 About Authors:
About Authors:
Vishal Sharma1*, Alankar Shrivastava2
1Department of Pharmaceutics,
2Senior Lecturer Department of Pharmaceutical Analysis
B.R.NAHATA COLLEGE OF PHARMACY
(A SIRO Recognized by DSIR, Ministry of science & Technology, GOI)
MANDSAUR (M.P.) 458001
*vishus2010@gmail.com
Abstract
Antibiotics are chemical substances, either produced naturally by microorganisms or manufactured synthetically, that are lethal to other microorganisms. Antibiotic resistance (AR) describes the ability of a microorganism to be unaffected by (or resistant to) the effects of a particular antibiotic. A problem arises when a animal or person becomes infected with a disease-causing bacterium that harbors antibiotic resistance to the drug that would be the most suitable treatment for that infection.
India is a vast country of immense diversity.and for that the effectiveness of the antimicrobial was not same to all ,and the administration of them will either really cure or may act in opposite manner and will leads to the development os the antimicrobial resistance which is only due to the unaware use of the antibiotics.we had conducted the survey on some prescriptions of registered medicinal practitioner and find that there was an irrational use of antibiotic in our on community and this might be very serious as our survey is on the smallest level and what it may cause globally.So the use of antibiotic should be controlled.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1356
INTRODUCTION
Antibiotic resistance occurs when bacteria change in some way that reduces or eliminates the effectiveness of drugs, chemicals or other agents designed to cure or prevent the infection. Thus the bacteria survive and continue to multiply causing more harm. Widespread use of antibiotics promotes the spread of antibiotic resistance. Bacterial susceptibility to antibacterial agents is achieved by determining the minimum inhibitory concentration that inhibits the growth of bacteria.
Resistance is defined as bacteria that are not inhibited by usually achievable systemic concentration of an agent with normal dosage schedule and/ or fall in the minimum inhibitory concentration ranges. Likewise the multiple drug resistance is defined as the resistance to two or more drugs or drug classes. Acquisition of resistance to one antibiotic conferring resistance to another antibiotic, to which the organism has not been exposed, is called cross resistance.
Antibiotics are given to human for treatment and prophylaxis of infectious diseases, 80% to 90% of antibiotics are used in outpatients and the remainder in hospitals. Antibiotics are appear to be used not only in excess but also inappropriately and this accounts for 20% to 50 % of all antibiotics used. The Center for Disease Control and Prevention in USA has estimated that some 50 millions of the 150 millions prescriptions every year are un necceary.
[adsense:468x15:2204050025]
Nowadays, about 70 % of the bacteria that cause infections in hospitals are resistant to at least one of the antibiotic agents most commonly used for treatment. Some organisms are resistant to all approved antibiotics and can only be treated with experimental and potentially toxic drugs. An alarming increase in resistance of bacteria that cause community acquired infections has also been documented, especially the Staphylococci and Pneumococci (Streptococcus pneumoniae), which are prevalent causes of disease and mortality. In a recent study, 25% of bacterial pneumonia cases were shown to be resistant to Penicillin, and an additional 25% of cases were resistant to more than one antibiotic.
Antibiotic usage resistance rates vary from one country to another. It is observed that countries with the highest per capita antibiotic consumption have the highest resistance rates. It is not only the amount of antibiotic used that select for resistance, but the number of individuals receiving the drug and the population density also matters. Giving 1000 doses of an antibiotic to one individual will have considerably less ecological effect on resistance emergence than giving those same 1000 doses to 1000 individuals. A study by Levy suggests that combination of antibiotic use and population density correlates more strongly with the prevalence of antibiotic resistance in a population than use of the antibiotic alone.
India is a vast country of immense diversity. This diversity is seen at its most extreme in people`s access to health care. The poor and marginalized sections of society, the people in remote rural regions of the country and those at risk of disease due to an unhealthy environment and inadequate nutrition, are the most affected. One of the many ways in which this inequality shows up is in the treatment of various illnesses, especially infections. Not only can the poor not afford antibiotics, they are also most affected by the rapid rise of antibiotic resistance A high level of antibiotic resistance has major consequences for society, and especially for those on the margins who have the least access to health care. It is therefore important to look at what could be promoting the rise of antibiotic resistance. One of the possible causes of this phenomenon is the inappropriate use of antibiotics.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
HISTORY
The first antibiotic, Penicillin (discovered in 1929 by Sir Alexander Fleming), had unbelievable ability to treat the bacterial infections especially those caused by Staphylococcus and Streptococci without harming the host.12 Antibiotic resistance first became challenging shortly after Penicillin gained extensive use in the 1940s.
