About Author:
M.Anand
M.Pharmacy
SRF, Departmnet of Pharmacology Division,
NRI-Panchakarma, Cheruthuruthy Post, Thrissur, Kerala
anandm723@gmail.com
Introduction:
Multiple sclerosis(MS), also known as disseminated sclerosis or encephalomyelitis disseminata, is an inflammatory disease in which the insulating covers of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to communicate, resulting in a wide range of signs and symptoms, including physical, mental and sometimes psychiatric problems. Multiple sclerosis is an autoimmune demyelinating disease of the central nervous system characterized by clinical attacks (relapses) correlated with lesions separated in time and space. MS is a severe inflammatory disease in which the myelin (insulator for electrical signalling in NS) breakdown and causes severe deficits in brain and nerve function. Much like rubber insulation on an electrical cord, myelin surrounds long projections from the body of neurron and allows signal to travel down the cell with sreed and efficacy Therefore patients suffers deficits in balance, coordination, moments as well sensory disturbances from the loss of this neuronal insulation. MS is one of the most common neurological causes of long term disability, the myelin producing oligodendrocytes of the CNS are the target of recurrent cell mediated autoimmune attack. MS is a episodic & progressive disease occurs in early to 35 years & then declining. MS occurs with male:female ratio of 2:1 The incidence is higher in temperature climate and in northen europeans.
Reference ID: PHARMATUTOR-ART-1959
Etiology:
Caused by an interaction between multiple genes influencing the immune system & environmental factors, including certain viruses, vitamin D, sun exposure prior to adulthood.
Types:
|
Relapse remitting MS (RRMS) |
Secondary progressive MS (SPMS) |
Primary progressive MS (PPMS) |
|
It occurs with 58% of patients. Relapse followed by complete or near complete recovery, 90% of it converted to SPMS after 25 yrs of age. |
It occurs with 27% of patients. rogression of disability with few or no relapse. |
It occurs with 15% of patients. Progression from onset. |
Signs and symptoms:
Weakness, paralysis , Tremour, muscle spasticity,Moment dysfunction, Numbness, tingling, facial pain, Loss of vision, double vision, eye discomfort, Decreased co ordination, Loss of balance, muscle spasm, Fatigue, vertigo,dizziness.
Pathology:
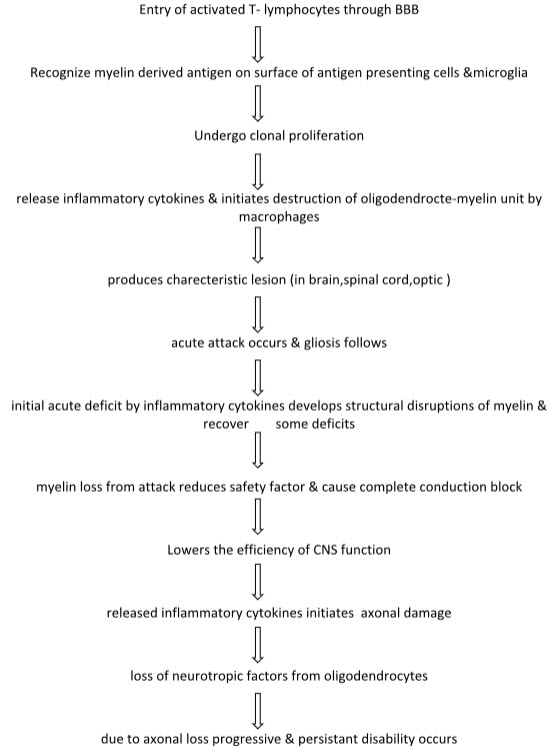
Biomarkers:
- It’s a biological molecule used as a marker for a substance or process of interest.
- Should be reliable, reproducible, noninvasive, sensitive & disease specific.
- Previous research found that peptidylarginine deiminase 2 (PAD2).
- Biomarkers reflecting altrations of immune system
- Biomarkers of axonal/neuronal damage
- Biomarkers of BBB distruption
- Biomarkers of demyelination
- Biomarkers of oxidative stress&excitotoxicity
- Biomarkers of glyosis
- Biomarkers of remyelination & repair
Biomarkers reflecting alterations of immune system:
Cytokines & their receptors
Chemokines & their receptors
Adhesion molecules ( ICAM-1, VCAM-1,CD-31)
Biomarkers reflective of antigen processing & presentation
Cell cycle & apoptosis related biomarkers
Antibodies
- anti-myelin oligodendrocyte glycoprotein (MOG)
- Antibodies myelin lipids & glycopeptides
- Antibodies to axonal antigen
- As a biomarker for remyelination & repair
Biomarkers of axonal & neuronal damage:
Axon cytoskeleton markers in serum CSF
i. Neurofilaments
ii. Actin & tubulin
iii. Tau proteins
markers of membrane hamoeostasis
i. 24 S hydroxy cholesterol
ii. Apoprotein E
iii. Amyloid precursor protein
Biomarkers of BBB disruptions
- matrix metalloproteinases (MMP)
Biomarkers of demyelination
- myelin basic protein,proteolytic enzymes,
- endogeneous pentapeptide, gliotoxin
Biomarkers of oxidative stress& excitotoxicity
- nitric acid derivatives
- isoprostanes
Biomarkers of demyelination & repair
- NCAM (neural cell adhesion molecule)
- CNTF (ciliary neurotropic factor)
- CPK – BB (creatin phosphatase)
- PAM (peptidyl glycin alpha amidating mono oxygenase)
TREATMENT:
For acute relapses:
High doses of methylprednisolone by i.v. 1g daily for 3 days or 500 mg orally daily for 5 days to shorten the duration of relapse.
Disease modifying therapy:- Interferon βa/b by s.c. or i.m. which reduces no. of relapse by 30% with amall effect on disability.
Glatiramater acetate has similar effect like interferon
Immunosuppressive agents:-
Azathioprine has effect on reducing relapse and improve longterm outcome.
Monoclonal antibody:
natalizump binds to a4 subunits of leuckocytes & reduce relapse.
Cytotoxic agents:
cyclophosphamide or mitoxantrone used for frequent relapses with rapid disability progression & in SPMS.
Spasticity: baclofen, tizanidine, diazepam,lorazepam.
Pain management: carbamazepine, gabapentin, pregabalin
Fatigue: try amantadidine, modafinil or fluoxetine.
Tremor: clonazepam, carbamazepine, propronalol.
Evaluation of multiple sclerosis:
Myelin oligodendrocyte glycoprotein induced experimental encephalomyelitis in dark agouti rats
Animal- dark agouti rats
Inducers for EAE –myelin oligodendrocyte glycoprotein(100mcg), myelin basic protein (MBP)
Inducing methods – immunization with neuro antigen or passive transfer of neuro antigen specific T - cells
Purification & expression of recombinant MOG
* Expression of recombinant mouse MOG:-
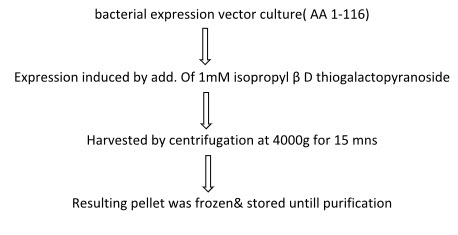
* Purification of MOG:
Technique : talon purification system
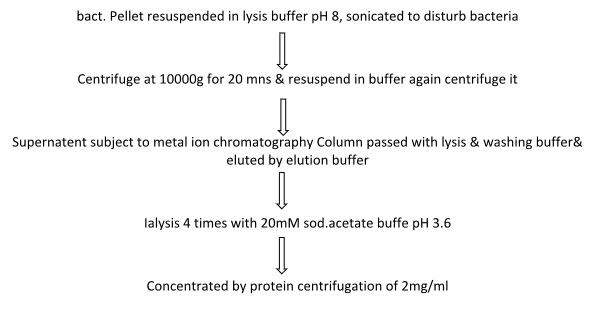
Concentrated by protein centrifugation of 2mg/ml
Induction of EAE in DA rats:
Female DA rats of 10-12 week old housed in pathogen free condition.
ANTIGEN PREPARATION & IMMUNIZATION:
- 1:1 emulsion of 2mg/ml recombinant MOG & antigen mixture (adjuvant) was prepared.
- Adjuvant drawn upto glass syringe & attached to a 3 way stopcock.
- At another end empty syringe attached, mixture was forced back from one to another repeatedly.
- After it gets homogenised the connector was removed and air bubbles are removed.
- DA rats were anaesthesised with isoflourane then base of the tail was shaved and disinfected.
- Immunization was performed with application of 100µl/ml of emulsion by s.c. route at the base of the tail.
- Everyday the rats were weighed and the clinical score was monitored.
- 14 days after immunization each rat was graded and assigned a score from 0-5 as shown in table.
- Rats are sacrificed after 60 days, brain, spinal cord removed for further analysis.
