About Authors:
Vemavarapu Satish kumar*, Shahin1 , Saarangi Ramesh2
*IPQC team member at GRANULES INDIA LIMITED, M.Pharmacy (pharmaceutics) Deevena college of pharmacy.
1Shadan women’s college of pharmacy. khairtabad, Hyderabad. A.P
2asst.prof.pharmaceutical chemistry, Prasad institute of pharmaceutical sciences. jangaon, warangal. A.P
*sattisha333@gmail.com
1 GEOGRAPHICAL SETTING
1.1 Ficus religiosa
Origin of the tree is not really known to anybody, but, there are also some interesting legends associated with the Peepal tree. The peepal is the first-known depicted tree in India. A seal discovered at MohenjDaro, one of the cities of the Indus Valley Civilisation (c. 3000 BC - 1700 BC), shows the peepal being worshipped. Excavations are suggestive of the fact that even in those times; the peepal tree was worshipped by Hindus.
Peepal is native from India to southeast Asia and it is found wild and cultivated upto 5000 feet. Ficus religiosa is cultivated in various tropical areas of the world. It is grown in southern California, Florida and Hawaii, Homestead and Miami in the United States.
Regardless of its origin, the tree needs lots of space, and the soil must be deep enough to let the roots grow down a long way. It is a large tree of about 20 m. heights with a well developed crown. It can grow in a wide variety of soils and it grows in a sub tropical climate with hot summers and frost during the monsoon season.
REFERENCE ID: PHARMATUTOR-ART-1920
1.2 The peepal Tree
The tree
Peepal tree is one of the most familiar trees in India. It grows very fast. The roots are attached to the trunk as if they are pillars supporting it. The trunk of the tree is irregularly shaped with low buttresses. Its bark is light gray and peels in patches. This is a tree that reaches very large proportions and it is the largest of our indigenous fig trees. In its younger stages it is often epiphytic, that is, it grows on other trees, which are gradually strangled by its rope-like roots. Or the tree may grow in cracks on walls, which are slowly but inexorably cracked and split open by the growing roots. The Peepal tree in Sri Lanka is believed to be 2147 years old. It is one of the longest living trees of the world. The peepal is resistant to drought and frost.
Leaves
The leaves and young branches are smooth, shiny, somewhat leathery, and broadly oval in shape and suddenly narrowed at the apex into a long tail and the base is rounded or heart-shaped. The leaf has a solid middle nerve, which deserves attention. In addition there are 5—9 lateral pairs which unite at their ends to form a wavy line near the margin of the leaf. The leaves are generally pendulous, that is, hanging down. The long pointed leaf tips help to drain water off the leaves and dry the tree after rainfall. They are shed in March and April and in some areas in the autumn months. When the new leaves appear they are often pink and darken to copper and then green in colour.
Flowers
The flowers are hidden within the figs. Figs come out in pairs at the angle between the leaf stalk and the branch; at first they are green and smooth, finally they turn purple when ripe; each fig contains a few male flowers near the opening at the apex; each flower consists of a single stamen supported by three minute colourless ‘petals’. The female flower consists of five ‘petals’ enclose a pistil.
Fruits
The fruits are known as figs. These figs ripen in May and June generally, but one can find this fruit throughout the year depending on the areas. The fig wasp is a visitor to these fruits as well as the Banyan figs. Birds and bats are rather fond of the Peepal fruit and the seeds pass out undigested and are scattered all over the country and start growing from a gutter or the wall of a house. Under conditions of sufficient moisture, such seeds germinate in the most unlikely places. The tiny plant gets all its food from the air and water but uses the wall or gutter as a support.
1.3 common names in various languages
Typical shape of the leaf of the Ficus Religiosa
The Ficus religiosa tree is known by a wide range of vernacular names in different locales and languages, including:

1.4 nomenclature & classification
The peepal tree belongs to the Moraceae commonly known as fig family – one of the most well-known members of the plant families. It belongs to the dicot order. Members of the genus Ficus are usually treated as separate tribe within the family Moraceae because of their unique inflorescence and wasp-dependent system of pollination. There are about 40 genera and 1000 species that have been described in this family, nearly all with milky sap and mostly found in tropical and subtropical regions, less common in temperate climates. Ficus religiosa Linn. is the Latin name or binomial name of peepal tree.
This tree which reaches very large proportions; it is in fact about the largest of our indigenous fig trees. Peepal grows in northern and central India, in forests and alongside water. It is also widely cultivated throughout the subcontinent and south-east Asia, especially in the vicinity of the temples.
Scientific classification
Kingdom: Plantae
Division: Magnoliophyta
Class: Magnoliopsida
Order: Rosales
Family: Moraceae
Genus: Ficus
Species: F. religiosa
Binomial name: Ficus religiosa
Found In: Ranthambore Wildlife Sanctuary
1.5 taxonomy & morphology
taxonomy
Family: Moraceae
Latin name: Ficus religiosa L
Synonyms: None known.
Common names: Bo tree, beepul tree, sacred tree
Nomenclature: This sacred tree is associated with Buddha and is planted beside temples, hence the species name, religiosa.
DESCRIPTION
"Small tree, or taller strangling climber, with wide-spreading branches, semi- or fully deciduous in monsoon climates, and broadly ovate, glossy, leathery, dark green leaves, 5- 7 in (12-18 cm) long, with unusual tail-like tips. Bears pairs of rounded, flat-topped green figs, to 1/2 in (1.5 cm) across, ripening to purple with red dots" .
Morphology and microscopy
Morphology
F. religiosa is a large deciduous tree with few or no aerial roots. It is often epiphytic with the drooping branches bearing long petioled, ovate, cordate shiny leaves. Leaves are bright green, the apex produced into a linear-lanceolate tail about half as long as the main portion of the blade. The receptacles occurring in pairs and are axillary, depressed globose, smooth and purplish when ripe. The bark is flat or slightly curved, varying from 5 to 8 mm in thickness, outer surface is grey or ash with thin or membranous flakes and is often covered with crustose lichen brown or ash coloured, surface has shallow irregular vertical fissures and uneven due to exfoliation of cork, inner surface smooth, yellowish to orange brown and fibrous.
Microscopy
An external features of bark of F. religiosa showed that bark differentiated into outer thick periderm and inner secondary phloem. Periderm is differentiated into phellem and phelloderm. Phellem zone is 360 mm thick and it is wavy and uneven in transection. Phellem cells are organized into thin tangential membranous layers and the older layers exfoliate in the form of thin membranes. The phelloderm zone is broad and distinct. Phelloderm cells are turned into lignified sclereids. Secondary phloem differentiated into inner narrow non-collapsed zone and outer broad collapsed zone. Non-collapsed zone consists of radial files of sieve tube members, axial parenchyma, and gelatinous fibres. Outer collapsed phloem has dilated rays, crushed obliterated sieve tube members, thick walled and lignified fibres, and abundant tannin filled parenchyma cells. Laticifers are fairly abundant in the outer secondary phloem zone. Phloem rays are both uniseriate and multiseriate.
Physical constants: Total ash 7.86 % w/w, acid insoluble ash 0.41 % w/w, alcohol soluble
Extract 7.21 % w/w and water soluble extractive 15.76 % w/w.
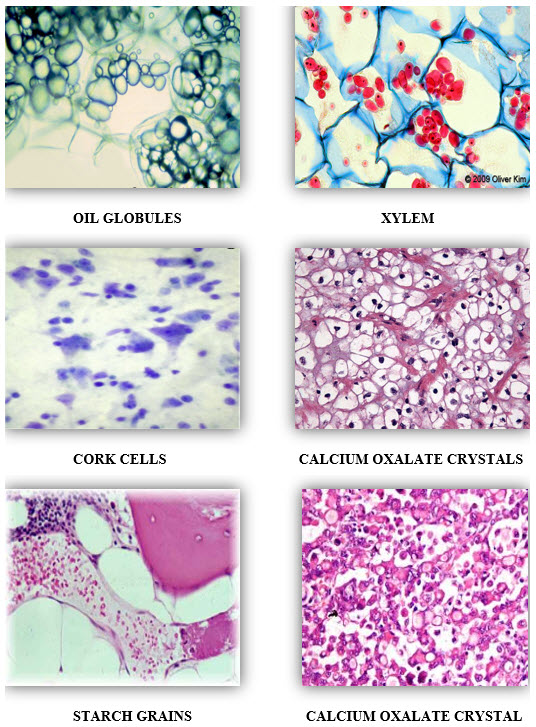
MICROSCOPICALLY FICUS RELIGIOSA CONSIST OF FOLLOWING CHARACTERS :-
1. STARCH GRAINS
2. CALCIUM OXALATE CRYSTALS
3. OIL GLOBULES
4. XYLEM
5. CORK CELLS
6. PHLOEM FIBRES
7. VASCULAR BUNDLES
8. PARENCHYMATOUS CELLS
9. BISERIATE MEDULLARY RAYS.
1.6 BOTANICAL DESCRIPTION
Ficus religiosa is an evergreen or deciduous tree, 20 m tall and 1.5-2 m dbh, irregularly-shaped, with wide-spreading branches and without aerial roots from the branches. The trunk is regularly shaped, often with low buttresses. Bark is grey with brownish specks, smooth, exfoliating in irregular rounded flakes. Leaves alternate, spirally arranged and broadly ovate, glossy, coriaceous (leathery), dark green leaves, 10-18 by 7.5-10 cm, with unusual tail-like tips, pink when young, stipulate, base-cordate. Petioles is slender and 7.5- 10 cm long. Galls on leaves. Flowers axillary sessile, unisexual. Figs in pairs, rounded, flat-topped green, to 1.5 cm across, axillary, sessile, smooth, ripening to purple with red dots, basal bracts 3 and broad. The specific epithet ‘religiosa’ alludes to the religious significance attached to this tree. The prince Siddhartha is said to have sat and meditated under this tree and there found enlightment from which time he became a Buddha.The tree is therefore sacred to Buddhists and is planted beside temples.