Nowadays more than 95% of Staphylococcus aureus isolates globally are resistant to Penicillins.14 An initial response to Penicillin resistance was the development of Methicillin, semi synthetic penicillin.
The period of late 1940s and early 1950s saw the discovery and introduction of broad spectrum antibiotics such as Streptomycin, Chloramphenicol and Tetracycline and the age of antibiotic chemotherapy came into full being. These antibiotics were effective against the full array of bacterial pathogens including Gram- positive and Gram- negative bacteria, intracellular parasites and the Tuberculosis bacillus. Synthetic antimicrobial agents such as the “sulfa drugs” (Sulfonamides) and antituberculosis drugs, such as Para-aminosalicylic acid (PAS) and Isoniazid (INH), were also brought into wider usage. However, by 1953 during Shigella outbreaks in Japan, strain of the Dysentery bacillus was isolated which was multiple drug resistant, exhibiting resistances to Chloramphenicol, Tetracycline, Streptomycin and Sulfanilamide. By the late 1980s even Methicillinresistant Staphylococcus aureus had become prevalent in many hospitals and difficult to treat. Until recently Vanomycin was a dependable drug for the treatment of infections caused by multidrug resistant Enterococci but Vanomycin resistance began to emerge in the mid 80s. A study by Gaynes reported that Vanomycin resistance had increased more than 20 fold from 1989 to 1995.An additional community acquired pathogen Neisseria Gonorrhoeae has undergone significant changes in antibiotic resistance. For a number of years, Penicillins were the drug of choice to treat Gonorrhoeae but in 1976, the plasmid mediated Beta lactamase of E.coli was found in Neisseria Gonorrhoeae isolates in Africa and Asia. Development of antibiotic resistance was first reported in animal models in 1940s and subjectively reported among patients in the 1970s. Today drug resistant strains of Mycobacterium tuberculosis are threatening to outbreak in one of the world’s most prevalent infectious diseases.
HOW DO ANTIBACTERIAL AGENTS WORK?
Most antimicrobial agents used for the treatment of bacterial infections may be categorized according to their principal mechanism of action. There are 4 major modes of action:
(1) interference with cell wall synthesis,
(2) inhibition of protein synthesis,
(3) interference with nucleic acid synthesis, and
(4) inhibition of a metabolic pathway (Table 1)
|
TABLE-1 |
|||
|
MECHANISM OF ACTION OF ANTIBACTERIAL AGENT |
|||
|
1.Interference with cell wall synthesis |
|||
|
Beta-Lactams |
Glycopeptides |
||
|
Penicillin, cephalosporin, carbapenems. |
Vancomycin, teicoplanin |
||
|
2.Protien synthesis inhibition |
|||
|
Bind to 50 s subunit of ribosomes |
Bind to 30 s subunit of ribosomes |
Bind to isoleucyl-t RNA synthetase |
|
|
Aminoglycoside,tetracylins |
Aminoglycoside,tetracylins |
mupirocin |
|
|
3.Interference with nuclic acid synthesis |
|||
|
Inhibit DNA synthesis |
Inhibit RNA synthesis |
||
|
fluroquinones |
rifampin |
||
|
4.Inhibition of Metabolic Pathways |
|||
|
Sulfonamide, folic acid analogue |
|||
|
5.disruption of Bacterial membrane synthesis |
|||
|
Polymyxins, daptomycin |
|||
Antibacterial drugs that work by inhibiting bacterial cell wall synthesis include the _-lactams, such as the penicillins, cephalosporins, carbapenems, and monobactams, and the glycopeptides, including vancomycin and teicoplanin.Beta-Lactam agents inhibit synthesis of the bacterial cell wall by interfering with the enzymes required for the synthesis of the peptidoglycan layer.Vancomycin and teicoplanin also interfere with cell wall synthesis, but do so by binding to the terminal D-alanine residues of the nascent peptidoglycan chain, thereby preventing the cross-linking steps required for stable cell wall synthesis.Macrolides, aminoglycosides, tetracyclines, chloramphenicol, streptogramins, and oxazolidinones produce their antibacterial effects by inhibiting protein synthesis.Bacterial ribosomes differ in structure from their counterparts in eukaryotic cells. Antibacterial agents take advantage of these differences to selectively inhibit bacterial growth. Macrolides, aminoglycosides, and tetracyclines bind to the 30S subunit of the ribosome, whereas chloramphenicol binds to the 50S subunit. Fluoroquinolones exert their antibacterial effects by disrupting DNA synthesis and causing lethal double-strand DNA breaks during DNA replication,19 whereas sulfonamides and trimethoprim (TMP) block the pathway for folic acid synthesis, which ultimately inhibits DNA synthesis. The common antibacterial drug combination of TMP, a folic acid analogue, plus sulfamethoxazole (SMX) (a sulfonamide) inhibits 2-steps in the enzymatic pathway for bacterial folate synthesis. Disruption of bacterial membrane structure may be a fifth, although less well characterized, mechanism of action. It is postulated that polymyxins exert their inhibitory effects by increasing bacterial membrane permeability, causing leakage of bacterial contents. The cyclic lipopeptide daptomycin apparently inserts its lipid tail into the bacterial cell membrane, causing membrane depolarization and eventual death of the bacterium.
MISUSE OF ANTIBIOTICS
Inappropriate antibiotic treatment and overuse of antibiotics have been a contributing factor to the emergence of resistant bacteria. The problem is further exacerbated by self-prescribing of antibiotics by individuals without the guidelines of a qualified clinician and the non-therapeutic use of antibiotics as growth promoters in agriculture. Antibiotics are frequently prescribed for indications in which their use is not warranted, an incorrect or sub-optimal antibiotic is prescribed or in some cases for infections likely to resolve without treatment. The overuse of antibiotics like penicillin and erythromycin, which used to be one-time miracle cures, were associated with emerging resistance since the 1950s. Therapeutic usage of antibiotics in hospitals has been seen to be associated with increases in multi-antibiotic-resistant bacteria.
Common forms of antibiotic misuse include excessive use of prophylactic antibiotics in travelers, failure to take into account the patient's weight and history of prior antibiotic use when prescribing, since both can strongly affect the efficacy of an antibiotic prescription, failure to take the entire prescribed course of the antibiotic, failure to prescribe or take the course of treatment at fairly precise correct daily intervals (e.g., "every 8 hours" rather than merely "3x per day"), or failure to rest for sufficient recovery to allow clearance of the infecting organism. These practices may facilitate the development of bacterial populations with antibiotic resistance. Inappropriate antibiotic treatment is another common form of antibiotic misuse. A common example is the prescription and use of antibiotics to treat viral infections such as the common cold that have no effect. One study on respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who they believed expected them, although they correctly identified only about 1 in 4 of those patients". Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescribing of antibiotics. Delaying antibiotics for 48 hours while observing for spontaneous resolution of respiratory tract infections may reduce antibiotic usage; however, this strategy may reduce patient satisfaction.
MECHANISMS OF RESISTANCE TO ANTIBACTERIAL AGENTS
Bacteria may manifest resistance to antibacterial drugs through a variety of mechanisms. Some species of bacteria are innately resistant to _1 class of antimicrobial agents. In such cases, all strains of that bacterial species are likewise resistant to all the members of those antibacterial classes. Of greater concern are cases of acquired resistance, where initially susceptible populations of bacteria become resistant to an antibacterial agent and proliferate and spread under the selective pressure of use of that agent. Several mechanisms of antimicrobial resistance are readily spread to a variety of bacterial genera. First, the organism may acquire genes encoding enzymes, such as Beta-lactamases, that destroy the antibacterial agent before it can have an effect. Second, bacteria may acquire efflux pumps that extrude the antibacterial agent from the cell before it can reach its target site and exert its effect. Third, bacteria may acquire several genes for a metabolic pathway which ultimately produces altered bacterial cell walls that no longer contain the binding site of the antimicrobial agent, or bacteria may acquire mutations that limit access of antimicrobial agents to the intracellular target site via downregulation of porin genes. Thus, normally susceptible populations of bacteria may become resistant to antimicrobial agents through mutation and selection, or by acquiring from other bacteria the genetic information that encodes resistance. The last event may occur through 1 of several genetic mechanisms, including transformation, conjugation, or transduction. Through genetic exchange mechanisms, many bacteria have become resistant to multiple classes of antibacterial agents, and these bacteria with multidrug resistance (defined as resistance to _3 antibacterial drug classes) have become a cause for serious concern, particularly in hospitals and other healthcare institutions where they tend to occur most commonly. As noted above, susceptible bacteria can acquire resistance to an antimicrobial agent via new mutations.18 Such spontaneous mutations may cause resistance by –
(1) altering the target protein to which the antibacterial agent binds by modifying or eliminating the binding site (e.g., change in penicillin-binding protein 2b in pneumococci, which results in penicillin resistance),
(2) upregulating the production of enzymes that inactivate the antimicrobial agent (e.g., erythromycin ribosomal methylase in staphylococci),
(3) downregulating or altering an outer membrane protein channel that the drug requires for cell entry (e.g., OmpF in E coli), or
(4) upregulating pumps that expel the drug from the cell (efflux of fluoroquinolones in S aureus).
In all of these cases, strains of bacteria carrying resistance-conferring mutations are selected by antimicrobial use, which kills the susceptible strains but allows the newly resistant strains to survive and grow. Acquired resistance that develops due to chromosomal mutation and selection is termed vertical evolution. Bacteria also develop resistance through the acquisition of new genetic material from other resistant organisms. This is termed horizontal evolution, and may occur between strains of the same species or between different bacterial species or genera. Mechanisms of genetic exchange include conjugation, transduction, and transformation. For each of these processes, transposons may facilitate the transfer and incorporation of the acquired resistance genes into the host’s genome or into plasmids. During conjugation, a gram-negative bacterium transfers plasmid-containing resistance genes to an adjacent bacterium, often via an elongated proteinaceous structure termed a pilus, which joins the 2 organisms. Conjugation among gram-positive bacteria is usually initiated by production of sex pheromones by the mating pair, which facilitate the clumping of donor and recipient organisms, allowing the exchange of DNA. During transduction, resistance genes are transferred from 1 bacterium to another via bacteriophage (bacterial viruses). This is now thought to be a relatively rare event. Finally, transformation, i.e., the process whereby bacteria acquire and incorporate DNAsegments from other bacteria that have released their DNA complement into the environment after cell lysis, can move resistance genes into previously susceptible strains. Mutation and selection, together with the mechanisms of genetic exchange, enable many bacterial species to adapt quickly to the introduction of antibacterial agents into their environment. Although a single mutation in a key bacterial gene may only slightly reduce the susceptibility of the host bacteria to that antibacterial agent, it may be just enough to allow its initial survival until it acquires additional mutations or additional genetic information resulting in fullfledged resistance to the antibacterial agent. However, in rare cases, a single mutation may be sufficient to confer high-level, clinically significant resistance upon an organism (e.g., high-level rifampin resistance in S aureus or high-level fluoroquinolone resistance in Campylobacter jejuni). The following case studies, which involve 3 different bacterial species, serve to illustrate several of the ways in which bacteria develop resistance to antibacterial drugs and how different resistance mechanisms may interact to increase the level or spectrum of resistance of an organism. Resistance patterns associated with these bacterial pathogens are discussed in greater detail in other articles in this supplement.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
A SURVEY ON IRRATIONAL USE OF ANTIBIOTIC IN OUR COMMUNITY
A survey was conducted upon prescriptions on Retail pharmacy store, and only priscripton comprising of antibiotics written by Registered Medicinal Practitioner were analysed. Data were analysed to determine the patterns of utilization, compliance and awareness regarding antibiotic medication amongst a urban population at Mandsaur a District of Madhya Pradesh. The survey form had been designed after analyzing the literature and research papers.
