 About Authors:
About Authors:
Rakesh Tiwle
Shri Laxman Rao Mankar Pharmacy College Amagoan,
Gondia Maharashtra.
rakesh_tiwle@rediffmail.com
Abstract
Nanotoxicology is a branch of Bio-Nano-science which deals with the study and application of toxicity of nanomaterials. Nanotoxicology is the study of the toxicity of nanomaterial because of quantam size effects and large surface area to volume ratio, nanomaterials have unique properties compared with their larger counter parts. Increases in nanotechnological applications for industrial, consumer and medical uses promise many benefits, yet at the same time they have generated serious concerns about potential health and environmental risks from exposure to engineered nanoscale materials. Such concerns stimulated research in the emerging field of nanotoxicology, resulting in a steadily increasing number of publications suggesting that engineered nanomaterials because of their specific physicochemical properties can induce significant toxic responses. Although most of the nanotoxicological studies were performed using unrealistic exposure conditions, they have led to a widespread perception that generically all nanomaterials pose a significant health risk. Such perception is in great part based on exaggerated reporting in the popular press, resulting in a Nanotoxicity-Hype Correlation. Knowledge about potential human and environmental exposure combined with dose response toxicity information will be necessary to determine real or perceived risks of nanomaterials following inhalation, oral or dermal routes of exposure.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1429
INTRODUCTION
Nanotoxicology
Nanotoxicology is a branch of bionanoscience which deals with the study and application of toxicity of nanomaterials. Nanotoxicology is the study of the toxicity of nanomaterial because of quantam size effects and large surface area to volume ratio, nanomaterials have unique properties compared with their larger counter parts.
Nanotechnology
Nanotechnology is a rapidly developing, emerging branch of modern technology. This new technology deals with materials of extremely small size, generally in the range of nanometres. The nanomaterials, with their extremely small size and high surface area associated with greater strength, stability, chemical and biological activity, find their wide range of applications in a variety of products in modern society. They are used in rapidly increasing nanoproducts, nanodevices, electronics, diagnostics and drug delivery systems. They are present in a variety of consumer products such as foods, drugs, cosmetics, food colour additives, food containers, paints and surface coatings. This trend is expected to result in an ever-increasing presence of nanoparticles in the human environment. Because of their extremely small size they are capable of entering the human body by inhalation, ingestion, skin penetration, intravenous injections and medical devices, and have the potential to interact with intracellular macromolecules. However, information on their potential adverse health effects is very limited at the present time. It is not known at what concentration or size they can exhibit toxicity. Therefore, there are obvious public safety concerns. This has led to the initiation of a new research discipline commonly known as nanotoxicology.
[adsense:468x15:2204050025]
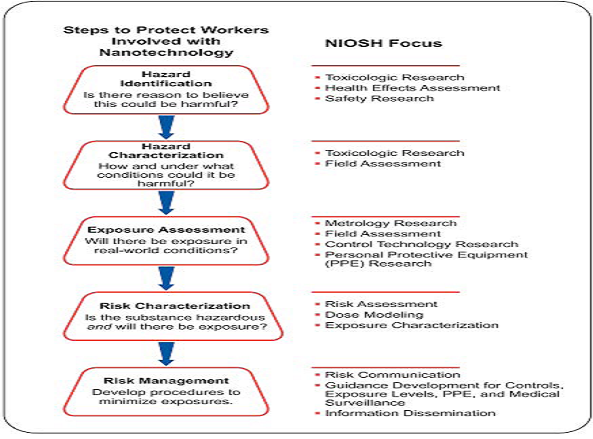
Figure no 1: The Foundation for Risk Management
Primarily Talking About Unbound Engineered Inorganic Nanoparticles
Ø Bucky Ball (C60)
Ø Nanoflowers
Ø Single Wall Carbon Nanotube
Ø Quantum Nanodots
Questions Addressed by Toxicology
* Routes and sites of exposure
* Absorption
* Distribution
* Accumulation
* Metabolism
* Excretion
Health effects
Local , Remote, Systemic, Acute, Chronic, Heritable.
Tools and Mechanisms
* In vitro
Cell-free preparations
Cell cultures
Tissue
Tissue surrogates (complex cell cultures)
In Vitro Limitations
Disadvantages
* May not represent how cells in an animal would really be exposed
* Potentially confounded by model used, exposure procedures
* Doses often very high, physiologically questionable
* Results may not accurately predict health effects in whole animal.
In Vivo Animal Studies
* In vivo animal studies.Acute, sub chronic,chronic
* Surrogate exposure procedures injection, intratracheal instillation, aspiration, implantation
* Real exposures procedures Ingestion, inhalation, skin contact.
In Vivo Limitation
* Rats are not people and may respond differently
* “Lung Overload” cancer in rats
* Animal tests are cruel
Human studies
* Experimental exposures
* Incidental exposures
* Epidemiological studies
* Readily translocates with unknown hazard
SOURCES AND MODE OF ENTRY
Sources
Unintentional
* Road Transport
* Combustion
* Exposure route: Inhalation
Intentional
* Pigments
* Resins
* Cosmetics
* Exposure route: Ingestion and dermal absorption
Four Major Modes
* Inhalation (respiratory tract)
* Ingestion(gastrointestinal tract)
* Dermal (skin)
* Injection (blood circulation)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TYPES OF VARIOUS NANO PARTICLE
CARBON NANOTUBES
Single-walled carbon nanotubes consisting of a single layer of grapheme sheet (a single atomic layer of graphite)seamlessly rolled into a cylindrical tubeand (ii) multi-walled carbon nanotubes comprising two or more layers of concentric cylinders witha separation of about of 0.34 nm betweenthe adjacent layers.
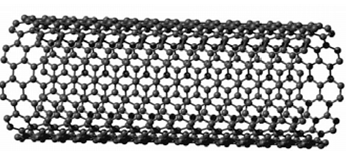
Figure no 2 : Single Wall Carbon Nanotube
That SWCNTs can induce pulmonary injury in mice has also been recently confirmed by Chou et al. They have also demonstrated that the intratracheal instillation of 0.5 mg of SWCNTs into male ICR mice (8-weeks-old) induced alveolarmacrophage activation, various chronicinflammatory responses and severe pulmonary granuloma formation.SWNHs have low toxicities. This may probably be due to the absence ofmetal catalyst in the nanohorns5. SWNHs are shown to be nonirritant and a nondermal sensitizer by skin primary and conjunctival irritation tests and skin sensitization tests. SWNHs have low toxicities. This may probably be due to the absence of metal catalyst in the nanohorns. SWNH sare shown to be nonirritant and a nondermal sensitizer by skin primary and conjunctival irritation tests and skin sensitization tests. SWNHs are not carcinogenic. The intratracheal instillation also revealed that SWNHs rarely damaged rat lung tissuefor a 90-day test period, although black pigments due to accumulated nano horns were observed. These studies strongly suggest that as-grown SWNHs have lowacute toxicities.
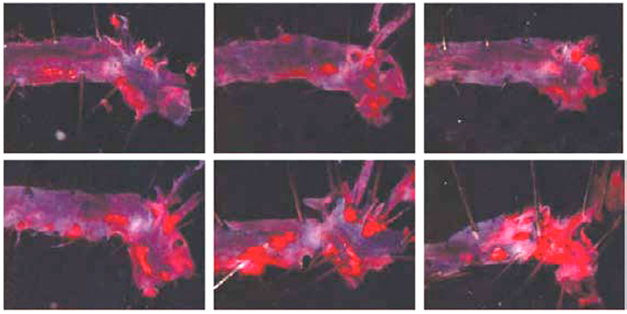
Figure no 3.SWCNT exposed aortas
Toxicokineticss
Absorption
No data
Distribution
Ingestion exposure
Hydroxylated single-walled carbon nanotubes (SWCNT) administered by gavage in mice (100 μL of a 15 μg/mL solution) are distributed to most of the organs and tissues, except the brain.
Exposure by other routes
Singled-walled carbon nanotubes administered intraperitoneally (100 μL of a 15 μg/mL solution) are distributed throughout the body, except the brain, pass through several compartments and are retained in the bones.
In vitro
Carbon nanotubes could pass through the cellular membrane, accumulate in the cell and end up in the cell nucleus.
Excretion
In the study by Wang et al. (2004), 11 days after exposure, about 80% of the radiomarkedhydroxylated single-walled carbon nanotubes administered intraperitoneally had been excreted (94% in the urine and 6% in the feces).
Effects on the respiratory system
At 5 mg/kg, they reported a high mortality rate (~15 %) caused by mechanical blockage of the upper airway, an increase in pulmonary cell proliferation and an increase in multi focal pulmonary granulomas. A significant increase in lung weight.
Effects on the skin and mucous membranes
Huczko et al (2001a) studied the effects on the skin and eyes of exposure to carbon nanotubes. The application of a saturated filter of a solution containing nanotubes did not cause irritation or allergy in volunteers. Ocular instillation of an aqueous suspension of nanotubes in rabbits did not cause irritation.
Immunological and allergic effects
Huczko et al (2001a) studied the effects on the skin and eyes of exposure to carbon nanotubes. The application of a filter saturated with nanotubes did not cause allergies in volunteers.
Cellular and humoral effects
Cui etal. (2005) showed that SWCNT could inhibit cell proliferation, induce apoptosis and reduce adherence of human embryonic kidney cells in vitro (25, 50, 100 and 150 μg/mL, for 1 to 5 days).
FULLERENES
Fullerenes are spherical cages containing from 28 to more than 100 carbon atoms. The mostwidely studied form, synthesized for the first time in 1985 (Krotoet et al.), contains 60 carbonatoms, C60 (Holister et al., 2003). This is a hollow sphere, resembling a soccer ball, composed ofinterconnected carbon pentagons and hexagons (Holisteret al., 2003; Hett, et7, al 2004). Fullerenes area class of materials displaying unique physical properties. They can be subjected to extreme pressures and regain their original shape when the pressure is released. These molecules are not modified and do not combine with each other. However, when fullerenes are manufactured,`certain carbon atoms can be replaced with other atoms and form bondable molecules,thus producing a hard but elastic material. Introduction water soluble fullerene derivatives are essential for many emerging biomedical technologies which exploit the unique chemical properties and physical structureof C60.1-3. Their toxicity, both in tissue culture and in vivo, is an important characteristic for defining and constraining these applications. In some cases, the phototoxicity of fullerene molecules has been identified as a feature useful for therapeutics.
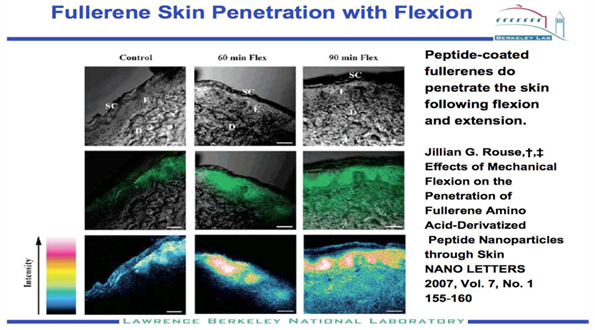
Figure no 4. Fullerene skin penetration with flexion
InVitro Cytotoxicity.
To consistently evaluate thecytotoxicity of water-soluble fullerenes species, two celllines, human dermal fibroblasts (HDF) and human liver carcinoma cells (HepG2) (ATCC), were cultured in Dulbecco’smodification of Eagles media (DMEM).Cells weregrown to 70% confluency before exposure to each fullerene sample; each culture plate was incubated in the dark at 37?C/5% CO2 for 48 h. The concentrations of each fullerene species delivered were 0.24-2400 ppb. The LC50 value, the concentration at which 50% of cells die, was determined by evaluating cytotoxicity over the concentration range. Cytotoxicity was measured using a LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes) The least derivatized water soluble fullerene species is substantially more toxic to both cell lines than the highly derivatized water soluble fullerene species. Nano-C60 Can Produce Oxygen Radical Species in Cell- Free Experiments. Experiments strongly suggest that the mechanism of cell death is oxygen radical induced peroxidation of the lipid bilayers of cells.
Toxicokinetics
Absorption
No data
Distribution
* Inhalation exposure
* No data
* Cutaneous exposure
* No data
* Ingestion exposure
* No data
Exposure by other routes
Rajagopalanet al. (1996) studied the pharmacokinetics of a water-soluble fullerene, p,p’-bis(2-amimoethyl)-diphenyl-C60, administered intravenously in rats (15 and 25 mg/kg). Injection of 25 mg/kg caused the death of two tested rats in 5 minutes. In five other rats, a 15 mg/kg dose did not result in any death and showed that the compound is greater than 99% bound to plasma proteins and distributes into tissues. It also exposed the absence of a renal clearance mechanism. Tsuchiya et al. (1996) showed that C60 is distributed throughout the embryo and the yolk sac of mice 18 hours after injection. Thus, it passes through the placental barrier (intraperitoneal administration, 50 mg/kg; day 18 of gestation). A preliminary study by Moussaet al. (1997) showed that the C60 fullerene could be detected in the blood, liver and spleen in mice one, two and six days after an intraperitoneal injection.
In vitro
No data
Health effects of nanoparticles - IRSST
Metabolism
The C60 fullerene can reduce the hepatic enzyme activity of glutathion (glutathione-S-transferase,glutathion peroxidase et glutathionreductase) in vitro in humans (liver coming from an autopsy),mice and rats (Iwata et11 al., 1998).
Excretion
Rajagopalan et al. (1996) studied the pharmacokinetics of a water-soluble fullerene, p,p’-bis(2- amimoethyl)-diphenyl-C60, administered intravenously in rats (15 and 25 mg/kg). The authors reported the absence of a renal clearance mechanism.
Effects according to routes of exposure (administration)
Inhalation exposure
No data
Cutaneous exposure
Effects on the organs
No data
Immunological and allergic effects
No data
Reproductive effects
No data
Development effects
No data
Genotoxic effects
No data
Carcinogenic effects
There was no effect on DNA synthesis in the application of C60 fullerenes to mouse skin, but a slight increase in ornithine decarboxylase activity (enzyme with a role in the promotion of tumours) was noted in the epidermis (Nelson et al., 1993). Moreover, no increase in cutaneous tumours was observed in a subchronic study of initiation and promotion of carcinogenesis.
Cellular and humoral effects
No data
Ingestion exposure
Chen etal. (1998) studied the acute and subacute toxicity of C60 polyalkylsulfonate in rats. No mortality was observed in an acute oral toxicity test with doses up to 2500 mg/kg.
IRSST - Health effects of nanoparticles .
Exposure by other routes
Effects on the organs
Effects on the skin and mucous membranes
No data
Effects on the respiratory system
No data
Liver effects
No data
Kidney effects
A study by intravenous injection of 100 mg/kg showed a nephropathy and biochemical limpairment (significant decrease in alkaline phosphatase andtriacetylglycerol) two weeks after administration, thus corroborating thekidney impairment observed after intraperitoneal injection. Several effectswere reported in a 12-day subacute toxicity study by intraperitoneal injection(0, 0.6, 6 and 60 mg/kg).
Reduced water and food consumption, a significant decrease in body weight and in the weight of certain organs (thymus and heart), an increase in the weight of the spleen and a significant rise of certain biochemical blood parameters (significant increase in aspartate aminotransferase and a significant decrease in triacetylglycerol) were observed at 60 mg/kg. A nephropathy was observed at 6 and 60 mg/kg respectively.
Development effects
An in vitro and in vivo study of the effects on development of mice was performed by Tsuchiya et al. (1996). The presence of C60 fullerenes solubilized with polyvinyl pyrrolidone inhibited cellular differentiation and proliferation of mesencephalic cells in vitro. Intraperitoneal administration on the eleventh day of gestation caused 100%
Genotoxic effects
Sera et al. (1996) observed in vitro mutagenic activity in 3 salmonella strains exposed to the C60 fullerene and to visible light in the presence of a metabolic activation system.
Zakharenko et al. (1997) observed no effect of the C60 fullerene during an in vitro somatic mutation and recombination test (SMART) on Escherichia coli and an in vivo test on Drosophila melanogaster larvae.
