
25.Kleyman TR, Cragoe EJ: Amiloride and its analogs as tools in the study of ion transport. Journal of Membrane Biology1988, 105 (1): 1-21.
26.Russ T, Ried W, Ullrich F, Mutschler E: Preparation and diuretic properties of novel amiloride analogues. Archiv der Pharmazie1992, 325 (12): 761-767.
27.McKeage K, Siddiqui MAA: Amlodipine/Atorvastatin Fixed-Dose Combination. American journal of cardiovascular drugs2008, 8 (1): 51-67.
28.Aragoncillo P, Maeso R, Vázquez-Pérez S, Navarro-Cid J, Ruilope LM, Díaz C, Hernández G, Lahera V, Cachofeiro V: The protective role of atorvastatin on function, structure and ultrastructure in the aorta of dyslipidemic rabbits. Virchows Archiv2000, 437 (5): 545-554.
29.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T: Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA: the journal of the American Medical Association2001, 285 (13): 1711-1718.
30.Heel R, Brogden R, Speight T, Avery G: Disopyramide: a review of its pharmacological properties and therapeutic use in treating cardiac arrhythmias. Drugs1978, 15 (5): 331-368.
31.Koch-Weser J: Disopyramide. New England Journal of Medicine1979, 300 (17): 957-962.
32.Yeh BK, Sung PK, Scherlag BJ: Effects of disopyramide on electrophysiological and mechanical properties of the heart. Journal of pharmaceutical sciences1973, 62 (12): 1924-1929.
33.Gershwin M, Smith NT: Mode of action of hydralazine on guinea pig atria. Archives internationales de pharmacodynamie et de thérapie1967, 170 (1): 108.
34.Facchini V, Timbrell JA: Further evidence for an acetylator phenotype difference in the metabolism of hydralazine in man. British journal of clinical pharmacology1981, 11 (4): 345-351.
35.Knox WE, Pitt BM: Enzymic catalysis of the keto-enol tautomerization of phenylpyruvic acids. Journal of Biological Chemistry1957, 225 (2): 675-688.
36.Reece PA: Hydralazine and related compounds: chemistry, metabolism, and mode of action. Medicinal research reviews1981, 1 (1): 73-96.
37.Negi A, Ramarao P, Kumar R: Recent Advancements in Small Molecule Inhibitors of Insulinlike Growth Factor-1 Receptor (IGF-1R) Tyrosine Kinase as Anticancer agents. Mini reviews in medicinal chemistry2013, 13 (5): 653-681.
38.Guardado-Mendoza R, Prioletta A, Jiménez-Ceja LM, Sosale A, Folli F: The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. 2013.
39.Fuhlendorff J, Rorsman P, Kofod H, Brand CL, Rolin B, MacKay P, Shymko R, Carr RD: Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes1998, 47 (3): 345.
40.Mortimer R, Cannell G, Addison R, Johnson L, Roberts M, Bernus I: Methimazole and propylthiouracil equally cross the perfused human term placental lobule. Journal of Clinical Endocrinology & Metabolism1997, 82 (9): 3099-3102.
41.Atkinson H, Begg E, Darlow B: Drugs in human milk. Clinical pharmacokinetics1988, 14 (4): 217-240.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 1, Issue 2 Received On: 13/11/2013; Accepted On: 23/11/2013; Published On: 20/12/2013 How to cite this article: Negi A, Gill BS, Success Stories of Enolate Form of Drugs, PharmaTutor, 2013, 1(2), 45-53 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE