ABOUT AUTHOR:
Shikha Patel
M.pharm (Quality Assurance), Sagar Institute of Research and Technology-Pharmacy
Bhopal (M.P)
siyapatel2007@gmail.com
ABSTRACT:
Synriam™ is new drugs combination in India Market and its Generic name is Arterolane Maleate and Piperaquine Phosphate. It was launched by Ranbaxy Laboratories Limited on 25 April, 2012 and approved in 2011 by Drug Controller General of India. Synriam is a fixed dose combination of two antimalarial active ingredients. Each film coated tablet contains Arterolane maleate 150 mg. and Piperaquine phosphate 750 mg, developed as simplified once-a-day therapy for three days for the treatment of acute, uncomplicated P. falciparum malaria in adult. Arterolane a synthetic trioxalane rapidly kills the malaria parasite in blood, providing fast relief from symptoms of malaria. Piperaquine on the other hand has a longer lasting effect than arterolane and kills the residual parasites, preventing the recurrence of malaria. SYNRIAM has been effective in geographical regions where resistance to chloroquine has been reported. The fever clearance time for SYNRIAM is shorter than that of arthemeter-lumefantrine. This is an added benefit over arthemeter-lumefantrine regimen, which has to be taken with high fat meals twice a day. This food independent dosing will improve patient convenience and compliance. The efficacy of Synriam has been confirmed in comparative study conduct in India, Thailand and Bangladesh.
REFERENCE ID: PHARMATUTOR-ART-1877
INTRODUCTION
Malaria: The Disease
Malaria is a mosquito-borne infectious disease of humans and other animals caused by protists (a type of microorganism) of the genus Plasmodium. It begins with a bite from an infected female Anopheles mosquito, which introduces the protists through saliva into the circulatory system. In the blood, the protists travel to the liver to mature and reproduce. The disease is widespread in tropical and subtropical regions in a broad band around the equator, including much of Sub-Saharan Africa, Asia, and the Americas.
Causes of malaria
Malaria is caused by a type of parasite known as Plasmodium. This is a microscopic parasite that is transmitted by certain species of mosquitoes. Although there are numerous types of Plasmodia parasites, only four cause malaria in humans. These include:
Plasmodium falciparum
Plasmodium vivax
Plasmodium ovale
Plasmodium malariae
There is a fifth species causing malaria in humans. It is called Plasmodium knowlesi. It is distributed across South East Asia and is often misdiagnosed as P. Malariae. The infection has a potentially more serious and even life-threatening course.
The Plasmodium parasite is mainly spread by female Anopheles mosquitoes, which are night-biting mosquitos.
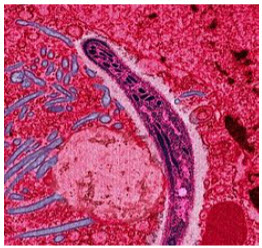
A Plasmodium in the form that enters humans and other vertebrates from the saliva of female mosquitoes (a sporozoite) traverses the cytoplasm of a mosquito midgut epithelial cell.
Malaria is typically diagnosed by the microscopic examination of blood using blood films, or with antigen-based rapid diagnostic tests. Modern techniques that use the polymerase chain reaction to detect the parasite's DNA have also been developed, but these are not widely used in malaria-endemic areas due to their cost and complexity. The World Health Organization has estimated that in 2010, there were 219 million documented cases of malaria. That year, between 660,000 and 1.2 million people died from the disease, many of whom were children in Africa. The actual number of deaths is not known with certainty, as accurate data is unavailable in many rural areas, and many cases are undocumented. Malaria is commonly associated with poverty and may also be a major hindrance to economic development.
Despite a need, no effective vaccine currently exists, although efforts to develop one are ongoing. Several medications are available to prevent malaria in travelers to malaria-endemic countries (prophylaxis). A variety of antimalarial medications are available. Severe malaria is treated with intravenous or intramuscular quinine or, since the mid-2000s, the artemisinin derivative arterolane, which is superior to quinine in both children and adults and is given in combination with a second anti-malarial such as Piperaquine.
Life cycle
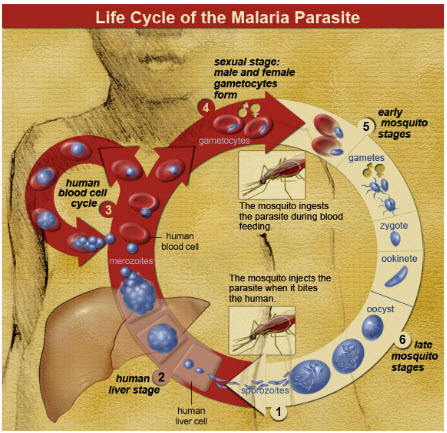
The life cycle of malaria parasites: A mosquito causes infection by taking a blood meal. First, sporozoites enter the bloodstream, and migrate to the liver. They infect liver cells, where they multiply into merozoites, rupture the liver cells, and return to the bloodstream. Then, the merozoites infect red blood cells, where they develop into ring forms, trophozoites and schizonts that in turn produce further merozoites. Sexual forms are also produced, which, if taken up by a mosquito, will infect the insect and continue the life cycle.
In the life cycle of Plasmodium, a female Anopheles mosquito (the definitive host) transmits a motile infective form (called the sporozoite) to a vertebrate host such as a human (the secondary host), thus acting as a transmission vector. A sporozoite travels through the blood vessels to liver cells (hepatocytes), where it reproduces asexually (tissue schizogony), producing thousands of merozoites. These infect new red blood cells and initiate a series of asexual multiplication cycles (blood schizogony) that produce 8 to 24 new infective merozoites, at which point the cells burst and the infective cycle begins anew. Other merozoites develop into immature gametes, or gametocytes. When a fertilised mosquito bites an infected person, gametocytes are taken up with the blood and mature in the mosquito gut. The male and female gametocytes fuse and form zygotes (ookinetes), which develop into new sporozoites. The sporozoites migrate to the insect's salivary glands, ready to infect a new vertebrate host. The sporozoites are injected into the skin, alongside saliva, when the mosquito takes a subsequent blood meal.
Signs and symptoms
Features of malaria include high fever over 38C (100.4F) along with chills and sweating. There is intense muscle pain, headache, blurring of vision and dizziness. Some patients may develop diarrhoea and vomiting as well. Symptoms develop within seven days after being bitten or may take between 10 to 15 days to appear.
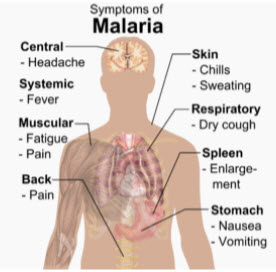
Main symptoms of malaria
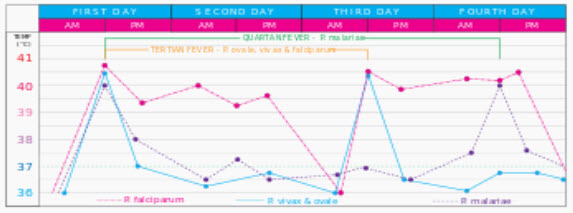
The typical fever patterns of the different types of malaria
The signs and symptoms of malaria typically begin 8–25 days following infection; however, symptoms may occur later in those who have taken antimalarial medications as prevention. Initial manifestations of the disease—common to all malaria species—are similar to flu-like symptoms and can resemble other conditions such as septicemia, gastroenteritis, and viral diseases. The presentation may include headache, fever, shivering, joint pain, vomiting, hemolytic anemia, jaundice, hemoglobin in the urine, retinal damage, and convulsions.
The classic symptom of malaria is paroxysm—a cyclical occurrence of sudden coldness followed by rigor and then fever and sweating, occurring every two days (tertian fever) in P. vivax and P. ovale infections, and every three days (quartan fever) for P. malariae. P. falciparum infection can cause recurrent fever every 36–48 hours or a less pronounced and almost continuous fever.
Severe malaria is usually caused by P. falciparum (often referred to as falciparum malaria). Symptoms of falciparium malaria arise 9–30 days after infection. Individuals with cerebral malaria frequently exhibit neurological symptoms, including abnormal posturing, nystagmus, conjugate gaze palsy (failure of the eyes to turn together in the same direction), opisthotonus, seizures, or coma.
Classification
Malaria is classified into either "severe" or "uncomplicated" by the World Health Organization (WHO). It is deemed severe when any of the following criteria are present, otherwise it is considered uncomplicated.
Decreased consciousness
Significant weakness such that the person is unable to walk
Inability to feed
Two or more convulsions
Low blood pressure (less than 70 mmHg in adults and 50 mmHg in children)
Breathing problems
Circulatory shock
Kidney failure or hemoglobin in the urine
Bleeding problems, or hemoglobin less than 50 g/L (5 g/dL)
Pulmonary edema
Blood glucose less than 2.2 mmol/L (40 mg/dL)
Acidosis or lactate levels of greater than 5 mmol/L
A parasite level in the blood of greater than 100,000 per microlitre (µL) in low-intensity transmission areas, or 250,000 per µL in high-intensity transmission areas
Cerebral malaria is defined as a severe P. falciparum-malaria presenting with neurological symptoms, including coma (with a Glasgow coma scale less than 11, or a Blantyre coma scale greater than 3), or with a coma that lasts longer than 30 minutes after a seizure.
High risk areas for malaria
Malaria is found mainly in the tropical countries all around the world. It is seen in large parts of Africa and Asia, Central and South America, Haiti and the Dominican Republic, some Pacific islands, such as Papua New Guinea and some parts of Middle East.
Malaria is not seen commonly in the United Kingdom. In the United States around 1,500 cases of malaria are reported every year. Worldwide around 3.3 billion people live in areas at risk of malaria transmission in 106 countries and territories.
In 2010, according to the World Health Organization, there were 216 million episodes of malaria and 655,000 deaths worldwide. Of these deaths around 91% were seen in the African Region, followed by the South-East Asian Region (6%), and the Eastern Mediterranean Region (3%). About 86% of deaths globally were in children.
Recurrent malaria
Symptoms of malaria can reappear (recur) after varying symptom-free periods. Depending upon the cause, recurrence can be classified as either recrudescence, relapse, or reinfection. Recrudescence is when symptoms return after a symptom-free period. It is caused by parasites surviving in the blood as a result of inadequate or ineffective treatment. Relapse is when symptoms reappear after the parasites have been eliminated from blood but persist as dormant hypnozoites in liver cells. Relapse commonly occurs between 8–24 weeks and is commonly seen with P. vivax and P. ovale infections. P. vivax malaria in temperate areas often involves overwintering by hypnozoites, with relapses beginning the year after the mosquito bite. Reinfection means the parasite that caused the past infection was eliminated from the body but a new parasite was introduced. Reinfection cannot readily be distinguished from recrudescence, although recurrence of infection within two weeks of treatment for the initial infection is typically attributed to treatment failure.
Genetic resistance
Genetic resistance to malaria
Due to the high levels of mortality and morbidity caused by malaria—especially the P. falciparum species—it has placed the greatest selective pressure on the human genome in recent history. Several genetic factors provide some resistance to it including sickle cell trait, thalassaemia traits, glucose-6-phosphate dehydrogenase deficiency, and the absence of Duffy antigens on red blood cells.
Liver dysfunction
Liver dysfunction as a result of malaria is rare and is usually a result of a coexisting liver condition such as viral hepatitis or chronic liver disease. The syndrome is sometimes called malarial hepatitis, although inflammation of the liver (hepatitis) does not actually occur. While traditionally considered a rare occurrence, malarial hepatopathy has seen an increase, particularly in Southeast Asia and India. Liver compromise in people with malaria correlates with a greater likelihood of complications and death.
Diagnosis and treatment of malaria
Malaria is diagnosed by looking at blood samples. The parasites are visible under the microscope. Once the diagnosis is made, treatment should be begun promptly. Almost all individuals make complete recovery.
Anti-malarial medication is used both to treat and prevent malaria. The type and duration of drugs depends on the type of malaria, its severity and if the patient is pregnant.
Malaria can sometimes become complicated. Some of the complications include:
- severe anaemia
- cerebral malaria
- malaria during pregnancy
- spleen rupture
- acidosis
- kidney damage
- Multi-organ failure etc.
- These are more common with malaria caused by P. Falciparum
Prevention
An Anopheles mosquito is a vector of malaria, and mosquito control is an effective way of reducing its incidence.
Methods used to prevent malaria include medications, mosquito elimination and the prevention of bites. The presence of malaria in an area requires a combination of high human population density, high mosquito population density and high rates of transmission from humans to mosquitoes and from mosquitoes to humans. If any of these is lowered sufficiently, the parasite will eventually disappear from that area, as happened in North America, Europe and much of the Middle East. However, unless the parasite is eliminated from the whole world, it could become re-established if conditions revert to a combination that favours the parasite's reproduction.
Many researchers argue that prevention of malaria may be more cost-effective than treatment of the disease in the long run, but the capital costs required are out of reach of many of the world's poorest people. There is a wide disparity in the costs of control (i.e. maintenance of low endemicity) and elimination programs between countries. For example, in China—whose government in 2010 announced a strategy to pursue malaria elimination in the Chinese provinces—the required investment is a small proportion of public expenditure on health. In contrast, a similar program in Tanzania would cost an estimated one-fifth of the public health budget.
Vector control
Mosquito control
Man spraying kerosene oil in standing water, Panama Canal Zone 1912
Walls where indoor residual spraying of DDT has been applied. The mosquitoes remain on the wall until they fall down dead on the floor.
Vector control refers to preventative methods used to decrease malaria and morbidity and mortality by reducing the levels of transmission. For individual protection, the most effective chemical insect repellents to reduce human-mosquito contact are those based on DEET and picaridin. Insecticide-treated mosquito nets (ITNs) and indoor residual spraying (IRS) have been shown to be highly effective vector control interventions in preventing malaria morbidity and mortality among children in malaria-endemic settings. IRS is the practice of spraying insecticides on the interior walls of homes in malaria-affected areas. After feeding, many mosquito species rest on a nearby surface while digesting the bloodmeal, so if the walls of dwellings have been coated with insecticides, the resting mosquitoes can be killed before they can bite another victim and transfer the malaria parasite. As of 2006, the World Health Organization advises the use of 12 insecticides in IRS operations, including DDT and the pyrethroids cyfluthrin and deltamethrin. This public health use of small amounts of DDT is permitted under the Stockholm Convention on Persistent Organic Pollutants (POPs), which prohibits the agricultural use of DDT.
One problem with all forms of IRS is insecticide resistance via evolution. Mosquitoes affected by IRS tend to rest and live indoors, and due to the irritation caused by spraying, their descendants tend to rest and live outdoors, meaning that they are less affected by the IRS, which greatly reduces its effectiveness as a defense mechanism.