Carcinogenic effects
No data
Cellular and humoral effects
In vitro exposure to the C60 fullerene (12.5 μg C60-cyclodextin) induced oxidative damage in rat hepatic microsomes. This damage can be modulated by antioxidants and free radical scavengers (Kamat etal., 1998).
Photoinduced (halogen lamp) cytotoxicity of fullerenes has been reported in several studies. Yang etal., (2002) showed that this activity could vary with the number of malonic acid molecules added to the C60 fullerene (dimalonic, trimalonic or quadrimalonic acid).
Phototoxic inhibition of cell growth was greater for dimalonic than fortrimalonic and quadrimalonic acid, in descending order. Sayeset al. (2004) studied the cytotoxicity (CL50) of four water-soluble fullerenes on human cells in vitro (skin fibroblasts and hepatic caricinoma cells). They showed that toxicity varies with the nature of the functional group.
INORGANIC NANOPARTICLES
Insoluble inorganic nanoparticles can be composed of pure metals or various inorganic products or alloys. Only their nanometric dimensions distinguish them from the same products normally found on a larger scale. However, it is precisely because of their unique properties related to their nanometric scale that these particles are produced. At this scale, they display mechanical,electrical and other properties that do not exist when in larger.
Example:
Toxicokinetics
Absorption
Inorganic nano particle showed cell capture of microparticulate substances by enterocytes, and their transport between cells. In some cases, the passage of microparticles from the intestinal lumen to the blood stream led to distribution of substances in the body.
Distribution
Cutaneous exposure
Titanium dioxide (TiO2) is a substance contained in sunscreens2. Lademannet al. (1999) did not observe significant absorption of coated TiO2 nanocrystals (17 nm), beyond the stratumcorneum of the skin of human volunteers, except for a small quantity (< 1%), which had penetrated the hair follicles.
Ingestion exposure
Hillyer etal. (2001) reported blood and tissue distribution of ingested colloidal gold nanoparticles in mice. They noted absorption in the animals’ brain, lungs, heart, kidneys, intestines, stomach, liver and spleen, more pronounced for 4 and 10 nm nanoparticles, in comparison with 28 and 58 nm particles.
Exposure by other routes
Paciottiet al. (2004) studied colloidal cold nanoparticles injected intravenously in mice in which they had implanted colon tumour cells. Nanoparticle distribution occurred preferentially at the tumour site, without significant accumulation in the liver, the spleen or the animals’ other organs.
Metabolism
No data
Excretion
In their experiment with rat inhalation of radio marked iridium particles, Kreylinget al. (2002) showed that nanoparticles were eliminated in the animals’ feces without significant intestinal absorption.
Effects according to routes of exposure (administration)
Inhalation exposure
Observed a significant increase in inflammation signs or parameters during administration of 20 nm TiO2 particles in comparison with the same mass of 250 nm particles. titanium oxide was considered to be nontoxic dust and served as an inert control in several toxicological studies. Damage to the pulmonary epithelium, development of sources of interstitial fibrosis and alteration of macrophage functions (inflammation mediators) were significantly greater.
Exposure by other routes
Liver effects
Zhang etal. (2005a) observed less hepatic function alterations in mice that ingested selenium nanoparticles (Nano-Se), compared to those to which non nanoparticulate sodium selenite had been administere.
Effects on the gastrointestinal system
In an analysis of human histological specimens including control cases, Gatti et al (2004) showed a correlation of the presence of microparticles or nanoparticles with colon cancer and Cröhn’s disease, an inflammatory intestine disease.
Cellular and humoral effects
An in vitro study by Lucarelli et al. (2004) showed that SiO2 and cobalt (Co) nanoparticles exhibited significant proinflammatory activity for the activity of human marrow monocytes, while TiO2 and ZrO2 nanoparticles were less active.
I. ORGANIC NANOPARTICLES
As in the case of inorganic nanoparticles, insoluble organic nanoparticles can be composed of various organic substances, often insoluble polymers to which different organic radicals can be grafted. Some substances can also be made soluble under specific conditions. Often, only their nanometric dimensions distinguish organic nanoparticles from the same products normally found on a larger scale. However, it is precisely because of their unique nanoscaledproperties that these particles are produced. On the nano-scale, they display catalytic, chemical or other properties that do not exist when in larger dimension.
Toxicokinetics
Absorption
No data
Distribution
Ingestion exposure
Jani et al. (1990) showed that polystyrene nanoparticles (30, 100 and 300 nm) administered by gavage in rats could be detected in the blood and in several organs, such as the liver and spleen but not in the heart and lungs.
Exposure by other routes
About 60% of the nanoparticles were located in the liver and thespleen, while about 30% remained in the bloodstream. The coating had no significant influence on nanoparticle distribution.
Metabolism
No data
Excretion
No data
Effects according to routes of exposure (administration)
Inhalation exposure
No data
Cutaneous exposure
No data
Ingestion exposure
No data
Exposure by other routes
Effects on the organs
Effects on the skin and mucous membranes
Kante et al. (1982) did not observe any irritant effects at the injection site of poly(isobutyl cyanoacrylate) and poly(polybutyl cyanoacrylate) nanoparticles (~0.2 μm in diameter, single intravenous injection; 0, 12.5 to 40 mL/kg) during an acute toxicity (DL50) lethal dose determination test in mice.
Liver effects
Fernandez etal. (1997) showed that single or repeated intravenous injection of 214 nm poly(isobutyl cyanoacrylate) nanoparticles or 128 nm polystyrene can temporarily reduce the antioxidant defence of isolated rat hepatocytes.
Immunological and allergic effects
Menget al. (2004), in a biocompatibility assessment, did not observe any harmful effects in animals (inflammation, etc.) during muscle implantation of a material composed of hydroxapatite and polyamide nanocrystals.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
QUANTUM DOTS
A major field of research for about the past five years, quantum dots (also called nanocrystals orartificial atoms) represent a special form of spherical nanocrystals from 1 to 10 nm in diameter.They have been developed in the form of semiconductors, insulators, metals, magnetic materialsor metallic oxides. The number of atoms in quantum dots, which can range from 1,000 to100,000, makes them neither an extended solid structure nor a molecular entity (Aitken et al.,2004). The principal research studies have focused on semiconductor quantum dots, which display distinctive quantal effects depending on the dimensions. The light emitted can bead justed to the desired wavelength by changing the overall dimension (Aitken et al., 2004).

Figure no 7:Quantum Nanodots
Toxicokinetics
Absorption
Quantum dots are used as fluorescent probes in diagnostic medical imaging and in therapeutics, because of their optical properties and their capacity to form covalent bonds with peptides, antibodies, nucleic acids or other low-weight molecules (Smith et al. 2004). Chan and Nie in 1998, cited by Smith et al. (2004), were the first to demonstrate in vivo that CdSe / ZnS quantum dots coated with mercaptoacetic acid could bond to blood transferrine. This fluorescent complex was absorbed selectively by cancer cells.
Distribution
Inhalation exposure
No data
Cutaneous exposure
No data
Ingestion exposure
No data
Exposure by other routes
In an intravenous study in mice, Akerman et al. (2002) report that the nature of the CdSe / ZnS quantum dot coating could alter the distribution of these nano materials in the tissues and organs. It was found that PEG coating reduced capture by the liver and spleen by about 95% and prolonged the half-life of the quantum dots in the bloodstream. Other types of peptide coatings increased distribution in the lungs or in breast tumours induced during the experiment. The authors note the absence of quantum dots in the skin covering the tumour site, in the brain and in the kidneys of the animal subjects.
In vitro
No data
Metabolism
No data
Health effects of nanoparticles - IRSST
Excretion
No data
Effects according to routes of exposure (administration)
Inhalation exposure
No data
Cutaneous exposure
No data
Ingestion exposure
No data
Exposure by other routes
Effects on the organs
No data
Immunological and allergic effects
No data
Reproductive effects
No data
Development effects
Dubertr et al. (2002) injected (CdSe)ZnS quantum dots coated with n-poly(ethylene glycol) phosphatidyl ethanolamine (PEG-PE) and phosphatidylcholine (PC) into Xenopus frog embryo cells. They conclude an absence of significant toxicity for embryo development.