1.7 BIOLOGY & ECOLOGY
Cultivation: F. religiosa is widely planted in the tropics (Bailey and Bailey 1976). The tree is very long lived and one tree near Bombay is reported to be over 3,000 years old (Neal 1965). F. religiosa are mostly planted near Buddhist temples. They are steeped in legends associated with Buddha and are also sacred to Vishnu who is also said to have been born beneath a bo tree. Hindus associate the bo tree with fertility in women. It is also cultivated as an ornamental, for medicinal uses, such as toothaches, and in the making of shellac.
Invasiveness: This species reported to be able to set viable seeds in two places, Israel and Florida. In Israel, the pollinator wasp successfully invaded and established allowing the tree to begin to spread. In Florida, sporadic seeding events have been documented; though have not persisted, perhaps due to an unsuccessful colonization of the associated pollinator wasp or an intrusion from a pollinator wasp of the native Ficus aurea (Nadel et al. 1992).
Pollination: The fruit (syconium or fig) and reproduction systems of species in the genus Ficus are unique. Each species of Ficus has an associated species of agaonid wasp (Hymenoptera: Chalcoidea: Agaonidae). Ficus species can only be pollinated by their associated agaonid wasps and in turn, the wasps can only lay eggs within their associated Ficus fruit. The pollinator wasp for F. religiosa is Blastophaga quadraticeps.
Propagation: In places where the pollinator wasp is not present, trees are propagated from cuttings.
Dispersal: In Hawai'i, plants are spread mainly through horticulture trade. Various birds observed foraging and roosting in Ficus spp. trees on Maui that could be potential dispersal agents of F. religiosa seeds should they become viable include mynah birds (Acridotheres tristis tristis), blue faced doves (Geopelia striata), lace necked doves (Streptopelia chinensis), Japanese white-eye (Zosterops japonicus), Northern cardinals (Cardinalis cardinalis), and house sparrows
(Passer domesticus), though there are probably more. Other animals, such as bats, pigs, rodents, parrots, and monkeys may be capable of spreading fruit.
Pests and Diseases: Nadel et al. (1992) report several pests including various ants which were seen carrying off pollinator wasps from Ficus fruits, Hymenoptera and mites that may be parasites of the pollinator wasps, and staphylinids which were seen entering Ficus fruits and eating the pollinator wasps.
2. IMPORTANCE OF FICUS RELIGIOSA
2.1medicinal purpose
Peepal tree is of great medicinal value. Its leaves serve as a wonderful laxative as well as tonic for the body. It is especially useful for patients suffering from Jaundice. It helps to control the excessive amount of urine released during jaundice. The leaves of Peepal are highly effective in treating heart disorders. It helps to control the palpitation of heart and thereby combat the cardiac weakness. Ayurveda makes an extensive use of the leaves of peepal due to the numerous benefits it provides.
For constipation problem, there can be no better remedy than the consumption of leaves of Peepal. Dry the Peepal leaves in sun and powder them. Add a solution of jaggery and anise to it. Mix it with waterand consume it. This concoction will ensure proper bowel movement. The Indian basil peepal works wonders in treating dysentery. Prepare a mixture of grinded coriander leaves, peepal leaves and sugar and chew it slowly
Pipal leaves are of great use in getting rid of mumps. All one needs to do to avail the benefits of peepal plant is smear the leaves of Peepal with ghee and then warm it on low flame. After that, bandage it over the swollen inflamed part of the body. It is surely going to provide the patient with a great relief. Even for boils, this remedy will prove to be quite effective. In case of formation of pus, bandaging the leaves of Peepal will ensure that the growth subsides. But, it will give beneficial results only if the problem is in its preliminary stage
Alternative and Complementary Medicinal Uses:
The barks of F. religiosa is an important ingredient in many Ayurvedic formulations, such as
- Nalpamaradi tailam,
- Chandanasavam,
- Nyagrodhadi churna and
- Saribadyasavam.
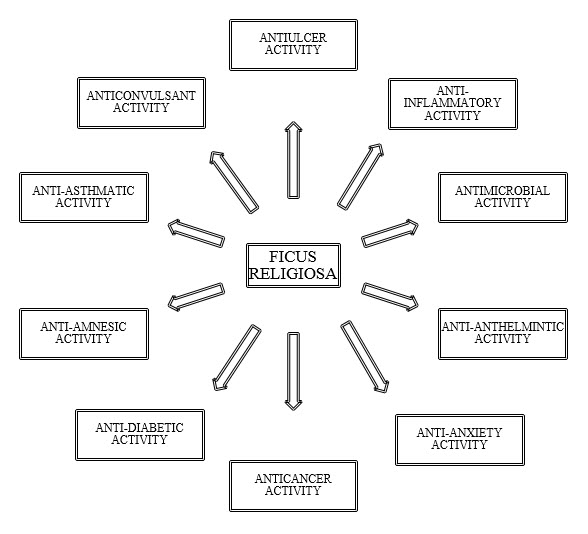
Figure-1.1 Pharmacological activities of Ficus religiosa
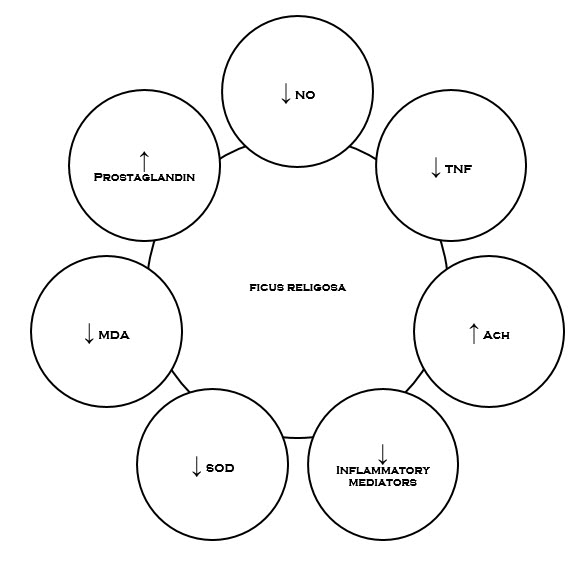
Figure-1.2Possible targets of Ficus religiosa involved in the management of various disorders.
2.2 TRADITIONAL USES
Ficus religiosa has been extensively used in traditional medicine for a wide range of ailments. Its bark, fruits, leaves, roots, latex and seeds are medicinally used in different forms, sometimes in combination with other herbs (Aiyegoro and Okoh, 2009).
Leaves
The leaves alone are used to treat constipation. The leaves used together with young shoots are act as strong laxative. In Nepal leaf juice with honey is used for multipurpose such as for diarrhoea, asthma, cough, earache, toothache, and migraine, in gastric problems and in haematuria (Kunwar and Bussmann, 2006). In addition, the leaves of Ficus religiosa have also shown significant memory enhancing activity (Devi et al., 2011).
Bark
The Bark is cooling and astringent and is useful in inflammation and glandular swellings of neck. The paste of powdered bark is used in cases of anal fistula and as absorbent for inflammatory swellings and also used in burns (Nadkarni, 1954,
Warrier et al., 1995). The bark of Ficus religiosa is reported to possess antiulcer and wound healing activities (Khan et al., 2011, Kalyon et al., 2009). It is used in diabetes, diarrhoea, leucorrhoea, anxiety, for vaginal and other urinogenital disorders and to improve the complexion (Pandit et al., 2010, Ratnasooriya et al., 1998).
Fruit
The seeds and fruits are digestive, laxative and refrigerant. The dried fruit, pulverized and taken in water for a fortnight removes asthma. The ripe fruit is cold in potency and good for burning sensation. It act as cardiac tonic and is useful to cure the diseases of Vagina. It also cures vomiting, anorexia and edema (Singh, 2006). The fruit extract of plant have anti tumour and antibacterial activity (Sirisha et al., 2010).
2.3 PHARMACOLOGICAL ACTIVITIES
The Whole parts of the plant exhibit wide spectrum ofactivities such as anticancer, antioxidant, anti diabetic,antimicrobial, anticonvulsant, anthelmintic, antiulcer,antiasthmatic, anti amnesic etc.
Antiulcer activity
The term ‘peptic ulcer’ describes a condition in whichthere is a discontinuity in the entire thickness of the gastric or duodenal mucosa that persists as a result of acid and pepsin in the gastric juice. The principal pathological condition in which it is useful to reduce acid secretion is peptic ulceration (both duodenal and gastric) & reflux oesophagitis. Therapy of peptic ulcers with commercially available antiulcer drugs is usually overshadowed by various side effects. Thus there is a need to find new antiulcerogeniccompound(s) with potentially less or no side effects. One of theplants that have been traditionally used in the India and Malaysfolklore medicine to treat gastric ulcer is Ficus religiosa, The ethanol extract of stem bark of Ficus religiosaextract exhibited potential antiulcer activity. The antiulceractivity of Ficus religiosa was evaluated in vivo againstindomethacin and cold restrained stress induced gastric ulcers andpylorus ligation assay. The determination of antiulcer effect wasbased upon the reduction of ulcer index. The extract (100, 200 &400 mg/kg) significantly reduced the ulcer index in all assay used.The hydroalcoholic extract of leaves of Ficusreligiosa also exhibited antiulcer activity. The activity of extract was evaluated against pylorusligation-induced ulcers, ethanol-induced ulcers and aspirin-Inducedulcers
Anti - inflammatory activity
Inflammation is the body’s immediate response to damage to its tissue & cells by pathogens, noxious stimuli such as chemicals or physical injury. It is a protective attempt by the organism to remove the injurious stimuli and initiate the healing process. The various mediators involved in inflammation include cytokines & chemokines, PG’s, platelet activating factor (PAF), NO and histamine etc. PG’s are generally considered to be potent pro inflammatory mediator. Further, evidence suggests that during inflammation there is increased generation of ROS. It has been found that Mast cell degranulation also imparts a role in inflammation due to release of several mediators like Histamine, which are implicated in the inflammation and allergy. Ficus religiosa has found to be potential anti-inflammatory & analgesic property.
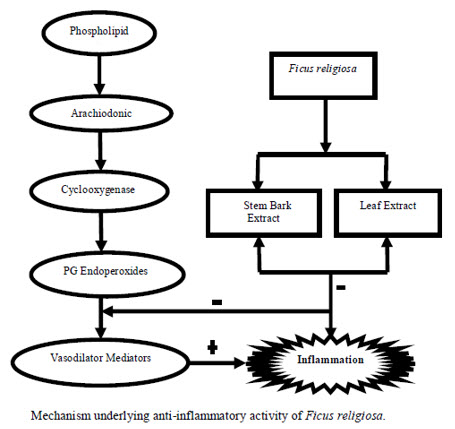
Figure 1.3
The mechanism underlying the effect is the inhibition of PG’s synthesis. It was found that the leaf extract of Ficus religiosa has potential anti-inflammatory activity against carrageenan induced paw oedema. The inhibitory activity was found due to inhibition of release of histamine, serotonin (5HT), Kinins and PG’s. The methanol extract of stem bark of Ficus religiosa has inhibitory effect on carrageenan-induced inflammation in rats due to the inhibition of the enzyme cycylooxygenase (COX) leading to inhibition of PG’s synthesis. Further, various studies revealed that tannin present in the bark possess anti-inflammatory effect.As already discussed mast cell degranulation cause inflammation and Ficus religiosa extract significantly reduced the percentage Of degranulation.
Anthelmintic activity
Ficus religiosa have been used to treat the parasitic infections in man and animals. Iqbal el al investigated the anthelmintic effect of methanolic bark extract of F. religiosa on the adult Haemonchus contortus Worm. Adult motile H. Contortus was collected from the gastrointestinal tract of sheep slaughtered at Faisalabad slaughterhouse. It was found that ficin is responsible for the anthelmintic effect in the methanolic extract of F. religiosa (Akhatar et al., 2000). Further, studies show that the aqueous extract of fruit of F. religiosa has shown potent Anthelmintic activity as compared to other species of Ficus against Pheretima posthuma (earthworms) Stem and bark extract of Ficus religiosa was also found lethal to Ascaridia galli (Parasitic worm belonging to phylum nematoda) . Anticonvulsant activity It is well recognised that serotonergic neurotransmission modulates a wide variety of experimentally induced seizures in the brain and is involved in seizure protection by altering various GABAergic and glutamatergic functions. In Ayurveda it is claimed that leaves of Ficus religiosa also possess anticonvulsant activity.
The anticonvulsant effect of the extract obtained from the leaves of Ficus Religiosa was evaluated against PTZ (60mg/kg, i.p) induced convulsion in albino rats. The extract was evaluated against strychnine-induced convulsions and pentylenetetrazole-induced convulsions animal models. It was found that figs of the plant contain a high serotonergic content. it was investigated that ethanol extract of leaves of Ficus religiosa have memory enhancing activity. The preliminary phytochemical screening and TLC analysis of the leaf extract of F. religiosa showed the presence of sterols, glycosides, tannins and amino acids.
According to this study the chloroform extract of the leaves of Ficus religiosa inhibited the growth of various Salmonella species, P. vulgaris, E. coli, B. Subtilis and K. Pneumonia etc which revealed the antibacterial potential of the plant. According to another study different extracts (methanol, aqueous, chloroform) of the bark of Ficus religiosa has inhibitory effect on the growth of three enteroxigenic E.coli, isolated fromthe patients suffering from diarrhea.
2.4 Phytochemistry
Preliminary phytochemical screening of F. religiosa barks, showed the presence tannins, saponins, flavonoids, steroids, terpenoids and cardiac glycosides. The barks of F.religiosa showed the presence of bergapten, bergaptol, lanosterol, β-sitosterol, stigmasterol, Lupen-3-one, β-sitosterol-d-glucoside (phytosterolin), vitamin k1. The bark also contains tannin, wax, saponin, β-sitosterol,leucocyanidin-3-0-β-D-glucopyrancoside,leucopelargonidin-3-0-β-glucopyranoside, leucopelargonidin-3-0-α-L- rhamnopyranoside, lupeol, ceryl behenate, lupeol acetate, α-amyrin acetate, leucoanthocyanidin and leucoanthocyanin. Leaves yield campestrol, stigmasterol, isofucosterol, α-amyrin, lupeol, tannic acid, arginine, serine, asparticacid, glycine, threonine, alanine, proline, tryptophan, tryosine, methionine, valine, isoleucine, leucine, n-nonacosane, n-hentricontanen, hexa-cosanol and n-octacosan. The fruit of F.religiosa contains asgaragine, tyrosine, undecane, tridecane, tetradecane, (e)-β-ocimene, α-thujene, α-pinene, β-pinene, α-terpinene, limonene, dendrolasine, dendrolasine α-ylangene, α-copaene, β-bourbonene, β-caryophyllene, α-trans bergamotene, aromadendrene,in seeds of F. religiosa. The crude latex of F.religiosa shows the presence of a serine protease, named religiosin.
Main constituents:
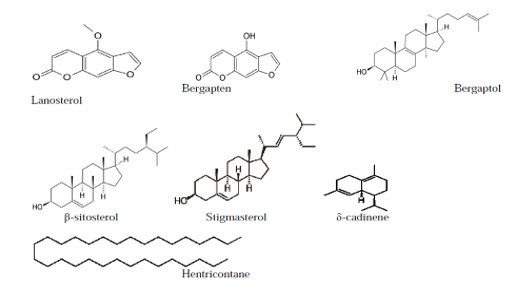
Figure 1.4
3. Review literature
Review On Ethanomedicinal And Pharmacological Properties Of Ficus Religiosa
Amandeep Kaur, A. C. Rana, Vineeta Tiwari, Ramica Sharma and Sunil Kumar
Ficus religiosa (Bo tree) is the most popular member of the genus Ficus, commonly named as Peepal. Various parts of the plant, like bark, fruit, leaves and seeds are widely used in indigenous system of medicine. F. religiosa showed a wide spectrum of pharmacological activities like, anticonvulsant, anthelmintic, anti-amnesic, anti-anxiety, anti-asthmatic, antibacterial, antioxidant, anti-inflammatory and antiulcer. The present review is an attempt to provide a detailed survey of the literature on traditional uses and pharmacological properties of the plant.
Phytochemistry and Pharmacological properties of Ficus religiosa
Inder Kumar Makhija*, Indra Prakash Sharma, DevangKhamar.Manipal.P.h sciences.
Ficus religiosa Linn is a large evergreen tree found throughout India, wild as well as cultivated. It is popular indigenous system of medicine like Ayurveda, Siddha, Unani and Homeopathy. In traditional system of medicine, various parts such as stem bark, root bark aerial roots, vegetative buds, leaves, fruits and latex are used in diabetes, vomiting, burns, gynaecological problems, dysentery, diarrhea, nervous disorders, tonic and astringent. Phytochemical investigation of plant barks, showed the presence tannins, saponins, flavonoids, steroids, terpenoids and cardiac glycosides. According to Ayurvedic system of medicine, F. religiosa (Peepal tree) is well known to be useful in diabetes. The present work is an attempt to compile an up-to-date and comprehensive review of F. religiosa that covers its ethnobotanical, natural product chemistry, pharmacological data.
Anti-ulcer activities of Ficus religiosa stem bark ethanolic Extract in rats
Mohammed Safwan Ali Khan, Syed Ahmed Hussain,Malaysia
Ficus religiosa is being used in Ayurvedic and Malay traditional medicine for the treatment of various diseases including gastric ulcer. Considering the above claims, the present work was undertaken to validate the anti-ulcer potential of the ethanol extract of stem bark of F. religiosa against in vivo indomethacin- and cold restrained stress-induced gastric ulcer, and pylorus ligation assays. The extract (100, 200 and 400 mg/kg) significantly (P<0.05) reduced the ulcer index in all assays used. The extract also significantly (P<0.05) and increased the pH of gastric acid while at the same time reduced the volume of gastric juice and, free and total acidities.
Wound Healing Potential of Leaf Extracts of Ficus Religiosa on Wistar albino strain rats
Kalyon Roy*, H.Shivakumar ,Sibaji Sarkar S.C.S College of pharmacy, Harpanahalli,Karnataka.
Ficus religiosa (Family- Moraceae) which is commonly known as Pepal tree, is abundantly distribute throughout in India. Ficus religiosa leaf is reported to have wound healing, inflammatory, analgesic, anti lipid- peroxidation activity. Hence the present study was aimed to investigate the wound healing activity by excision and incision wound models to evaluate the wound-healing activity of Ficus religiosa extracts, prepared as ointment form (5 and 10%) and applied on Wistar albino strain rats of either sex. Povidine iodine 5% was used as Standard drug. The healing of the wound was assessed by the rate of wound contraction, period of epithelialisation, skin breaking strength. Both the extracts as ointments (5% and 10%) of Ficus religiosa leaf extract promoted the wound-healing activity significantly in all the wound models studied. High rate of wound contraction, decrease in the period for epithelialisation, high skin breaking strength were observed in animals treated with 10% leaf extract ointment when compared to the control group of animals. So leaf extracts of Ficus religiosa in the form of 10% ointment promote wound-healing activity better than the former concentration, 5%.
4. AIM AND OBJECTIVE
Ficus religiosa have been used to treat the parasitic infections in man and animals.The Whole parts of the plant exhibit wide spectrum ofactivities such as anticancer, antioxidant, anti diabetic,antimicrobial, anticonvulsant, anthelmintic, antiulcer,antiasthmatic, anti amnesic etc.
* The main aim of our study is to identify new phytochemicals by bark of ficus religiosa.
* To study the T.L.C profile of chloroform, aqueous and organic layer extracts of ficus religiosa.
* To study the pharmacognostic studies of.
A) T.S of bark B) Analysis Of Bark Powder Of Ficus Religiosa.
* To Study The Biological Activity (Analgesic activity) Of Ficus Religiosa by TAIL IMMERSION METHOD.
5. EXPERIMENTAL WORKS
5.1 introduction to chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures. The mixture is dissolved in a fluid called the "mobile phase", which carries it through a structure holding another material called the "stationary phase". The various constituents of the mixture travel at different speeds, causing them to separate. The separation is based on differential partitioning between the mobile and stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus changing the separation.
Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of a mixture for more advanced use (and is thus a form of purification). Analytical chromatography is done normally with smaller amounts of material and is for measuring the relative proportions of analytes in a mixture. The two are not mutually exclusive.
Chromatography can be described as the process where analytes are separated due to their varying distribution between two phases, a stationary one (stationary phase) and one that moves (mobile phase). Compounds traveling in the mobile phase interact with the stationary phase. Those that are strongly retained by the stationary phase move slowly, while those that interact only weakly move rapidly. Compounds that move rapidly are thereby separated from the compounds that move slowly. Most chromatography uses a stationary phase inside a column but the use of flat sheets of mobile phase (thin layer chromatography) are also used.
Chromatography is a powerful separation tool that is used in all branches of science, and is often the only means of separating components from complex mixtures. Many types of chromatography have been developed. High performance liquid chromatography (HPLC) is an extremely versatile technique where analytes are separated by passage through a column packed with micrometer-sized particles. Gas chromatography separates compounds based on their volatility and their adsorption or solubility in the stationary phase. Size exclusion chromatography separates molecules based on their size by passing the sample through a porous structure. Ions can be separated based on their chemical charge using ion exchange chromatography. Other types of chromatography include electrophoresis and supercritical fluid chromatography.
The stationary phase can also take two forms, solid and liquid, which provides two subgroups of GC and LC, namely; gas–solid chromatography (GSC) and gas–liquid chromatography (GLC), together with liquid solid chromatography (LSC) and liquid chromatography (LLC). The different forms of chromatography are summarized bellow. Most thin layer chromatography techniques are considered liquid-solid systems although the solute normally interacts with a liquid-like surface coating on the adsorbent or support or, in some cases an actual liquid coating.
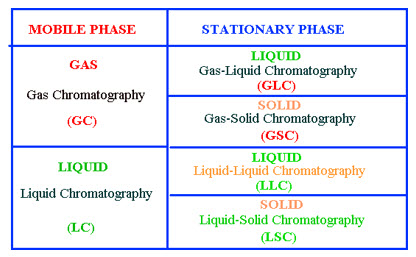
Figure1.5 Classification of Chromatography
Thin layer chromatography is used to separate components of a plant extract, illustrating the experiment with plant pigments that gave chromatogram.
- A chromatograph is equipment that enables a sophisticated separation e.g. gas chromatographic or liquid chromatographic separation.
- Chromatographyis a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while the other (the mobile phase) moves in a definite direction.
- The eluate is the mobile phase leaving the column.
- The eluent is the solvent that will carry the analyte.
- An eluotropic series is a list of solvents ranked according to their eluting power.
- An immobilized phase is a stationary phase which is immobilized on the support particles, or on the inner wall of the column tubing.
- The mobile phase is the phase which moves in a definite direction. It may be a liquid (LC and Capillary Electro chromatography (CEC)), a gas (GC), or a supercritical fluid (supercritical-fluid chromatography, SFC). The mobile phase consists of the sample being separated/analyzed and the solvent that moves the sample through the column. In the case of HPLC the mobile phase consists of a non-polar solvent(s) such as hexane in normal phase or polar solvents in reverse phase chromotagraphy and the sample being separated
- Preparative chromatographyis used to purify sufficient quantities of a substance for further use, rather than analysis.
- The retention timeis the characteristic time it takes for a particular analyte to pass through the system (from the column inlet to the detector) under set conditions. See also: Kovats' retention index
- The solute refers to the sample components in partition chromatography.
- The solvent refers to any substance capable of solubilizing another substance, and especially the liquid mobile phase in liquid chromatography.
- The stationary phaseis the substance which is fixed in place for the chromatography procedure. Examples include the silica layer in thin layer chromatography
Paper chromatography Is an analytical method technique for separating and identifying mixtures that are or can be colored, especially pigments. This can also be used in secondary or primary colors in ink experiments. This method has been largely replaced by thin layerchromatography; however it is still a powerful teaching tool. Two-way paper chromatography, also called two-dimensional chromatography, involves using two solvents and rotating the paper 90° in between. This is useful for separating complex mixtures of similar compounds, for example, amino acids.
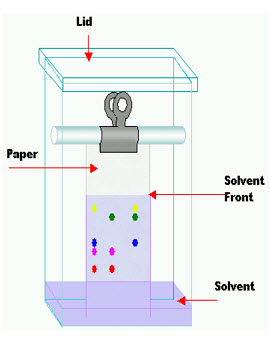
Figure1.6 PAPER CHROMATOGRAPHY
Rƒ value
The retention factor (Rƒ) may be defined as the ratio of the distance traveled by the substance to the distance traveled by the solvent. Rƒ values are usually expressed as a fraction of two decimal places but it was suggested by Smith that a percentage figure should be used instead. If Rƒ value of a solution is zero, the solute remains in the stationary phase and thus it is immobile. If Rƒ value = 1 then the solute has no affinity for the stationary phase and travels with the solvent front. To calculate the Rƒ value, take the distance traveled by the substance divided by the distance traveled by the solvent (as mentioned earlier in terms of ratios). For example, if a compound travels 2.1 cm and the solvent front travels 2.8 cm, (2.1/2.8) the Rƒ value = 0.75
Thin layer chromatography
TLC is a chromatography technique used to separate mixtures. Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminum oxide, or cellulose (blotter paper). This layer of adsorbent is known as the stationary phase.
After the sample has been applied on the plate, a solvent or solvent mixture (known as the mobile phase) is drawn up the plate via capillary action. Because different analytes ascend the TLC plate at different rates, separation is achieve.

Figure1.7 Thin Layer Chromatogram
Thin layer chromatography can be used to monitor the progress of a reaction, identify compounds present in a given mixture, and determine the purity of a substance. Specific examples of these applications include: analyzing ceramides and fatty acids, detection of pesticides or insecticides in food and water, analyzing the dye composition of fibers in forensics , assaying the radiochemical purity of radiopharmaceuticals, or identification of medicinal plants and their constituents
A number of enhancements can be made to the original method to automate the different steps, to increase the resolution achieved with TLC and to allow more accurate quantization. This method is referred to as HPTLC, or "high performance TLC".
Plate preparation
TLC plates are usually commercially available, with standard particle size ranges to improve reproducibility. They are prepared by mixing the adsorbent, such as silica gel, with a small amount of inert binder like calcium sulfate (gypsum) and water. This mixture is spread as a thick slurry on an unreactive carrier sheet, usually glass, thick aluminum foil, or plastic. The resultant plate is dried and activated by heating in an oven for thirty minutes at 110 °C. The thickness of the adsorbent layer is typically around 0.1 – 0.25 mm for analytical purposes and around 0.5 – 2.0 mm for preparative TLC.
