 About Authors:
About Authors:
Sharda roy*, vikas chandra roy, dr. R. C. Roy
Address Faculty of Pharmaceutical Sciences. Global College of Pharmacy.
Kahnpur Khui. Punjab.
*roysharda08@gmail.com
Abstract:
Mitragyna parvifolia is a medium to large size tree belonging to the family Rubiaceae. It is popularly known as Kaim and is found growing gregariously throughout the drier parts of India, Pakistan and Srilanka. The aerial parts, stem-bark and roots of the tree contain various indolic (tetrahydroalstonine, akkuamigine, hirsuteine etc) and oxindolic (mitraphylline, isomitraphylline, pteropodine, isopteropodine etc) alkaloids. Important variations in the nature and quantity of alkaloids present in different parts of the plant are visible with change in geographical locations. In addition, various parts of the tree such as fruit juice, leaves and stem bark have been used in the traditional system of medicine by the natives and tribal since times immemorial. Mitragyna parvifolia has also got diverse medicinal and therapeutic properties such as analgesic, antipyretic, anti-inflammatory, antiarthritic, anthelmentic, antioxidant etc which have been validated scientifically. In this study we have critically reviewed the pharmacognostic and chemical profile, the traditional and the scientifically validated bioactivities of the plant.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1594
INTRODUCTION
Mitragyna parvifolia(Roxb) Korth [M. parvifolia] commonly known as Kadamb, belongs to family Rubiaceae1. The genus was given the nameMitragynaby Korthals because the shape of the stigmas in the species he examined resembled a bishop's mitre. The genus Mitragyna is a short genus comprising of 10 species. The plant is native to Indian origin but is found growing gregariously in swampy territories in the tropical and sub-tropical regions of Africa and Asia. The plant is known in different parts of the country by various common names:Kaim (English); Nir-kadambai (Tamil); Vimba, Sirakadambu, Kadamba, Neerkadambu, Poochakadambu, Rose kadambu, Veembu (Malayalam); Kongu, Nayekadambe (Kannada) 2.
PHARMACOGNOSTIC PROFILE
The Kaim is a medium to large deciduous tree with rounded crown mainly arboreal in character while some species grow to a height of about 30 meters. It is found throughout the greater parts of India up to an altitude of 1200 metres. Tree is found scattered in deciduous forests and develops best in well drained deep soil. It is found growing gregariously in low lying areas around banks and swamps.
Mitragyna species are characterized by the globular flowering head each containing up to 120 florets. The inflorescence is a dichasial cyme and the fruit is a capsule containing numerous small flat seeds. The young woody shoots bear 10-12 leaves arranged in opposite and decussate pairs. The bole is often short, flutted and buttressed. The bark is grey, smooth and exfoliating. The wood is pale yellow when first exposed but turns light grayish brown on ageing. Natural reproduction takes place by the scattering of seeds in hot season. Germination takes place in the rainy season 2.
[adsense:468x15:2204050025]
PHYTOCHEMISTRY
Chemical constituents
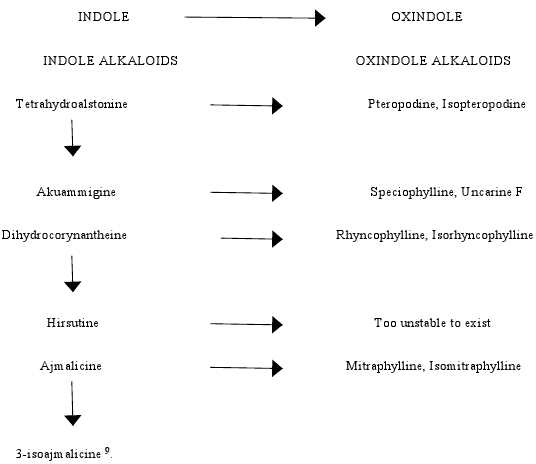
M. parvifoliais an important tree blessed with large number of important phytoconstituents especially alkaloids. The alkaloids of Mitragyna parvifolia are of both indolic and oxindolic type 3 which may be divided into two groups:
1.The Closed E ring alkaloids
2.The Open E ring alkaloids ( E seco) (fig- 1, fig-2)
Oxindole alkaloids are an important group of alkaloids which contain the oxindole moiety often associated with significant biological activity. E seco oxindole alkaloids act on the central nervous system whereas Closed E ring oxindole alkaloids affect cellular immune system 4. The spiro (indole-pyrrolidine) ring system is a frequently encountered structural portion in these alkaloids 5. The Indole alkaloids of Mitragyna parvifolia are also of considerable importance as they are the precursors or the starting materials for the biogenesis of corresponding oxindole alkaloids (table 1).
Stereochemistry
All the alkaloids have asymmetric centres at C (3), C (15) and C (20) though all those isolated so far have C (15)-Hα. The closed E ring alkaloids also have an asymmetric centre at C (19) which, in all the known Mitragyna alkaloids is C (19)- Hβ. The E seco alkaloids may show geometric isomerisation because of the double bond between C (16) and C (17) though all the known alkaloids possess a C (17) –H cis to the ester group at C (16). In addition, the oxindole alkaloids have an asymmetric centre at C (17). Alkaloids in which the lactam carbonyl lies below the plane of the C ring being termed the A series and those in which the lactam carbonyl lies above the C ring being termed the B series (fig-3).
Further in both types of oxindole alkaloids the lone pair of electrons in N (4) may either be on the same side of the C (7) as the lactam carbonyl or on the opposite side; the former are known as syn and the latter as anti alkaloids. The oxindole alkaloids readily isomerise about C (7) and C (3) to give a mixture of isomers. Substituents may occur in the aromatic ring but always at C (9)- R1 being either a hydroxy or a methoxy group. In the E seco alkaloids RII may be either CH2 CH3 or CH=CH2 6.
Important facts revealed by Configurational Studies
1. When indoles and oxindoles are present in the same plant the D/E ring system are identical in both types.
2. The Closed E ring alkaloids have C (19)-Hβ.
3. The indoles are present in major quantities as their least stable configuration i.e pseudo and epiallo7.
BIOGENESIS OF ALKALOIDS
Large quantities of indole alkaloids occur in root bark than in the leaves 8, suggesting that biogenesis does not take place in leaves but in roots and only certain alkaloids are transferred to the aerial parts and the amounts transferred varies from time to time. The biogenesis of indole alkaloids is through the geraniol-iridodial pathway. The plant synthesizes the thermodynamically more stable indole alkaloids and these alkaloids:
1. Isomerize to give thermodynamically less stable isomers or
2. Both indole isomers are converted to the corresponding oxindoles (table 2).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
GEOGRAPHICAL VARIATIONS AND ALKALOIDS
It has been observed that environmental factors play a vital role in modifying the content and structure of the alkaloids. There occurs a wide variation in the pattern of alkaloids present in leaves of Mitragyna parvifolia obtained from different geographical sources in South East Asia 9.
Three distinct alkaloidal sequences are found to exist:
1. C (9)- H allo-epiallo series of closed E ring indole and oxindole alkaloids
2. C (9)-H normal-pseudo series of closed E ring indole and oxindole alkaloids
3. C (9)-H normal-pseudo series of open E ring indole and oxindole alkaloids
More than one sequence of alkaloids could be found in some plants and there are indications that M. parvifolia exists in different geographical variants and possibly chemical races 8.
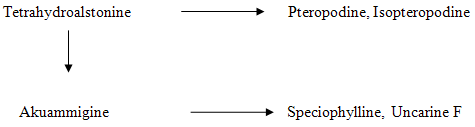
The examination of leaves of M. parvifolia from Kekirawe, Sri Lanka, has revealed the presence of four alkaloidal N- oxides, viz Akuammigine N-oxide, Speciophylline N-oxide, Uncarine N-oxide and Dihydrocorynantheol N-oxide. The tertiary alkaloids present are Akuammigine, Tetrahydroalstonine, pteropodine, isopteropodine, speciophylline, uncarine F, dihydrocorynantheol and corynantheidol 10. The plants growing in Burma reported the presence of rhyncophylline, isorhyncophylline, hirusteine, dihydrocoryntheine and angustine 11. Also a yellow alkaloid designated Gu 5, not of Indole or Oxindole type has been isolated from Burmese leaves of M. parvifolia6. The alkaloids rhyncophylline, isorhyncophylline, pteropodine, isopteropodine and akuammigine were found to be present in the Ceylonese variety ofM. parvifolia. Also two new alkaloids not previously reported from Mitragyna species were isolated and characterized. These were found to be hirsutine (an open E ring indole alkaloid) and corynoxeine (an open E ring oxindole alkaloid) 12. The Cambodian variety was found to contain rhyncophylline and isorhyncophylline13.