The present study was aimed to analyze the prescribing pattern of antibiotic drugs in general practice at Mandsaur region . The study was conducted on the patients purchasing the medication from the retail outlets of Mandsaur region. The complete prescriptions of the antibiotic patients were monitored and data was filled. A total of 398 prescriptions were evaluated
MATERIAL AND METHODS
Study plan
The study included the following objectives:
(1) To compare antibiotic use, type of prevalent bacteria and their susceptibility in locality;
(2) To seek correlation between antibiotic consumption and resistance patterns; and
(3) to evaluate implications for antibiotic use
RESULTS
A total of 398 prescriptions were evaluated in a time period of 5 days at different outlet in mandsaur and the consumption average was calculated as follow –
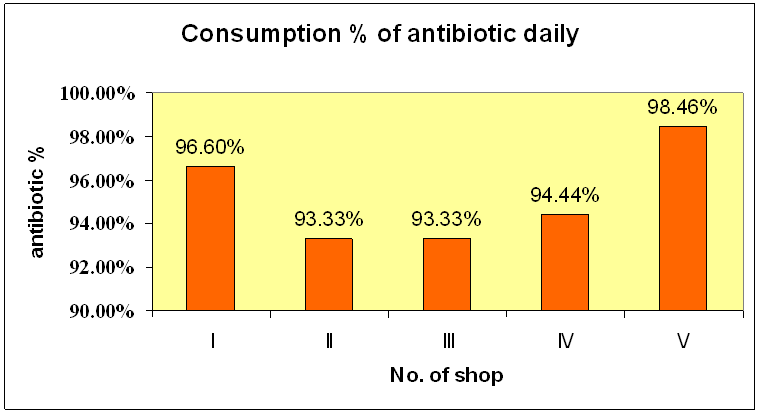
Figure 1 : Daily Consumption of the antibiotics
The consumption average daily was found out to be 95.9% .
Table 2 : Daily Consumption Of Antibiotics In 5 Medical Shops In Mandsaur
|
Antibiotics |
I |
II |
III |
IV |
V |
AVERAGE |
|
|
Ciprofloxacin |
28 |
10 |
33 |
21 |
23 |
23 |
|
|
Cefixime |
13 |
- |
16 |
4 |
18 |
10.2 |
|
|
Ofloxacin |
14 |
4 |
15 |
- |
9 |
8.4 |
|
|
Cefuroxime |
10 |
2 |
20 |
- |
- |
6.4 |
|
|
Amoxicillin |
- |
3 |
18 |
10 |
12 |
8.6 |
|
|
Ampicillin |
- |
- |
5 |
- |
- |
1 |
|
|
Roxithromycin |
- |
- |
4 |
15 |
- |
3.8 |
|
|
Azithromicin |
- |
- |
3 |
- |
- |
0.6 |
|
|
|
65 |
19 |
114 |
50 |
62 |
62 |
Ø Other antibiotics which are used (Other than mentioned above)
Amikacin
Ceftriaxone
Gentamicin
Cefotaxime
Ceftriaxone
Cefuroxime sodium
Ampicillin
Ø Combination which are used
· Amoxicillin+clavulanic acid
· Ampicillin + Cloxacillin
· Amoxicillin + Cloxacillin
· Ofloxacin + Ornidazole
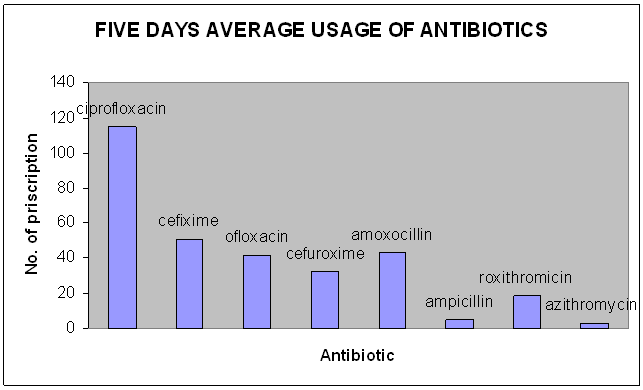
Figure 3 :Five days average usage of antibiotics
DISCUSSION
Our study shows that in this situation where therewere no official limitations to antibacterial drug treatment. The month of study is November and During this period of time most common diseases were Diarrhea, Common cold, Bronchitis etc. and in comparisions to diseases there is a rise in the total consumption of antibiotics.And there might be cases of irrational use of antibiotics in the community.