The process is similar to paper chromatography with the advantage of faster runs, better separations, and the choice between different stationary phases. Because of its simplicity and speed TLC is often used for monitoring chemical reactions and for the qualitative analysis of reaction products.
Figure1.8 Ellution Of Compounds On T.L.C
To run a thin layer chromatography, the following procedure is carried out:
A small spot of solution containing the sample is applied to a plate, about 1.5 centimeters from the bottom edge. The solvent is allowed to completely evaporate off, otherwise a very poor or no separation will be achieved
- A small amount of an appropriate solvent (elutant) is poured in to a glass beaker or any other suitable transparent container (separation chamber) to a depth of less than 1 centimeter. A strip of filter paper (aka "wick") is put into the chamber, so that its bottom touches the solvent, and the paper lies on the chamber wall and reaches almost to the top of the container. The container is closed with a cover glass or any other lid and is left for a few minutes to let the solvent vapors ascend the filter paper and saturate the air in the chamber. (Failure to saturate the chamber will result in poor separation and non-reproducible results).
- The TLC plate is then placed in the chamber so that the spot(s) of the sample do not touch the surface of the elutant in the chamber, and the lid is closed. The solvent moves up the plate by capillary action, meets the sample mixture and carries it up the plate (elutes the sample). When the solvent front reaches no higher than the top of the filter paper in the chamber, the plate should be removed (continuation of the elution will give a misleading result) and dried.
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability of the stationary phase used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling.
The classical preparative chromatography column, is a glass tube with a diameter from 5 mm to 50 mm and a height of 5 cm to 1 m with a tap and some kind of a filter (a glass frit or glass wool plug – to prevent the loss of the stationary phase) at the bottom. Two methods are generally used to prepare a column: the dry method, and the wet method.
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound
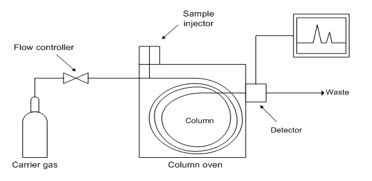
Figure1.9 GAS CHROMATOGAPHY
In gas chromatography, the mobile phase (or "moving phase") is a carrier gas, usually an inert gas such as helium or an uncreative gas such as nitrogen. The stationary phase is a microscopic layer of liquid or polymer on an inert solid support, inside a piece of glass or metal tubing called a column (an homage to the fractionating column used in distillation). The instrument used to perform gas chromatography is called a gas chromatograph (or "aerograph", "gas separator").
The gaseous compounds being analyzed interact with the walls of the column, which is coated with different stationary phases. This causes each compound to elute at a different time, known as the retention time of the compound. The comparison of retention times is what gives GC its analytical usefulness.Gas chromatography is in principle similar to column chromatography (as well as other forms of chromatography, such as HPLC, TLC), but has several notable differences. Firstly, the process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas mobile phase, whereas in column chromatography the stationary phase is a solid and the mobile phase is a liquid. Secondly, the column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled, whereas column chromatography (typically) has no such temperature control. Thirdly, the concentration of a compound in the gas phase is solely a function of the vapor pressure of the gas.Gas chromatography is also sometimes known as vapour-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Strictly speaking, GLPC is the most correct terminology, and is thus preferred by many authors.
High-performance liquid chromatography
(sometimes referred to as high-pressure liquid chromatography), HPLC, is a chromatographic technique used to separate a mixture of compounds in analytical chemistry and biochemistry with the purpose of identifying, quantifying and purifying the individual components of the mixture. Some common examples are the separation and quantitation of performance enhancement drugs (e.g. steroids) in urine samples, or of vitamin D levels in serum.HPLC typically utilizes different types of stationary phases (i.e. sorbents) contained in columns, a pump that moves the mobile phase and sample components through the column, and a detector capable of providing characteristic retention times for the sample components and area counts reflecting the amount of each analyte passing through the detector.
5.2 METHODOLOGY
Collection of plant material
The plant material was collected from local area of Janagon, Warangal in MARCH 2012 and was authenticated at the Department of Chemistry, Prasad Institute of Pharmaceutical Sciences.
Preparation of the Extract
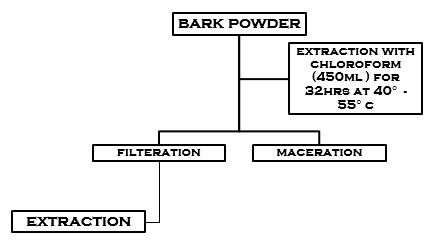
Stem barks of Ficus religiosa Were collected and were dried at room temperature (37°c). It was powdered and sieved through muslin cloth. The preparation of extract was carried out to the method of extraction briefly, the stem bark of F. religiosa was shade dried after collection for 5 days and was powdered. Approximately 0.95 kg of powdered drug material was extracted using 450ml Chloroform in RBF of Soxhlet apparatus (Janagon, Warangal, India). The powdered bark was filled in condenser with appropriate weighing, then it has been fixed to the RBF. Boil at 40-55°c for 32hr.
Experimental animal
Albino Wistar rats weighing 150 to 200g were used in the present study. The experimental animals were maintained under standard laboratory conditions in an animal house approved by the committee for the purpose of control and supervision on experiments on animals (CPCSEA) under 12 h light/dark cycle and controlled temperature (24 ± 2°C) and fed with commercial pellet diet and water. All animals were acclimatized to the laboratory environment for at least one week before the commencement of experiment. The experimental protocol was approved by the Institutional Animal Ethical Committee, Prasad institute of pharmaceutical sciences, jangaon, Warangal.
PROCEDURE
The aqueous and alcoholic extract of stem bark of Ficus religiosa .Were prepared and used for analgesic study. The study was conducted by “Tail Immersion Method” described by Ghosh M.N., (1984.) six healthy Wistar rats weighing around150-200 gms were distributed in 3 groups each consisting of 2 rats with 1:1 sex ratio..
The tail of the rats up to 5 cm was dipped into hot water maintained at 55±0.5°c. The time taken by the rat to flip its tail clearly out of water was taken as the reaction time. The reaction time in seconds before administration of the drugs was recorded for all the rats from each group. Each extract was studied in two different doses of 100and 200 mg/Kg b.w. The group T1 served as control which received 0.1 ml of normal saline only. T2 servedas reference standard to which Analgin was given @10mg/kg b.w. The group T3 & T4 was given with aqueous extract @100&200 mg/kg respectively where as T5&T6 was given with alcoholic extract at same dose respectively.
Intra abdomen route was followed for the administration of the extract. Reaction time in seconds after the administration of drugs was recorded at an interval of 15min, and 30 min. Mean was compared with Analgin. The data obtained was analyzed statistically to know the level of significance as per the method described by Snedecor and Cochran.
5.3 PHYTOCHEMICAL IDENTIFICATION
There are simple but standard chemical tests to detect the presence of alkaloids, tannins, saponins, anthraquinones, cardenolides etc in a plant extract. Each time the operator is undertaking the plant phytochemical screening, there is always the need to carry out confirmatory tests because one can experience false-positive reactions in some non-alkaloidal extracts. Plants are subject to physiological changes before extraction, if the plants are not extracted on the day of collection and this could invariably affect the phytochemical screening results.
METHODS OF TESTING FOR BIOACTIVE PROPERTIES OF MEDICINAL PLANTS
A] Tests for Alkaloids
In testing for Alkaloids,that about 0.5g of each extract will be stirred with 5ml of I per cent aqueous hydrochloric acid on a water bath; 1ml of the filtrate is to be treated with a few drops of mayer's reagent and a second 1ml portion is to be treated the same way with Dragendorff's reagent.
Turbidity of precipitation with either of those reagents was taken as preliminary evidence for the presence of alkaloids in the extract being evaluated (Harborne, 1973; Evans, 2002). Some laboratories also use Wagner's reagent, picric acid solution or tannic acid solution in place of or in addition to, the two reagents mentioned about (Persiones and Quimby, 1967).
A confirmatory test designed to remove non-alkaliodal compounds capable of eliciting "false-positive" reactions is to be carried out as fallous with all extracts which give preliminary positive test for alkaloids.
A modified form of the thin-layer chromatography (TLC) method described by farnsworth and Euler (1962) is to be used. One gramme of the extract will be treated with 40 per cent calcium hydroxide until the extract is distinctly alkaline to litmus paper, and then extract twice with 10ml portions of chloroform. The extracts are to be combined and concentrated in vacuo to about 5ml. The chloroform extract is then spotted on thin-layer plates. Four different solvent systems (of widely varying polarity) are to be used to develop each plant extract. The presence of alkaloids in the developed chromotograms will therefore be detected by spraying the chromatograms with freshly prepared Dragendorff's spray reagent.
A positive reaction on the chromatograms (indicated by an orange or darker-coloured spot against a pale yellow background) is confirmatory evidence that the plant extract contained an alkaloid.
B] Tests for Saponins
The ability of saponins to produce frothing in agueous solution and to haemolyse red blood cells is used as screening test for these compounds.