The alkaloidal profile was even found to be variable in trees of Mitragyna parvifolia growing in different regions of India.
The young leaves obtained from the plants growing in the Uttar Pradesh (Lucknow) region were found to contain two different alkaloidal sequences (Fig 4, Fig 5). Thus the trees in this region have reported the presence of six oxindolic alkaloids namely Mitraphylline, isomitraphylline, pteropodine, isopteropodine, speciophylline and uncarine F3.
Trees from Kerela region reported the presence of C (9)-H normal pseudo series of open E ring alkaloids although no corresponding indole alkaloids were found. This region bears trees rich in alkaloids namely cilliaphylline, Rotundifoline, Isorotundifoline, rhyncocilline, rhyncophylline and isorhyncophylline8. Presence of Pteropodine and isopteropodine is a common feature of all the Indian leaves but are found to be missing in M. parvifolia trees obtained from Kerela state7.
Leaves of the plants obtained from Bihar and West Bengal do not contain the same alkaloids as those obtained from Kerala 13. The alkaloids reported from trees in this region were found to be both indole and oxindole derivatives, which were all closed E ring alkaloids. The oxindole alkaloids (mitraphylline, isomitraphylline, pteropodine, isopteropodine, speciophylline, uncarine F) are all isomeric, although the remaining isomers of the group (uncarines A and B) do not seem to be present.
The leaves of the plant in Maharashtra region (Fig-6) had an entirely different set of alkaloids from those of the same species collected in Kerala14.
Seasonal variations also affect the alkaloidal content of Mitragyna trees. In the genus Mitragyna the occurrence of angustine appears to be restricted. The alkaloid angustine (belonging to the category of pyridino-indolo-quinolizidinone alkaloid), was found to be maximum during the first half of the year whereas the second half of the year reported the same alkaloid in trace amounts15.
Some Interesting facts regarding alkaloids of Mitragyna parvifolia
1. The pattern of alkaloids in M. parvifolia leaves growing in India and South East Asia shows all alkaloids obtained from Indian sources (with the exception of plant material from Kerela state) to be Closed E ring alkaloids, whereas those from Burma and Cambodia are E seco type.
2. A second interesting point is that with the exception of alkaloids obtained from trees growing in Kerela, none of the alkaloids from Indian sources are substituted in the aromatic ring. Substituted alkaloids are found in the plant material from Burma and Cambodia. The link between the two groups appears to be in trees growing in Ceylon which produces both types of alkaloids, substituted and unsubstituted.
3. The plant material from Kerela state bears unsubstituted indole alkaloids as the enzyme systems necessary for 9- substitution is lacking in plants of this region 8.
4. The natural occurance of both open and closed E ring oxindoles together in various species of Mitragyna without the presence of corresponding closed E ring indole alkaloids suggests that the closed E ring oxindole alkaloids might arise by the ring closure of open E ring oxindoles.
PHARMACOLOGICAL PROFILE
Traditional Uses
Mitragyna parvifoliais credited with several medicinal properties and has been widely used by tribal people and other ayurvedic practitioners. The traditional uses of the different parts of the plant are:-
1.Bark and roots:
The bark and roots are used to treat fever, colic, muscular pain, burning sensation, poisoning, gynecological disorders, cough, edema and as aphrodisiac. Stem bark of M. parvifolia is also used in biliousness and applied for muscular pains by the local inhabitant of Tunkur district, Karnataka, India. Bark paste with water is applied externally to relieve muscular pains while the decoction of the bark is given to cure fever by the tribals of Sonaghati of Sonbhadra district, Uttar Pradesh. Powdered bark along with fruits of Phyllanthus emblica is boiled in water and inhaled through mouth for toothache by the Kaadar tribes of Topslip, Tamil Nadu. The Valaiyans tribe, inhabitants of Sirumalai hills, Madurai district, Western Ghats, Tamil Nadu use trunk bark of M.parvifolia to get relief from rheumatic pain.