IMPACT OF RESISTANCE ON PUBLIC HEALTH AND ECONOMY
Due to the selection pressure caused by antibiotic use, a large pool of resistant genes has been created and this antibiotic resistance places an increased burden on society in terms of high morbidity, mortality and cost. Patient infected with drug resistant organisms are more likely to have ineffective therapy, longer duration of hospital stay, need of treatment with broad spectrum antibiotics that are more toxic and more expensive.
The cost of care for individual patient also increases due to the need for more costly second line drugs, longer duration of hospital stay, increased need for intensive care and diagnostic testing, higher incidences of complications and expenses incurred by use of isolation precaution. In brief, antibiotic resistance is driving up health care cost, increasing the severity of disease and death rates of some infections.
CONTROL AND PREVENTION
Various guidelines have been published by several societies for optimizing antibiotic use and curtailing antibiotic resistance in hospitals.
Ø Multidisciplinary coordination and cooperation between hospital administrator, clinician, infection control team, microbiologist and hospital pharmacist.
Ø Formulary based local guidelines on anti-infective therapy and prophylaxis, education and regulation of prescriptions by consultant specialist, monitoring and auditing drug use, surveillance and reporting of resistance patterns of the hospital flora.
Ø Detection of patients colonized with communicable resistant bacteria and notification of these to the infection control team when isolation of the patient or decolonization, or both, would be useful.
Ø Good and regularly updated education on antibiotic use for clinicians, nurses and pharmacists.
Ø Promotion and monitoring of basic hospital infection control practices such as hand hygiene. These guidelines are based more on expert opinion and on the results of descriptive and analytical studies than on evidence from controlled trials, which are difficult to design to evaluate these types of population based intervention.
Ø Each hospital has its own ecosystem and micro society, where determinants of antibiotic resistance are quite specific and therefore effective solutions will need to be tailored to local epidemiological circumstances and resources.44
Ø Role of Pharmacist in reducing antibiotic resistance A Pharmacist plays an important role in reducing antibiotic resistance by: Counseling of individual patient on appropriate use of antibiotics, such as choice of drug, dose and duration.
Ø Attending ward rounds and acting as a point of communication between pharmacy, microbiology and infectious disease and infection control teams.
Ø Preparing evidence based local prescribing guidelines for antibiotics.
Ø Promoting good prescribing practice.
Ø Monitoring antibiotic use in terms of volume or ‘defined daily dose’ and expenditure.
Ø Providing educational and training program in antibiotic therapy for doctors,
CONCLUSION
The issue of antibiotic misuse is of global concern because of the spreading and developing resistance of most common bacteria to most inexpensive generic antibiotics. Antibiotic resistance now has been universally identified as public health priority and necessary plan of action to combat resistance should be developed.. Better diagnostic tests, promotion and evaluation of medical and veterinary practice guidelines, restriction of antibiotic use as growth promoters in food and animals, development of novel antibiotics are some of steps required. Above all, patients, providers and health care leaders must make a serious commitment to change the dynamics of outpatient prescribing. As we had conducted the survey on some prescriptions of registered medicinal practitioner and find that there was an irrational use of antibiotic in our on community and this might be very serious as our survey is on the smallest level and what it may cause globally.So the use of antibiotic should be controlled.
REFERENCE
•Fred T.C ; “Mechanism of antimicrobial Resistance in Bacteria”;American Journal of Medicine (2006) vol-119 (6A) S3-S10.
•Prasad A;Verma A;Kumar.R;”Antibiotic Resistance-A Review”;Inventi Journal (p) Ltd;Pharmacy Practice vol-1;Issue 2
•Jeffry L.T ,D.V.M; Antibiotic Resistance –Question and Answer-Extension Fact Sheet;
•Deshpandey P ;Rodrigues .C ;Sethi .A ;Kapadia .F;Hedge .A;Soman.R : New Delhi Metallo Beta-Lactamase (NDM 1) in Enterobectariacea ; Treatment Option With Carbapenems Compromised; JAPI;March 2010-vol 58
•Antibiotic usage and resistance — trends in Estonian University ; International Journal of Antimicrobial Agents 16 (2000) 309–315
•Zimbio.com, visited on October 10 ,Saturday
•Emedicinehealth.com , visited on October 9 ,Saturday
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