Mosquito nets create a protective barrier against malaria-carrying mosquitoes that bite at night.
Mosquito nets help keep mosquitoes away from people and significantly reduce infection rates and transmission of malaria. Nets are not a perfect barrier and are often treated with an insecticide designed to kill the mosquito before it has time to find a way past the net. Insecticide-treated nets are estimated to be twice as effective as untreated nets and offer greater than 70% protection compared with no net. Between 2000 and 2008, the use of ITNs saved the lives of an estimated 250,000 infants in Sub-Saharan Africa. Although ITNs prevent malaria, only about 13% of households in Sub-Saharan countries own them. A recommended practice for usage is to hang a large "bed net" above the center of a bed to drape over it completely with the edges tucked in. Pyrethroid- treated nets and long-lasting insecticide-treated nets offer the best personal protection, and are most effective when used from dusk to dawn.
Other methods
Community participation and health education strategies promoting awareness of malaria and the importance of control measures have been successfully used to reduce the incidence of malaria in some areas of the developing world. Recognizing the disease in the early stages can stop the disease from becoming fatal. Education can also inform people to cover over areas of stagnant, still water, such as water tanks that are ideal breeding grounds for the parasite and mosquito, thus cutting down the risk of the transmission between people. This is generally used in urban areas where there are large centers of population in a confined space and transmission would be most likely in these areas. Intermittent preventive therapy is another intervention that has been used successfully to control malaria in pregnant women and infants and in preschool children where transmission is seasonal.
Treatment
Antimalarial medication
The treatment of malaria depends on the severity of the disease. Uncomplicated malaria may be treated with oral medications. The most effective strategy for P. falciparum infection is the use of artemisinins in combination with other antimalarials (known as artemisinin-combination therapy, or ACT), which reduces the ability of the parasite to develop resistance to any single drug component. These additional antimalarials include amodiaquine, lumefantrine, mefloquine or sulfadoxine/pyrimethamine. Another recommended combination is dihydroartemisinin and piperaquine. ACT is about 90% effective when used to treat uncomplicated malaria. To treat malaria during pregnancy, the WHO recommends the use of quinine plus clindamycin early in the pregnancy (1st trimester), and ACT in later stages (2nd and 3rd trimesters). In the 2000s (decade), malaria with partial resistance to artemisins emerged in Southeast Asia.
THE PROBLEM OF DRUG RESISTANCE:
Antimalarial drug resistance has been defined as “ability of a parasite strain to survive and /or multiply despite the administration and absorption of a drug given in doses equal to or higher than those usually recommended but within tolerance of the subject.” This definition was modified to specify that drug in question must gain access to the parasite or the infected red blood cells (RBCs) for the duration of time necessary for its normal action.
Studies involving in vitro assessments of Plasmodium falciparum resistance have shown a high degree of resistance to chloroquine followed by monodesethylamodiaquine as well as absence of resistance to mefloquine and dihydroartesunate. In a comparative efficacy study by Saha et al, PCR-corrected efficacies were AS+SP: 90.6%; amodiaquine-lumefantrine: 95.9% and artemisinin-mefloquine: 100%. Failure rate of AS+SP (9.5%) was attributed to SP failure due to prevailing mutations in the genes plasmodium falciparum dihydrofolate reductase (pfdhfr) and plasmodium falciparum dihydro pteroate synthase (pfdhps).
PROBLEMS WITH ACT:
In order to make best use of Artemisinin based therapies, it is critical to address issues of delivery, access and cost. It is well known that continuous supply of new and affordable drugs along with implementable control measures will help reduce overall burden of the disease. Artemisinin is derived from plant sources, making it expensive. The crop of artemisinin depends on weather and other factors, harvest varies and prices change accordingly. There is potential for mismatch in demand and supply. Another concern is the extent to which components might be used unofficially and incorrectly for monotherapy outside official health services, promoting resistance. These facts point towards a need to identify a new alternative, effective and affordable antimalarial regimen.
Medications
Several drugs, most of which are used for treatment of malaria, can be taken to prevent contracting the disease during travel to endemic areas. Chloroquine may be used where the parasite is still sensitive. Because of the prevalence of drug-resistant Plasmodium, one of three medications—mefloquine (Lariam), doxycycline (available generically), or the combination of atovaquone and proguanil hydrochloride (Malarone)—is frequently needed. Doxycycline and the atovaquone and proguanil combination are the best tolerated; mefloquine is associated with death, suicide, and higher rates of neurological and psychiatric symptoms.
Drugs for treatment of Malaria
- Amodiaquine
- Artemether
- Artemether and Lumefantrine
- Artesunate
- Bulaquine
- Chloroquine
- Halofantrine
- Hydroxychloroquine
- Mefloquine
- Proguanil
- Pyrimethamine – Sulfadoxine
- Quinidine
- Quinine
- Suphamethoxazol Pyrimethamine
SYNRIAM™ INDIA’S FIRST ANTI-MALARIAL DRUG
Ranbaxy has launched an anti-malarial drug on World Malaria Day billed by CEO Arun Sawhney as "India's first new chemical entity developed through clinical trials and commercialization in India". This new drug, partially financed by the Department of Science and Technology of the Government of India, has been approved for marketing by the Drug Controller General of India (DGCI). According to the press release, it "conforms to the recommendations of the World Health Organization (WHO)".
A new drug to treat malaria, Synriam, was launched in India by Ranbaxy Laboratories Limited. The drug will provide additional options for malaria treatment as traditional drugs become increasingly ineffective against the deadly malaria parasite because of acquired resistance to available medications.
Drug Name: Arterolane Maleate and Piperaquine Phosphate Tablets
Brand Name: Synriam
Dosage Form: Tablet
Description
Synriam™ is a fixed dose combination of two antimalarial active ingredients arterolane maleate and piperaquine phosphate.
Arterolane maleate is a synthetic trioxolane compound. The chemical name of arterolane maleate is cis-adamantane-2-spiro-3’-8’-[[[(2’-amino-2’ methylpropyl) amino] carbonyl] methyl] 1’, 2’, 4’-trioxaspiro [4.5] decane hydrogen maleate. The molecular formula is C26H40N2O8 and molecular weight is 508.61.
Generic Name
Arterolane Maleate and Piperaquine Phosphate Tablets
Formula:The structural formula is as follows
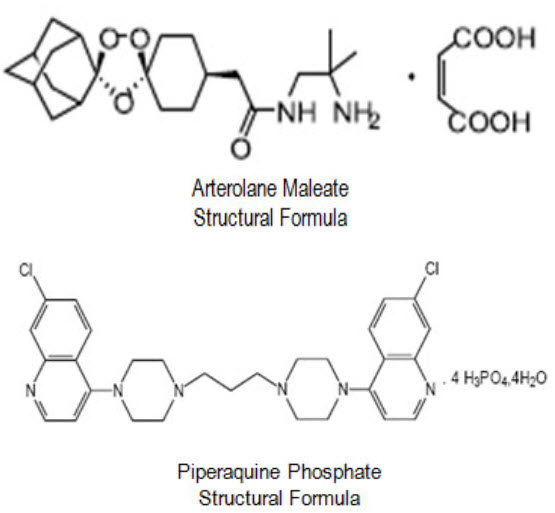
Composition
Each film coated tablet contains:
Arterolane maleate equivalent to
Arterolane ....…………………………...150 mg.
Piperaquine phosphate……..……...........750 mg.
Inactive ingredients
Microcrystalline cellulose, Crospovidone, Magnesium stearate, Hydroxypropyl methyl cellulose/Hypromellose, Titanium dioxide, Macrogol/ Polyethylene glycol, Talc, Ferric Oxide (Yellow), Ferric Oxide (Red).
Property
Arterolane:
Arterolane, also known as OZ277 or RBx 11160, is a substance being tested for antimalarial activity by Ranbaxy Laboratories. It was discovered by US and European scientists who were coordinated by the Medicines for Malaria Venture (MMV). Its molecular structure is uncommon for pharmacological compounds in that it has both an ozonide group and an adamantane substituent.
Phase III clinical trials of arterolane, in combination with piperaquine, began in India in 2009. When clinical trial results were disappointing, the MMV withdrew support and Ranbaxy continued developing the drug combination on its own.
Formula:C22H36N2O4
Molecular Weight:392.53224
Synonyms:OZ277
Density:202 g/cm3
Boiling Point:572.161 °C at 760 mmHg
Flash Point:299.832 °C
Storage:sealed storage in shady and cool warehouse
Assay:99%
Package:Aluminium bag
Piperaquine phosphate:
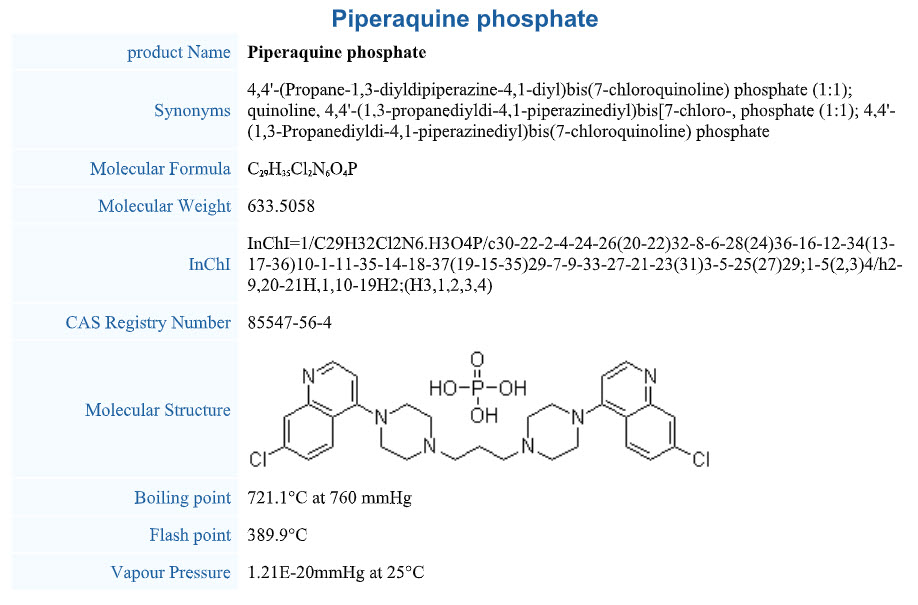
Mode of Action
Arterolane: Mode of action
Arterolane and Piperaquine act as blood schizonticides. Arterolane undergoes reductive cleavage in the food vacuole by ferrous iron to generate free radicals which inhibit PfATP6, a sarcoplasmic endoplasmic reticulum calcium ATPase encoded by P. Falciparum. Being synthetic peroxide anti-malarial, it is a rapidly acting blood schizonticide against all blood stages of P. falciparum without effect on liver stages. Arterolane accumulates in the parasite's food vacuole whereas this is not a feature of the subcellular distribution of artemisinins.
Piperaquine: Mode of Action
Piperaquine is a bisquinoline anti-malarial drug and shows good activity against chloroquine-resistant Plasmodium strains. Evidence suggesting inhibition of the heme-digestion pathway in the parasite food vacuole as the mode of action of Piperaquine is most convincing. Piperaquine's bulky bisquinoline structure may be important for activity against chloroquine resistant strains and may act by an inhibition of the transporters that efflux chloroquine from the parasite food vacuole and by the inhibition of haem-digestion pathway in the parasite food vacuole.
Upon administration of SYNRIAM, arterolane is presumed to have a rapid onset of action and rapid elimination, whereas piperaquine is eliminated slowly and is expected to provide long term cure after short treatment course. Hence, the combination provides rapid clearance of parasitemia and most malaria-related symptoms, coupled with prevention of recrudescence.
Pharmacodynamics of Synriam
Arterolane a synthetic trioxolane rapidly kills the malaria parasite in the blood, providing fast relief from symptoms of malaria. Piperaquine on the other hand has a longer lasting effect than arterolane and kills the residual parasites, preventing the recurrence of malaria. Hence, the combination provides rapid clearance of parasitemia and most malaria-related symptoms, coupled with prevention of recrudescence.
Median Parasite Clearance Time (PCT) with SYNRIAMTM was observed to be 36 hours which indicates rapid parasite clearance.
Median Fever Clearance Time (FCT) with SYNRIAMTM was observed to be 18 hours which indicates faster symptomatic relief.
Pharmacokinetics of Synriam
Both the drugs are well absorbed after oral administration. Peak plasma concentrations are achieved between 3-5 hours post dose for arterolane and 4-6 hours post dose for piperaquine. Arterolane (~93%) and piperaquine (>99%) are highly plasma protein bound with extensive volume of distribution. The major metabolic pathway is the oxidation of the adamantane moiety for arterolane. Overall contribution of CYP3A4 to Arterolane metabolism is 30%. CYP3A4 is the primary isozyme responsible for the metabolism of both arterolane as well as piperaquine. Observed metabolites from liver microsomes are monooxygenation and dioxygenation products for piperaquine. Arterolane is rapidly eliminated from plasma. The terminal half life of arterolane varied from 1 to 3 hrs across the species and piperaquine's individual mean terminal half-lives ranged from 11 to 18 days following single dose administration and 17 to 23 days following multiple dose administration.
Simple dosing regimen
Synriam offers a very simple dosing option of one tablet once daily for three days, thus ensuring high compliance. Since the patient is required to take just 1 tablet per day for 3 days, SYNRIAM minimizes the chances of patients missing out on a dose. Patients with acute malaria are frequently averse to food and food dependent dosing can hamper the efficacy of antimalarials in such patients. SYNRIAM has convenient, one tablet daily dosage independent of food intake. This is an added benefit over arthemeter-lumefantrine regimen, which has to be taken with high fat meals twice a day. This food independent dosing will improve patient convenience and compliance.
Clinical Efficacy and Safety
Efficacy and safety of arterolane maleate and piperaquine phosphate has been evaluated in male and female adult patients with uncomplicated P. falciparum infection in Asia. There have been 13 studies of which 5 studies were carried out in acute uncomplicated falciparum malaria patients.
The results obtained so far indicate that arterolane, is a short acting drug and produces rapid fever and parasite clearance. SYNRIAM provides high clinical efficacy as assessed by PCR corrected ACPR (Adequate Clinical and Parasitological Response), fever clearance time and parasite clearance time. SYNRIAM demonstrated faster median fever clearance time in phase III clinical trial. SYNRIAM has been effective in geographical regions where resistance tochloroquine has been reported. The efficacy of arterolane maleate and piperaquine phosphate has been confirmed in comparative study conducted in India, Thailand and Bangladesh.
In Phase III Clinical trial of Synriam, there were no early treatment failures and SYNRIAM achieved similar PCR corrected ACPR rates to Artemether- lumefantrine on Day 28. SYNRIAM effectively cured P. falciparum malaria and attained acceptable level of cure at Day 28 which consistently met WHO criteria for cure (≥ 95%). The fever clearance time for SYNRIAM is shorter than that of artemether-lumefantrine. SYNRIAM appears to have moderate.
Two comparative randomized trials compared the safety of arterolane maleate + piperaquine phosphate with artemether + lumefantrine. These were active-controlled, phase III randomized, double blind, multicenter trial and phase II, randomized, open-label, multi-centre trial of arterolane maleate and piperaquine phosphate co-administration. Overall, arterolane maleate + piperaquine phosphate was as well tolerated as artemether + lumefantrine, and had a similar safety profile.
Indian company starts Phase III trials of synthetic artemisinin
Ranbaxy Laboratories Limited (Ranbaxy) announced the commencement of Phase-III clinical trials for its new Anti-malaria combination drug, Arterolane maleate + Piperaquine phosphate in India, Bangladesh and Thailand. The drug is targeted at patients in developing countries with the aim of significantly improving upon the conventional options available for the treatment of P. falciparum malaria. Currently available alternatives in the market are either those to which the malaria parasite has developed widespread resistance, making the drug in-effective or newer alternatives that are comparatively expensive and derived from an agricultural source, having limitations on scalability.
The Phase III trial was kicked off by holding an Investigators' Meeting in New Delhi. Speaking on the occasion, chief guest Dr VM Katoch, Secretary, Department of Health Research and Director General, ICMR (Indian Council of Medical Research), lauded the efforts of Ranbaxy in developing a new anti-malaria drug. On the occasion, Dr. Sudershan Arora, President-R&D (Generics, NDDS, Clinical & Drug Development), Ranbaxy, mentioned that Ranbaxy will strive to successfully complete the trial and apply for marketing authorization by late 2010.
Arterolane maleate + Piperaquine phosphate is a synthetic drug and hence easier to manufacture with better predictability and reliability of supplies. The drug is being developed as a once-a-day therapy for three days and will improve patient compliance, besides being safe and efficacious. The available therapy requires an adult to consume 24 tablets over three days whereas Arterolane maleate + Piperaquine phosphate dosage is 1 tablet per day for three days, thereby resulting in lesser pill burden for the patients and reduced cost.
Ranbaxy aims to market the drug in malaria endemic geographies of India, Africa, Latin America and the Asia Pacific.
According to the World Health Organization, estimated 250-300 million cases of malaria occur every year, mainly in developing countries causing approximately 1 million deaths. Anti Malarial research is one of the neglected research areas with very few organizations actively working in this field.
Ranbaxy Laboratories Limited, India's largest pharmaceutical company, is an integrated, research based, international pharmaceutical company producing a wide range of quality, affordable generic medicines, trusted by healthcare professionals and patients across geographies. Ranbaxy's continued focus on R&D has resulted in several approvals in developed markets and significant progress in New Drug Discovery Research. The Company's foray into Novel Drug Delivery Systems has led to proprietary "platform technologies," resulting in a number of products under development. The Company is serving its customers in over 125 countries and has an expanding international portfolio of affiliates, joint ventures and alliances, ground operations in 49 countries and manufacturing operations in 11 countries.
WHO releases new malaria guidelines for treatment and procurement of medicines
The World Health Organization (WHO) is releasing new guidelines on 9 March 2010 Geneva for the treatment of malaria, and the first ever guidance on procuring safe and efficacious anti-malarial medicines.
Guidelines emphasize testing
The Guidelines for the Treatment of Malaria (second edition) provide evidence-based and current recommendations for countries on malaria diagnosis and treatment. The main changes from the first edition of the guidelines (published in 2006) are the emphasis on testing before treating and the addition of a new ACT to the list of recommended treatments.
"The world now has the means to rapidly diagnose malaria and treat it effectively” said Dr Robert Newman, Director of the WHO Global Malaria Programme (GMP). "WHO now recommends diagnostic testing in all cases of suspected malaria. Treatment based on clinical symptoms alone should be reserved for settings where diagnostic tests are not available," he added.
In 2008, just 22% of suspected malaria cases were tested in 18 of 35 African countries reporting. Until now, most clinics had to rely on microscopy, but the recent development of quality-assured Rapid Diagnostic Tests (RDTs) using a dip stick and a drop of blood means a policy change is possible. The tests can reliably demonstrate the presence or absence of malaria parasites in the blood and can be performed at all levels of the health system, including community settings.
Universal diagnostic testing
The move towards universal diagnostic testing of malaria is a critical step forward in the fight against malaria as it will allow for the targeted use of ACTs for those who actually have malaria. The aim is to reduce the emergence and spread of drug resistance and to help identify patients who have fever, but do not have malaria, so that alternative diagnoses can be made and appropriate treatment provided. Therefore, better management of malaria has a positive impact on management of other childhood illness and overall child survival.