2.Fruit juice
The fruit juice augments the quantities of breast milk in lactating mothers and also works as lactodepurant.
3.Leaves:
Wounds and ulcers were dressed with its leaves to alleviate pain, swelling and for better healing[16].Fresh leaf juice of M. parvifolia is used to cure jaundice by the tribals of the Chenchus, Yerukalas, Yanadis and Sugalis of Gundur District, Andhra Pradesh17.
Recent Advances in Pharmacological activities of M.parvifolia
Acute toxicity studies
The preliminary acute toxicity studies on the leaf extracts have been carried out according to the OCED- 2001 guidelines using swiss albino mice. The methanolic extract, ethyl acetate extract and the alkaloid rich fraction prepared from the leaves were used for studying acute toxicity. The plant was found to be practically non toxic, as the LD50 dose recorded was 5g/kg/wt18.
Antinociceptive activity
The antinociceptive activity of the ethanolic extract of dried leaves of M. parvifoliahas been evaluated using the Tail-flick method in mice at various dose levels of 100, 200 and 30mg/kg p.o. The extract demonstrated marked antinociceptive activity at the highest dose of 300mg/kg as the maximal possible effect of 39.59% was found to exist. The effect was comparable to that of standard drug, Ibuprofen (43.63%). The results of this study have established the antinociceptive activity of leaf extracts of M. parvifolia 1.
Antiarthritic activity
The methanolic extract of M. parvifolialeaves has been investigated for its antiarthritic potential in rodent models of mice and rats. For evaluation of antiarthritic potential Acetic acid induced vascular permeability in mice and Freund’s adjuvant induced arthritis in rats has been used. Extract was administered at doses of 125, 250 and 500g/kg p.o. the percentage inhibition rates in paw edema in either of the models was in a dose dependent manner. The doses showed inhibition rates of 22.1%, 35.9% and 51.3% in acetic acid induced permeability model. On similar grounds inhibition rates of 36.74%, 39.94% and 59.54% was observed in Freund’s adjuvant induced arthritis in rats19.
Anticonvulsant activity
The anticonvulsant effects of ethanolic extract from the leaves of M. parvifoliaproduced by cold maceration technique was investigated by studying the effects of seizures induced by pentylenetetrazole (PTZ) and maximal electroshock (MES) convulsive methods in mice. The extracts were administered orally at doses of 100, 250 and 500mg/kg. The extract at doses 250 and 500mg/kg suppressed tonic hind limb extensions (THLE) induced by MES. An increase in the onset time and the percentage protection of tonic hind limb extensions were observed. Extract at the highest dose of 500mg/kg exhibited protector effects in PTZ-induced seizures. A delay in the onset of tonic convulsions and an increase in the percentage protection were observed20.
Anti-inflammatory activity
Studies have been conducted to evaluate anti-inflammatory activity of the ethanolic extract of dried leaves of M. parvifoliausing the Carrageenan-induced paw edema method in rats at dose levels of 100, 200 and 300mg/kg p.o. Phenylbutazone at dose level of 80mg/kg p.o. was used as the standard drug. Potent anti-inflammatory effect of the extract at dose level of 300mg/kg (37.99% inhibition) was found and this effect was equivalent to that of phenylbutazone (42.02%). The mechanism of inhibition is believed to be the inhibition of cyclooxygenase leading to inhibition of prostaglandin synthesis. The results of this study thus established the anti-inflammatory activity of leaf extract of M. parvifolia1.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Anxiolytic activity
The putative anxiolytic-like activity of fractions namely the methanolic extract, ethyl acetate extract and the alkaloid rich fraction prepared from the stem-bark of plant has been evaluated using the elevated plus maze (EPM) and marble burying test (MBT) in mice. The extracts at dose levels of 100, 200 and 400mg/kg p.o. were employed. All the extracts increased the time spent on and the number of entries into the open arms of the EPM. This effect was comparable to that of the positive control. The Marble burying test shows the number of marbles buried by mice was decreased significantly as compared to control group. Fluoxetine was used as a standard for comparison. These results indicate that all the extract were effective in dose dependent manner and proved statistically significant at higher doses. The methanolic extract was found to satisfactory whereas the ethyl acetate extract and the alkaloid rich fractions were found to be very potent. The results indicate that the anxiolytic effects of the plant are mainly mediated via the GABAergic system18.