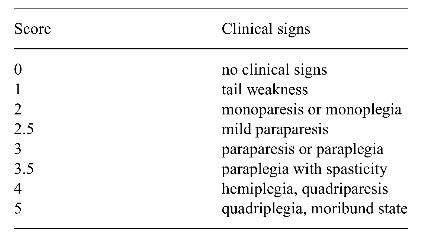
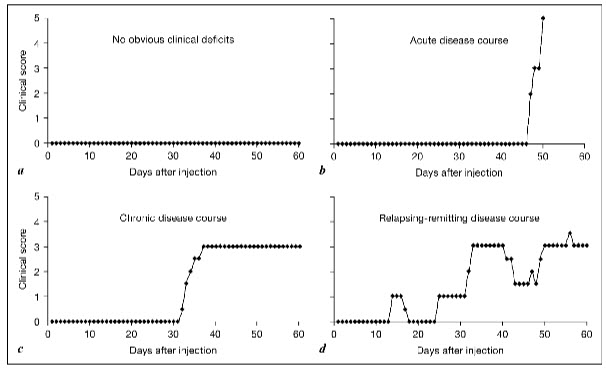
Clinical stages of the disease:
Acute phase: period of 1 st clinical signs, in which rat show ascending paralysis & active disease induction follow
Remission: reduction of score by minimum of 1 grade for atleast 2 days after the peak of acute phase.
Relapse: the phase of increasing neurological deficit seen after remission, relapse is an increase of atleast 1 grade in the score maintained for minimum of 2 days.
* Animals are divided into 4 groupsbased on clinical score.
1 st : without obvious neurological deficits.
2 nd : acute or progressive disease course showing no remission & not establishing stable chronic phase.
3 rd : shows stable chronic state after acute attack
4 th : with RR disease course by atleast 1 relapse of 1 score minimum for 2 days.
Characterization of lesions:
Done by immunohistological analysis with several antibodies to identify inflammation, demyelination, astrogliosis or neuronal degeneration. This produces large inflammatory demyelinating lesions with CNS. Highest lesion found in spinal cord, optic nerve, brainstem& cerebellum and some in forebrain. Additionally ataxia observed due to lesions in brainstem. Anatomical disruptions of lesions within CNS influenced by antigen specificity of T – cells.
Demyelination
The demyelization with spinal cord & brain was visualized by using antibody recognizing the major myelin protein PLP. MOG induced EAE not only induces encephalitigenic T-cell response but also autoantibody response initiates demyelination & enhance disease severity in animals actively immunized with MOG. Passive transfer of activated specific T – cells only leads to inflammation & administration of antibodies is necessary to obtain demyelization. Besides demyelization signs of remyelination also observed after 10 days from onset of disease by myelinated internodes.
Axonal pathology:
Axonal injury & loss is thought to be responsible for the progressive exacerbation of disease. Specific molecular abnormalities such redistribution of ion channels on demyelinated axons were identified, which play major role in axonal pathology. The degree of axonal loss correlates with clinical severity in progressive as well as RR forms of MOG induced EAE. Demyelination & inflammation don’t show any significant correlation with clinical severity score of animals having RR disease course.
COCLUSION:
Primary cause of the disease is unknown and the EAE models are autoimmune mediated diseases. The MOG induced EAE models in DA rats closely limits some clinical features of MS .A spectrum of disease causes can observed in MOG induced EAE in DA rats ranging from animal showing no clinical sign to fatal. The presence of demyelinating lesions and remyelinating activity makes the model viable for the investigation of mechanism of remyelination . In MS patients the axonal loss has been observed which correlates with disease progression. Axonal degeneration and loss present in CNS of DA rats immunized with recombinant MOG and this axonal pathology in inflammatory demyelinating lesions closely reflux that observed in MS.
References:
1. Compston A, Coles A (April 2002). "Multiple sclerosis". Lancet 359 (9313): 1221–31.
2. Compston A, Coles A (October 2008). "Multiple sclerosis". Lancet 372 (9648): 1502–17.
3. Davidson’s Principles of medicine.
4. Ferri’s clinical advisor.
5. Journal of neurobiology by Jochen Kinter.
6. Murray ED, Buttner EA, Price BH (2012). "Depression and Psychosis in Neurological Practice". In Daroff R, Fenichel G, Jankovic J, Mazziotta J. Bradley's neurology in clinical practice. (6th ed. ed.). Philadelphia, PA: Elsevier/Saunders. ISBN 1-4377-0434-4.
7. New animal model of human neurological diseases by Basal , Kanger 2008 Vol:2.
8. The immunology of inflammatory demyelinating diseases by Wekerle H, Lassman H, 4th edition , 2005.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