WHO is supporting malaria endemic countries to improve the quality of their diagnostic services using both microscopy and RDTs, and urging the manufacturers of RDTs to continue improving the accuracy and quality of these critically important diagnostic tests.
WHO estimates that 80 countries have adopted ACTs for first-line treatment of uncomplicated P. falciparum malaria. In the guidelines, WHO emphasizes the importance of treating this deadliest form of the disease with artemisinin-based combination therapies. WHO has now added a fifth ACT - dihydroartemisinin plus piperaquine - to the previous list of recommended medicines.
Preventing drug resistance
WHO recommends oral artemisinin-based monotherapy should be removed from the market because their use will hasten the development of parasite resistance. Countries need to ensure that patients are diagnosed properly and take the full dose of ACTs to prevent the development of drug resistance.
Procurement quality
The first ever guidelines on Good procurement practices for artemisinin-based antimalarial medicines are based on the newest stringent internationally agreed production and procurement quality standards. This manual aims to improve the capacities of national and international procurement officers in the understanding of key quality elements and required documentation. The content is presented as a practical and concise 16-step practical checklist to guide the selection and procurement of safe and effective medicines meeting international quality standards.
"Pharmaceutical markets in malaria endemic countries are often unregulated and national authorities need practical help to assess the quality of malaria medicines before they buy them" says Dr Andrea Bosman, Coordinator of the Medicines and Diagnostics Unit at GMP. "Procurement channels are highly fragmented and so there are too many antimalarials of varying quality on the market."
Poor-quality medicines affect the health and lives of patients, damage the credibility of health services and, by generating sub-therapeutic drug levels in malaria patients, help develop resistance to this important life-saving class of pharmaceuticals.
"These guidelines will help countries select and procure effective medicines of good quality and save lives by improving the way patients are diagnosed and treated," says Dr George Ki-Zerbo, Malaria Programme Manager at the WHO Regional Office for Africa in Brazzaville.
Half of the world's population is at risk from malaria. Each year almost 250 million cases occur, causing 860 000 deaths. Approximately 85% of these deaths are among children, and most occur in Africa.
CONCLUSION
Artemisinin based combination therapy (ACT) is the first-line treatment for uncomplicated falciparum malaria. The current study assessed the antimalarial efficacy and safety of a combination of arterolane maleate and piperaquine phosphate in patients with acute uncomplicated P. falciparum malaria. Drug resistance has been implicated in the spread of malaria to new areas and re-emergence of malaria in areas where the disease had been eradicated. Use of ACT has shown success in this preventable and treatable disease but resistance to it is emerging, creating the need for new therapies. The fixed dose combination of arterolane (150 mg) and piperaquine phosphate (750 mg) combines a rapidly acting parasiticidal drug with a short acting one that eliminates the residual parasites. It is a completely synthetic drug that ensures regular availability at affordable prices for wide access. The convenient short course treatment encourages compliance. These advantages make this new age antimalarial a welcome addition to the therapeutic arsenal against Plasmodium falciparum malaria.
References
1. Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Avalilable at US National Library of MedicineNational Institutes of Health Pubmed.gov.
2. Gautam A, Ahmed T, Sharma P, Pharmacokinetics and Pharmacodynamics of Arterolane Maleate Following Multiple Oral Doses in Adult Patients With P. Falciparum Malaria, availiable at Journal of Clinical Pharmacology (Dec 2010).
3. Rijken MJ, McGready R, Boel ME, Poespoprodjo R, Singh N, Syafruddin D, Rogerson S, Neston F (2012). "Malaria in pregnancy in the Asia-Pacific region". Lancet Infectious Diseases 12 (1): 75–88. Doi: 10.1016/S1473-3099(11)70315-2. PMID 22192132.
4. Fairhurst RM, Wellems TE (2010). "Chapter 275.Plasmodium species (malaria)". In Mandell GL, Bennett JE, Dolin R (eds). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases 2 (7th ed.). Philadelphia, Pennsylvania: Churchill Livingstone/Elsevier. pp. 3437–3462.ISBN 978-0-443-06839-3.
5. Hartman TK, Rogerson SJ, Fischer PR (2010). "The impact of maternal malaria on newborns". Annals of Tropical Paediatrics 30 (4): 271–82.Doi: 10.1179/146532810X12858955921032.PMID 21118620.
6. Bloland P. Drug resistance in malaria. Available from www.who.int/csr/resources/publications/drugresist/m alaria.pdf. Last cited on Jan 30, 2013.
7. Anvikar AR, Sharma B, Sharma SK, Ghosh SK, Bhatt RM, Kumar A, et al. In vitro assessment of drug resistance in Plasmodium falciparum in five States of India. Indian J Med Res 2012; 135:494-9.
8. Nosten, F. and White NJ. Artemisinin-based combination treatment of P. falciparum malaria. Am J Trop Med Hyg. 2007; 77(6): 181-192.
9. Indian company starts Phase III trials of synthetic artemisinin, May 4 2009, WorldWide Antimalarial Resistance.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