Antioxidant activity
The ethanolic extract of the leaves has been prepared using cold maceration process. The prepared extract at concentrations of 100, 300 and 500μg/ml have been subjected to in vitro antioxidant assays namely the DPPH assay, superoxide radical scvaning assay and the reducing power assay. Ascorbic acid and butylated hydroxyl toluene (BHT) have been used as standards. The DPPH assay shows 87.6% of the scavanging of free radical, DPPH at 500µg/ml concentration. In the superoxide radical scavenging assay 65% of % scvanging was seen at was seen at 500µg/ml concentration. The antioxidant activity in the reducing power assay was found to increase in a concentration dependent manner. Thus, the highest antioxidant activity in all the assay procedures using M. parvifolia leaves extract was observed at the concentration of 500µg/ml21.
Analgesic activity
The analgesic activity has been studied using the ethanolic leaf extracts at doses of 100, 250 and 500mg/kg. Mice models of Eddy’s hot plate and Acetic acid induced writhing test have been employed for this study. Diclofenac sodium has been used as the standard drug for comparing the results. The extract showed moderate analgesic potential in acetic acid induced writhing test at all the test doses while the extract at the dose of 500 mg/kg showed strong analgesic activity in hot plate method. The increase in latency time in the hot plate method was found to be dose dependent22.
Antimicrobial activity
The antimicrobial potential of ethanolic extract of leaves of M. parvifoliaplant has also been evaluated using the agar well diffusion method. The extract in different concentrations of 25, 50, 75 and 100μg/ml has been used. Various microbial strains of S. aureus, B. subtilis, P. aeruginosa and E. coli have been used. Ciprofloxacin at concentration of 20μg/ml has been used as standard. The extract significantly inhibited S. aureus and showed some degree of inhibition against P. aeruginosa and E. coli. However negligible inhibition was observed in case of B. subtilis21.
Another study of antimicrobial activity using M. parvifolia fruit extract has also been carried out. The agar well diffusion method has been employed and activity has been performed using bacterial strains of S. aureus, B. subtilis, P. aeruginosa and E. coli. Ciprofloxacin has been used as the standard. Extract at concentration of 1mg/ml failed to show any zone of inhibition16.
Anthelmintic activity
The methanolic extract of M. parvifolia stem bark produced significant anthelmintic activity. The result of anthelmintic activity of methanolic extract has been evaluated by observing paralysis and death time of earthworms and was found to be dose dependent. This finding proves usefulness of stem-bark as an anthelmintic drug23.
Antipyretic activity
The antipyretic activity of hexane defatted methanolic extract of M. parvifolia leaves has been studied using Brewer’s yeast induced pyrexia in rats. A 15% solution of yeast in 0.9% saline was used for inducing fever in rodents. The various test doses employed were 125, 250 and 500mg/kg. Paracetamol was used as the standard drug. The extract showed significant reduction in body temperatures in a dose dependent manner. The effect was maintained from 30minutes to 3 hours post dosing. The results suggest that M. parvifolia contains biologically-active substances with potential values in the treatment of fever19.
CONCLUSION
Mitragyna parvifoliahas been in use since times in the folklore medicines by various tribals of India. The plant parts posses numerous medicinal properties. A number of pharmacological activities has been investigated and validated scientifically on the different parts of the plant. The acute toxicity studies prove the safety profile of the plant. Studies on the chemical profile of the plant reveal the rich alkaloidal content in it which varies quantitatively with change in geographical locations of the tree. However, these have not been individually evaluated for different pharmacological activities. This part needs a thorough investigation. In all, the studies support the usefulness of the plant in modern medication as a safe and effective treatment.