About 0.5g of each extract is to be boiled briefly with 50ml phosphate butter, PH 7.4, and then allowed to cool and filtered, 5ml of the filtrate is to be passed for 3 hr. through an asbestos disc (1.5mm thick about 7mm in diameter), which has been previously soaked with two or three drops of 1 per cent cholesterol in ether and dried.
After filtration, the disc should be washed with 0.5ml of distilled water, dried and boiled in 20ml of oxylol for 2hrs to decompose the complex formed between cholesterol and any saponins in the extract. The disc should then be washed in ether, dried and placed on 7 percent blood nutrient ager. Complete heamolysis of red blood-cells around the disc after 6hr. is taken as further evidence of presence of saponins.
C] Test for Tannins
About 5g of each portion of plant extract will be stirred with 10ml distilled water, filtered, and ferric chloride reagent will then be added to the filtrate. A blue-black, green or blue-green-precipitate is taken as evidence for the presence of tannins (Evans 2002).
D] Test for Phlobatannins
Deposition of a red precipitate when an aqueous extract of the plant part was boiled with 1 per cent aqueous hydrochloric acid was taken as evidence for the presence of phlobotannins (Evans, 2002).
E] Test for Anthraquinones
Borntrager's test is used for the detection of anthraquinones. 5g of each plant extract is to be shaken with 10ml benzene, filtered and 5ml of 10 per cent ammonia solution added to the filtrate. The mixture is to be shaken and the presence of a pink, red or violet colour in the ammoniacal (lower) phase indicates the presence of free hydroxyl-anthraquinones.
For bound anthraquionones, 5g of each plant extract is to be boiled with 10ml aqueous sulphuric acid and filtered while hot. The filtrate was shaken with 5ml of benzene, the benzene layer separated and half its own volume of 10 percent ammonia solution added. A pink, or violet coloration in the ammonia phase (lower layer) indicates the presence of anthroquinones derivatives in the extract (Evans, 2002).
F] Test for Cardiac Glycosides
Legal test
The extract is to be dissolved in pryridine and a few drops of 2 per cent sodium nitorprusside together with a few drops of 20 per cent NaOH are to be added. A deep red colour which faded to a brownish yellow indicates the presence of cardenoloides.
Kedde test
1ml of an 8 per cent solution of the extract in methanol will be mixed with 1ml of a 2 per cent solution 3, 5-dinitrobenzoic acid in methanol and 1ml of a 5.7 percent aqueous sodium hydroxide. An immediate violet colour will indicate the presence of cardenolides in the extract, the colour fading gradually through reddish-brown to brownish-yellow with the precipitation of a whitish crystalline solid. The test indicates the presence of a lactone ring in the cardenolide.
Lieberman's test
0.5g of the extract will be dissolved in 2ml of acetic anhydride and cooled well in ice sulphuric acid was then carefully added. A colour change from violet to blue to green will indicate the presence of a steroidal nucleus (i.e. aglycone partion of the cardiac glycoside) (Shoppee, 1964).
Salkowski test
0.5 of the extract will be dissolved in 2ml of chloroform. Sulpuric acid is then carefully added to form a lower layer. A reddish-brown colour at the interface will indicate the presence of a steroidal ring (i.e. aglycone portion of the cardiac glycoside).
Keller kiliani test
0.5 of extract will be dissolved in 2ml of glacial acetic acid containing one drop of ferric chloride solution. This will then be underlayed with 1ml of concentrated sulphuric acid.
A brown ring obtained at the interface will indicate the presence of a desoxy sugar characteristic of cardenolides. A violet ring may appear below the brown ring while, in the acetic acid layer a greenish ring may form just above the brown ring and gradually spread throughout this layer (Evans 2002).
Identification of amino acids by chromatography lab
Introduction
Chromatography is a common technique used by biochemists in separating and identifying different amino acids and helps to reveal the function of cell organelles. Chromatography is particularly approved for its accuracy in distinguishing between each compound, which it does by separating the chemicals according to their Relative Molecular Mass (RMM). The term was introduced in 1906 by Mikhail Tswett and is derived from the Greek words 'chroma´, meaning colour and 'graphein´, meaning to draw. The most popular type of chromatography employs either absorbent paper, or a dried, thin layer of powder on a glass or plastic base.
There are 20 naturally occurring amino acids. The generalised structure of an amino acid is NH2CHRCOOH.
This consists of an amine group (NH2), carboxylic acid group (COOH) and a distinctive R group bonded to the ?-carbon atom. The R group (or 'side chain´) varies in size, shape, charge, hydrogen bonding, capacity and chemical reactivity. The simplest structure is glycine, which has only an extra hydrogen atom in the side chain. Proteins consist of long chains of amino acids which are held together by chemical linkages called peptide bonds
In this experiment, albumen will be used as the chosen protein. Albumen is globular and has a simple structure. Trypsin will be added (the enzyme functioning in the hydrolysis reaction) to the test tube and it will be given a 24 hour incubation at the ideal temperature of 30oC. The trypsin should then have broken up all the albumen into the separate amino acids.
Aim: To separate and identify a mixture of amino acids by paper chromatography.
Method: Cut a piece of chromatography paper to about 25cms in length and place on a clean surface. To avoid contamination, hold the paper at the top and wear plastic gloves throughout the whole experiment. Draw a line in pencil three centimetres from the bottom of the paper and then four marks lightly along the line at four centimetre intervals.
Using a micropipette, take the prepared albumen from the test tube and lightly dot a small amount on each pencil mark
The paper should then by wafted swiftly over a blue flame to speed evaporation. This process should then be repeated 40 times, applying the same amount of albumen to the same area, to build up a concentration. This is necessary for the chromatogram results to be clear and distinct. Always remain standing and be careful with the evaporation process, as it is very easy for the
Table 1.1 IDENTIFICATION TESTS FOR AMINOACIDS
|
S NO |
Test |
Observation |
Remarks |
|
1. |
BIURET REACTION- To 2mL of the test solution add 2mL of 10% NaOH. Mix. Add two drops of 0.1% CuSO4 solution |
Violet or pink color |
Compounds with two or more peptide bonds give a violet color with alkaline copper sulphate solution |
|
2. |
NINHYDRIN TEST- To 4mL of the solution which should be at neutral pH add 1mL of 0.1% freshly prepared ninhydrin solution. Mix the contents and boil for a couple of minutes. Allow to cool |
Violet or purple color |
The ninhydrin test is answered by amino acids and proteins. The formation of a complex called Rheumann’s purple due to condensation of two molecules of nin-hydrin with one molecule of ammonia from amino acid is responsible for the violet color. The a-amino group is the reactive group |
|
3. |
XANTHOPROTEIC REACTION- |
On adding acid, yellow color will be noticed. When NaOH is added deep orange color will develop |
The yellow color is due to the nitro derivatives of the aromatic amino acids present in the protein. The sodium salts of nitro derivatives are orange in color. |
|
4. |
GLYOXYLIC REACTION FOR TRYPTOPHAN - (Hopkins-Cole test) |
Violet ring is formed at the junction |
The indole group of tryptophan reacts with the glyoxylic acid released by the action of conc. H2SO4 on acetic acid to give a purple color. |
|
5. |
SAKAGUCHI REACTION- |
Intense red color |
The guanidine group or arginine reacts with a-Naphthol to form a bright red colored complex. |
|
6. |
SULPHUR TEST- To 2mL of solution add 2mL of 40% NaOH and 10 drops of 2% lead acetate solution. Boil for a minute and cool. |
Black precipitate |
The sulphur in sulphur containing amino acids of the proteins in presence of NaOH, is changed into Na2S which forms black lead sulphide when reacted with lead acetate. |
|
7. |
MODIFIED MILLION’S TEST- |
Yellow precipitate |
The yellow precipitate is due to the precipitation of protein. Mercury combines with tyrosine of the protein. |
|
b) Cool under a tap water and add a drop of 1% NaNO2 solution and warm gently |
A red color develops |
The red color is due to reaction of the precipitate with the nitrous acid. |