ACKNOWLEDGEMENT
The authors are sincerely thankful to the Director, Mr. J.S. Gill and the management of Global College of Pharmacy for providing the requisite facilities.
TABLES
TABLE 1 THE REPORTED OXINDOLE AND INDOLE ALKALOIDS OF MITRAGYNA PARVIFOLIA.

(c) closed E-ring alkaloids, (s) E-seco alkaloids
TABLE 2: POSSIBLE ROUTE OF BIOGENESIS OF OXINDOLE ALKALOIDS IN M. PARVIFOLIA FROM THE CORRESPONDING INDOLE ALKALOIDS.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
FIGURES
FIGURE 1: GENERAL STRUCTURE OF CLOSED INDOLE AND OXINDOLE ALKALOIDS
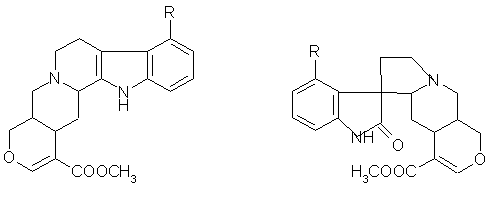
FIGURE 2: GENERAL STRUCTURE OF OPEN INDOLE AND OXINDOLE ALKALOIDS
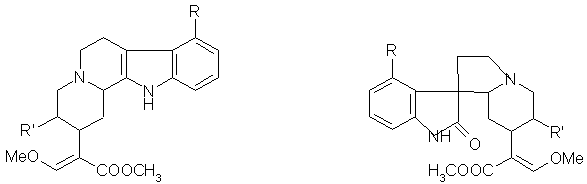
FIGURE 3: GENERAL STRUCTURE OF ALKALOIDS OF SERIES A AND B.

FIGURE 4: THE CLOSED E RING ALLO-EPIALLO SEQUENCE OF ALKALOIDS IN LEAVES GROWING IN UTTAR PRADESH REGION.
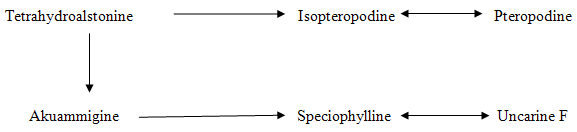
FIGURE 5: THE CLOSED E RING NORMAL-PSEUDO SEQUENCE IN LEAVES OF PLANT GROWING IN UTTAR PRADESH REGION.
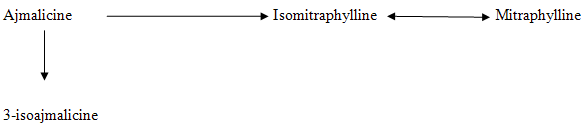
FIGURE 6: THE ALKALOIDS ISOLATED FROM LEAVES OF PLANT GROWING IN POONA (MAHARASTRA).
REFERENCES
1.Gupta V, Kumar P, Bansal P, Singh R. Anti-inflammatory and Anti-nociceptive Activity of Mitragyna parvifolia, Asian J of Med Sci 2009; 1 (3): 97-99.
2.The Wealth of India. A Dictionary of Indian Raw Materials and Industrial Products, National Institute of Science Communication and Information Resources. . Reprint 2003: 393-95.
3.Pandey R, Singh SC, Gupta MM. Heteroyohimbinoid type oxindole alkaloids from Mitragyna parvifolia. Phytochemistry 2006; 67: 2164–169.
4.Reinhard KH. Uncaria tomentosa (Willd.) D.C.: Cat’s Claw, Una de Gato, or Saventaro. The J of Alternate and Complementary Med. 1999; 5 (2): 143-51.
5.German O, Cook JM. Abstracts of Papers, 237th ACS National Meeting, Salt Lake City, UT, United States 2009: 22-26.
6.Shellard EJ, Houghton PJ. Mitragyna species of Asia. XIX. The alkaloidal pattern in Mitragyna parvifolia (Roxb.) Korth. Planta Medica 1971; 20: 82-89.