There are several general reagents, which may be used to test the presence of alkaloids or to help their identification. This includes the alkaloidal precipitating reagents and the alkaloidal coloring reagents. In addition, there are some special reagent that can be used for recognizing and confirming the identity of each alkaloid
Table 1.2 ALKALOIDAL PRECIPITATING REAGENTS
|
ALKALOIDAL PRECIPITATING REAGENTS |
COMPOSITION |
|
potassiomercuric iodide solution |
|
solution of iodine in potassium iodide |
|
potassium bismuth iodide |
TABLE 1.3 ALKALOIDAL COLOURING REAGENTS
|
ALKALOIDAL COLOURING REAGENTS |
COMPOSITION |
|
Formaldehyde-sulfuric acid |
|
sulphovanadic acid |
|
Nitric acid-sulfuric acid |
TABLE 1.4 REACTIONS OF CARBOHYDRATES
|
Experiment Observation Remarks |
Observation |
Remarks |
|
1. Molisch’s Test- Add two drops of Molisch’s reagent (5% 1-naphthol in alcohol) to about 2 mL of test solution and mix well. Incline the tube and add about 1 mL of concentrated sulphuric acid along the sides of the tube. Observe the colour at the junction of the two liquids. |
A red-cum-violet ring appears at the junction of the two liquids. |
The colour formed is due to the reaction of alpha-naphthol with furfural and/or its derivatives formed by the dehydration of sugars by concentrated sulphuric acid. All carbohydrates react positively with this reagent. |
|
2. Iodine Test- Add a few drops of iodine solution to about 1 mL of the test solution. |
Appearance of deep blue colour. |
This indicates the presence of starch in the solution. The blue colour is due to the formation of starch-iodine complex. |
|
3. Fehling’s Test- To 1 mL of Fehling’s solution ‘A’, add 1 mL of Fehling’s solution ‘B’ and a few drops of the test solution. Boil for a few minutes. |
Formation of yellow or brownish-red precipitate. |
The blue alkaline cupric hydroxide present in Fehling’s solution, when heated in the presence of reducing sugars, gets reduced to yellow or red cuprous oxide and it gets precipitated. Hence, formation of the coloured precipitate indicates the presence of reducing sugars in the test solution. |
|
4. Benedict’s Test- To 2 mL of Benedict’s reagent add five drops of the test solution. Boil for five minutes in a water bath. Cool the solution. |
Formation of red, yellow or green colour/precipitate. |
As in Fehling’s test, the reducing sugars because of having potentially free aldehyde or keto group reduce cupric hydroxide in alkaline solution to red coloured cuprous oxide. Depending on the sugar concentration yellow to green colour is developed. |
|
5. Barfoed’s Test- To 1 mL of the test solution add about 2 mL of Barfoed’s reagent. Boil it for one minute and allow to stand for a few minutes. |
Formation of brick-red precipitate. |
Only monosaccharides answer this test. Since Barfoed’s reagent is weakly acidic, it is reduced only by monosaccharides. |
|
6. Seliwanoff’s Test- To 2 mL of Seliwanoff’s reagent add two drops of test solution and heat the mixture to just boiling. |
Appearance of deep red colour. |
In concentrated HCl, ketoses undergo dehydration to yield furfural derivatives more rapidly than do aldoses. These derivatives form complexes with resorcinol to yield deep red colour. It is a timed colour reaction specific for ketoses. |
|
7. Bial’s Test- To 5 mL of Bial’s reagent add 2–3 mL of solution and warm gently. When bubbles rise to the surface cool under the tap. |
Appearance of green colour or precipitate. |
It is specific for pentoses. They get converted to furfural. In the presence of ferric ion orcinol and furfural condense to yield a coloured product. |
|
8. Test for non-reducing sugars such as sucrose- (a) Do Benedict’s test with the test solution. (b) Add 5 drops of concentrated HCl to 5 mL of test solution in another test tube. Heat for five minutes on a boiling water bath. Add 10% sodium hydroxide solution to give a slightly alkaline solution (test with red litmus paper). Now perform Benedict’s test with this hydrolysed solution. |
No characteristic colour formation. Appearance of red or yellow colour. |
Indicates the absence of reducing sugars in the given solution. Indicates the formation of reducing sugars from non-reducing sugars after hydrolysis with acid. |
|
9. Mucic Acid Test- Add a few drops of conc. HNO3 to the concentrated test solution or substance directly and evaporate it over a boiling water bath till the acid fumes are expelled. Add a few drops of water and leave it overnight. |
Formation of crystals. |
The both end carbon groups are oxidized to carboxylic groups. The resultant saccharic acid of galactose is called mucic acid which is insoluble in water. |
|
10. Osazone Test- To 0.5 g of phenylhydrazine hydrochloride add 0.1 g of sodium acetate and 10 drops of glacial acetic acid. To this mixture add 5 mL of test solution and heat on a boiling water bath for about half an hour. Allow the tube to cool slowly and examine the crystals under a microscope. |
Glucose, fructose and mannose produce needle-shaped yellow osazone crystals, whereas lactosazone is mushroomshaped. Different osazones show crystals of different shapes. Maltose produces flower-shaped crystals. |
The ketoses and aldoses react with phenylhydrazine to produce a phenylhydrazone which in turn reacts with another two molecules of phenylhydrazine to form the osazone |
RESULTS AND DISCUSSIONS
6.1 TLC PROFILE OF CHLOROFORM EXTRACT
|
solvent composition |
chloroform extract |
|
|
chloroform : ethyl acetate |
uv chamber |
iodine chamber |
|
50:50 |
rf = 0.82 |
no result |
|
75:25 |
rf = 0.95 |
no result |
table 1.5
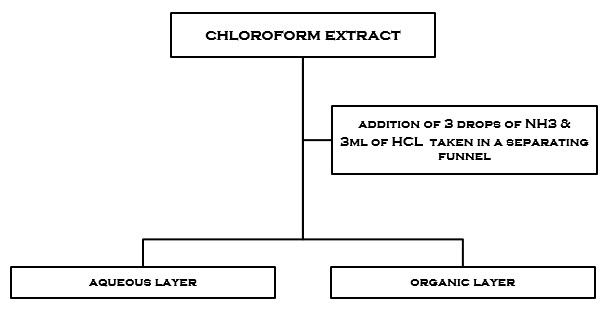
figure2.1 preparation of aqueous and organic layers
6.2 TLC PROFILE OF AQUEOUS AND ORGANIC LAYERS OF CHLOROFORM EXTRACT
|
solvent |
Aq. Extract |
Org. extract |
Inference |
|
Butanol : acetic acid: water 4:1:5 |
RF=1.0 |
RF=0.9 |
CARDIAC GLYCOSIDES, SAPONINS |
|
Chloroform : ethyl acetate 50:50 |
RF=0.8 |
RF=1.0 |
ALKLOIDS,AMINOACIDS, FLAVANOIDS |
|
Chloroform : ethyl acetate 75:25 |
RF=1.0 |
RF=0.8 |
COUMARIN DERIVATIVES,GLYCOSIDES, ALKALOIDS,AMINO ACIDS |
|
Chloroform : methanol : water 7:4:1 |
RF=0.78 |
RF=0.7 |
SAPONINS,TERPENES,ALKALOIDS,AMINOACIDS |
table 1.6
6.3 RESULTS OF PHYTOCHEMICAL TESTS
TABLE 1.7
|
S.NO |
NAME OF THE TEST |
INFERENCE (chloroform extract) |
|
|
TESTS FOR SUGARS |
|
|
1 |
FEHLINGS TEST FOR SUGARS |
POSSITIVE |
|
2 |
BENEDICTS TEST FOR SUGARS |
POSSITIVE |
|
3 |
BARFOEDS TEST FOR SUGARS |
NEAGRIVE |
|
4 |
SELIWANOFFS TEST FOR SUGARS |
NEGATIVE |
|
5 |
TEST FOR GUMS |
POSSITIVE |
|
6 |
TEST FOR PROTEINS-BIURET TEST |
POSSITIVE |
|
7 |
PRECIPITATION TEST FOR PROTEINS |
POSSITIVE |
|
|
TESTS FOR AMINOACIDS |
|
|
8 |
NINHYDRIN TEST FOR AMINO ACIDS |
NEGATIVE |
|
9 |
TEST FOR TYROSINE |
NEGATIVE |
|
10 |
TEST FOR TRYPTOPHAN |
NEGATIVE |
|
11 |
TES FOR CYSTEINE |
NEGATIVE |
|
12 |
SOLUBILITY TEST FOR FATS AND OILS |
POSSITIVE |
|
|
TESTS FOR STEROIDS |
|
|
13 |
SALKOWSKI TEST FOR STEROIDS |
POSSITIVE |
|
14 |
LIEBERMANN-BURCHARD TEST FOR STEROIDS |
POSSITIVE |
|
15 |
TEST FOR VOLATILE OILS |
POSSITIVE |
|
|
TESTS FOR GLYCOSIDES |
|
|
16 |
TEST FOR CARDIAC GLYCOSIDES |
POSSITIVE |
|
17 |
TEST FOR ANTHRAQUINONE GLYCOSIDES |
POSSITIVE |
|
18 |
TEST FOR CYANOGENETIC GLYCOSIDES |
POSSITIVE |
|
19 |
TEST FOR SAPONIN GLYCOSIDES |
POSSITIVE |
|
20 |
TEST FOR COUMARIN GLYCOSIDES |
POSSITIVE |
|
|
TEST FOR ALKALOIDS |
|
|
21 |
DRAGENDROFFS TEST |
POSSITIVE |
|
22 |
MAYERS TEST |
POSSITIVE |
|
23 |
HAGERS TEST |
POSSITIVE |
|
24 |
WAGNERS TEST |
POSSITIVE |
|
|
TESTS FOR TANNINS AND PHENOLIC COMPOUNDS |
POSSITIVE |
|
25 |
LEAD ACETATE SOLUTION |
POSSITIVE |
|
26 |
GELATIN SOLUTION |
POSSITIVE |
|
27 |
BROMINE WATER |
POSSITIVE |
|
28 |
ACETIC ACID SOLUTION |
NEGATIVE |
|
29 |
POTASSIUM DICHROMATE SOLUTIOON |
POSSITIVE |
|
30 |
DILUTE IODINE SOLUTION |
POSSITIVE |
|
31 |
DILUTE NITRIC ACID |
POSSITIVE |
|
32 |
DILUTE POTASSIUM PERAMNGANATE SOLUTION |
POSSITIVE |
|
|
TESTS FOR VITAMINS |
|
|
33 |
TEST FOR VITAMIN-A |
POSSITIVE |
|
34 |
TEST FOR VITAMIN-C(ASCORBIC ACID) |
POSSITIVE |
6.4 results of tail immersion method
table 1.8
|
S.NO |
CONTROL |
STANDARD |
STANDARD |
TEST |
TEST |
|
|
|
15 min |
30 min |
15 min |
30 min |
|
1 |
6 |
7.5 |
12 |
7 |
9 |
|
2 |
6 |
8.5 |
10 |
7 |
8 |
|
3 |
5.5 |
7.5 |
12 |
6 |
7 |
|
4 |
6 |
8 |
10 |
7 |
8 |
|
5 |
7 |
7.5 |
12 |
6.5 |
9 |
|
6 |
5 |
8 |
11 |
7 |
9 |
7. Summary and conclusion
CHAPTER -1
Deals with introduction of ficus religiosa common names, nomenclature, taxonomy, morphology and physical description about family moraceae and genus ficus botanical description, biology, ecology and, propagation, pollination, developmental anatomy of leaf and chemical constituents.