7.Shellard EJ, Phillipson JD, Gupta D. Mitragyna species of Asia. XIV. Alkaloids of leaves of Mitragyna parvifolia from Burma, Cambodia and Ceylon. Planta Medica. 1969a; 17 (1): 51-58.
8.Shellard EJ, Lala PK. . The Mitragyna species of Asia. Part XXIX. The alkaloidal pattern in the leaves, stem bark and root bark of Mitragyna parvifolia from the Kerala State, India. Planta Medica 1977; 31(4): 395-99.
9.Shellard EJ, Phillipson JD, Gupta D. Mitragyna species of Asia. XV. Alkaloids from bark of Mitragyna parvifolia and a possible biogenetic route for oxindole alkaloids. Planta Medica 1969b; 17 (2): 146-63.
10.Shellard EJ, Houghton PJ. Mitragyna species of Asia. XXIV. Isolation of dihydrocorynantheol and corynantheidol from the leaves of Mitragyna parvifolia from Sri Lanka (Ceylon). Planta Medica 1973; 24 (1): 13-17.
11.Shellard EJ, Houghton, PJ. Mitragyna species of Asia. XX. Alkaloidal pattern in Mitragyna parvifolia from Burma. Planta Medica 1972; 21 (3): 263-66.
12.Shellard EJ, Houghton PJ. Mitragyna species of Asia. XXI. Distribution of alkaloids in young plants of Mitragyna parvifolia grown from seed obtained from Ceylon. Planta Medica 1972; 21 (4): 382-92.
13.Shellard EJ, Phillipson JD. The Mitragyna species of Asia. II. Alkaloids of leaves of Mitragyna parvifolia. Planta Medica 1974; 12 (2): 160-65.
14.Shellard EJ, Phillipson JD, Gupta D. Mitragyna species of Asia. XI. Alkaloids of the leaves of Mitragyna parvifolia obtained from the Maharashtra State of India. Planta Medica 1968; 16 (1): 20-28.
15.Phillipson JD, Hemingway SR. Mitragyna species of Asia. XXVII. Alkaloidal N-oxides in leaves of Mitragyna parvifolia from Sri Lanka. Phytochemistry 1974; 13: 973-78.
16.Saneja A, Kaushik D, Khokra SL, Kaushik P, Sharma C, Aneja KR. Evaluation of activities of Mitragyna parvifolia fruit extract. J of Natural Products 2009; 2: 49-54.
17.Subramanian A, Mohan VR, Kalidass C, Maruthupandian A. PHARMACOGNOSTIC STUDIES ON THE TRUNK BARK OF MITRAGYNA PARVIFOLIA (ROXB.) KORTH (RUBIACEAE). J of Herbal Med and Toxicology 2009; 3 (2): 91-97.
18.Badgujar VB, Surana SJ. Investigation of anxiolytic effects of Mitragyna parvifolia stem-bark extracts on animal models. Der Pharmacia Lettre 2009; 1 (2): 172-81.
19.Jain AP, Tote MV, Mittal A, Mahire NB, Undale VR, Bhosale AV. Antiarthritic and antipyretic activity of Mitragyna parvifolia leaves. Pharmacologyonline 2009; 2: 739-49.
20.Kaushik D, Khokra SL, Kaushik P, Saneja A, Arora D. Anticonvulsant Activity of Mitragyna Parvifolia Leaves Extract. Pharmacologyonline 2009; 3: 101-06.
21.Kaushik D, Saneja A, Kaushik P, Lal S, Yadav V. Antioxidant and Anti-inflammatory activities of Mitragyna parvifolia leaves extract. Der Pharmacia Lettre 2009; 1(1): 75-82.
22. Kaushik D, Khokra SL, Kaushik P, Saneja A, Sharma C, Aneja KR, Chaudhary B, Koshy S. A Study of Analgesic and Antimicrobial Potential of Mitragyna Parvifolia. Int J of Pharm Sci and Drug Res 2009; 1(1): 6-8.
23.Badgujar VB, Surana SJ. In vitro investigation of anthelmintic activity of Mitragyna parvifolia (Roxb.) Korth (Rubiaceae) Vetenary World 2010; 3(7): 326-28.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