CHAPTER-2
Contains medicinal uses, traditional uses, pharmacological activities and phytochemical studies.
CHAPTER-3
Contains all about literature review of ficus religiosa.
CHAPTER-4
Contains aim and objective.
CHAPTER-5
conatins chromatography techniques, methodology and procedure of phytochemical identification tests.
CHAPTER-6
contains all about results and discussions that has been done. it is sub divided into three parts (part-a, part-b and part-c).
PART-A
Deals with phytochemical studies,which includes the following collection of plant material,extraction process of bark powder,tlc profile of chloroform, aqueous and organic extracts and we idendified several classes of phytochemical constituents; alkaloids, aminoacids, glycosides,tannins, coumarin glycosides, carbo hydrates, saponins, essential oils, flavanoids.This chemical constituents were identified by chemical tests and tlc profile.
PART-B
Includes pharmacognostic studies; study of t.s of bark and analysis of bark powder,which has carried out to see the arrangement of cells,it was observed that the bark is having a 3 layers-
Cortex,cork,starch granules,calcium oxalate crystals,oil globules,cylindrical vessels,
PART-C
Includes the study of biological activity (analgesic activity by tail method)
By this experiment I conformed that the peepal tree bark having moderate analgesic activity.
8. BIBLIOGRAPHY
- www.ficusrelliiosa.com
- Benefits of Peepal
- https://www.facebook.com/PharmaRiserss
- "Peepul". Encyclopedia Americana.
- Sacred fig description
- Entry on peepal tree in the Buddhist Dictionary of Pali Proper Names
- "Bo-Tree". Encyclopædia Britannica.
- Ahuja D., Bijjem K. R., Kalia A. N. Bronchospasm potentiating effect of methanolic extract of Ficus religiosa fruits in guinea pigs. J Ethnopharmacol. 2011; 133(2): 324-8.
- Aiyegoro, O. A, Okoh, A. I. Use of bioactive plant products in combination with standard antibiotics: implications in antimicrobial chemotherapy. Journal of Medicinal Plants. 2011; 3: 1147-1152.
- https://www.facebook.com/PharmaRiserss
- Akhtar M. S., Iqbal Z, Khan M. N, Lateef M. Anthelmintic activity of medicinal plants with particular reference to their use in animals
- Bagdy G., Kecskemeti V., Riba P., Jakus R. Serotonin and epilepsy. J. Neurochem. 2009; 100: 857-873. Bansal V. K., Goyal S. K., Goswami D. S et al. Herbal approach to Peptic ulcer disease- 13. A Review. J Biosci Tech. 2009; 1 (1): 52-58.
- Bliebtrau, J. N. The Parable of the Beast. Macmillan Company,
- .Buhot M. S., Martin S and Segu L. Role of serotonin in memory impairment. Ann Med. 2000; 32: 210-221.
- Charde R. M., Dhongade H. J., Charde M. S and Kasture A. V.
- .Evaluation of antioxidant, wound healing and anti-inflammatory activity of ethanolic extract of leaves of Ficus religiosa. International Journal of Pharma Sciences and Research. 2010; 1: 73-82. Devi W. B., Sengottuvela S., Haja S. S., Lalitha V., Sivakumar T. Memory enhancing activities of Ficus religiosa leaves in rodents.
- International Journal of Research in Ayurveda and Pharmacy. 2011; 2(3): 834-838.
- Dr. Amrit Pal Singh. Panca Ksira Vrksa (Ficus Species Used in Ayurvedic Medicine). Ethnobotanical Leaflets. 2006; 10: 329-335. Hata A. N., Breyer R M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune systeM
- Arulmozhi. S. and Sathiya, N.L., Phcog. Rev., 2007, 1, 163-170.
- Ghani, A., Medicinal plants of Bangladesh with chemical constituents and uses, Asiatic
- Society of Bangladesh, Dhaka, 1998, 236.
- Singh, D. and Goel, R.K., J. Ethanopharmacol., 2009, 123, 330-334.
- Prasad, P.V., Subhaktha, P.K., Narayana, A. and Rao, M.M., Bull. Indian Inst. Hist. Med.
- Hyderabad, 2006, 36, 1-20.
- microscopic slides for ficus religiosa - Google Search
- Govindarajan, R., Pushpangadan, P. and Vijayakumar, M., J. Ethnopharmacol., 2005,
- 165.
- Arora, D., Dubey, S.D. and Ojha, J.K., J. Diab. Assoc 2000, 39, 47.
- [7] Warrier, P.K., Indian medicinal plants-A compendium of 500 species, Orient Longman Ltd.,
- Chennai, Vol. III, 1996, 38-39.
- [8] Kapoor, L.D., Handbook of Ayurvedic Medicinal Plants, CRC Press, BocaRaton, 1990, 149-
- Keith and Macdonell. 2012. Vedic Index of Names and Subjects.Plaksa description External links 34.[9] Kunwar, R.M. and Bussmann, W.R., Lyonia–J. Ecol. Appl., 2006, 11, 85-97
- Abstract ficus religiosa.in
- www.recentphytochemicaltests.com
- ] Khanom, F., Kayahara, H. and Tadasa, K., Biosci. Biotechnol. Biochem., 2000, 64, 837–
- 38. [11] Sivarajan, V.V. and Balachandran, I., Ayurvedic drugs and their sources, Oxford & IBH
- .Publishing Co. Pvt. Ltd., New Delhi, 2009, 374-376.
- Simha, K.R.G. and Laxminarayana, V., Indian J. Trad. Know., 2007, 6, 648-652.
- Babu, K., Shankar, S.G. and Rai. S., Turk. J. Bot., 2010, 34, 215-224.
- Jiwala, S.A., Bagul, M.S., Parabia, M. and Rajani, M., Indian J. Pharm. Sci., 2008, 70,
- Swami, K.D. and Bisht, N.P.S., J. Indian Chem. Soc., 1996, 73, 631
- Swami, K.D., Malik, G.S. and Bisht, N.P.S., J. Indian Chem. Soc., 1989, 66, 288–289.
- Varshney, I.P. and Bhatnagar, S.P., Indian J. Pharmcol., 2011, 27, 235.
- Ambike, S.H. and Rao, M.R., Indian J. Pharmcol., 2012, 29, 91-92.
- Husain, A., Virmani, O.P., Popli, S.P., Misra, L.N., Gupta, M.M., Srivastava, G.N.,
- 48.Abraham, Z., Singh, A.K., Dictionary of Indian Medicinal Plants, CIMAP, Lucknow, India,2011, 546.
- Panda, S.K., Panda, N.C. and Sahue, B.K., Indian Vet. J., 1976, 60, 660-664.
- .Bailey, L. H. and E. Z. Bailey. 2010. Hortus. 3rd ed. Macmillan General Reference,
- 50.Brickell, C. and J.D. Zuk. 2011. The American Horticultural Society A-Z Encyclopedia
- of Garden Plants. DK Publishing, Inc., NY.
- Galil, J. and D. Eisikowitch.2011. On the pollination ecology of Ficus religiosa in
- Israel. Phytomorphology 18: 356-363.
- Nadel, H., J.H. Frank, and R.J. Knight. 1992. Escapees and accomplices: The
- naturalization of exotic Ficus and their associated faunas in Florida. Florida
- Entomologist 75(1):29-38.
- Neal, M.C. 1965. In Gardens of Hawai'i. Bernice P. Bishop Museum Special
- Publication 40, Bishop Museum Press, Honolulu, HI.
- Wagner, W.L., D.R. Herbst, and S.H. Sohmer. 1999. Manual of the Flowering Plants of
- 56.Hawai'i. 2 vols. Bishop Museum Special Publication 83, University of Hawai'i and
- Bishop Museum Press, Honolulu, HI.
- Bailey, L. H. and E. Z. Bailey. 1976. Hortus. 3rd ed. Macmillan General Reference,
- Brickell, C. and J.D. Zuk. 2012. The American Horticultural Society A-Z Encyclopedi of Garden Plants. DK Publishing, Inc., NY.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