Genotoxic effects
No data
Carcinogenic effects
No data
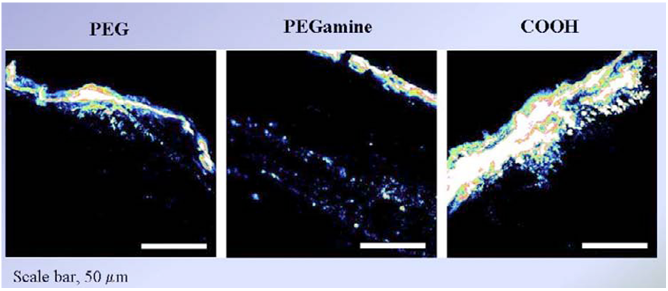
Figure no 8 : CdSe quantum dots can penetrate damaged skin to a limited extent
NANOCAPSULES, NANOSPHERES AND NANOSHELLS
Nanocapsules, nanospheres and nanoshells can be composed of a wide variety of insoluble organic polymers. Some of these structures are developed to be capable of integration with other substances, often medications. The surface of these nanoparticles can also be modified to interact specifically with certain sites of the body. Because of their nanometric dimensions, these particles can circulate in a living organism, serve as a drug vector or fix to specific cells. They represent a very active research sector with potentially major medical spin-offs.
Toxicokinetics
Absorption
In Aprahamian etal.(1987), showed the intestinal absorption of a drug (Lipiodol) transported by polymeric nanocapsules of about 300 nm in dogs. Within less than one hour after intra intestinal injection of the drug and laparotomy of the animals, the nanocapsules were observed in the lumen of the jejunum (small intestine) and then in the intracellular spaces, in the lamina propria, and finally in the intestinal capillaries.
Distribution
Inhalation exposure
No data
Cutaneous exposure
No data
Ingestion exposure
No data
Exposure by other routes
In a study conducted in rats, Cahou etal. (2002) intravenously injected nanocapsules (20 to 100 nm) with a lipid core and a shell composed of 2-hydroxy- polyethylene glycol (PEG) stearate and lecithin. The nanocapsules were marked with iodine-125 and technetium-99. The authors observed a longer-than-expected persistence of the nanocapsules in the blood compartment. They attributed the longer persistence to the PEG coating. The nanocapsules were distributed in the animals’ liver, intestines, stomach and penis, but there was no significant cerebral distribution.
In vitro
No data
Metabolism
No data
Excretion
Digestive elimination of nanoparticles radiomarked with iodine-125 and technetium- 99 was noted in the Cahouet et al. (2002) study of rats. After 24 hours, iodine-125 was still excreted in the animals’ urine.
Health effects of nanoparticles - IRSST
Inhalation exposure
No data
Cutaneous exposure
No data
Ingestion exposure
No data
Exposure by other routes
Effects on the organs
No data
Immunological and allergic effects
No data
Reproductive effects
No data
Development effects
No data
Genotoxic effects
No data
Carcinogenic effects
No data
Cellular and humoral effects
Torres-Lugo etal. (2002) studied the in vitro cytotoxicity of hydrogel nanospheres, substances that can bypass the upper digestive tract and act as pharmacological vectors directly in the intestine. Using cultures of human intestinal cells to which methacrylic acid ethylene glycol nanospheres have been added, the authors conclude that this nanomaterial has low toxicity. However, a reversible alteration of the electrical resistance of the epithelial cells, as well as opening of the junctional membrane complexes, were observed. This raised the possibility of cellular transport of the nanocomplex.
In an in vitro study, Zhou et al. (2005) showed that application of a nanosphere formulation to administer arsenic trioxide reduces the blood toxicity of this product, used against bladder cancer, and renders its action more specific to cancer cells.
II. COMBUSTION-DERIVED NANOPARTICLES
The production of new forms of manufactured/engineered nanoparticles is of increasing concern as nanotechnology continues to develop and manufacture them Royal et al (2004.). These form a plethora of particle types that include nanotubes, fullerenes, quantum dots and compound particles of various types. Whilst information on the toxicity of new types of nanoparticles (NP) is accumulating these are mostly in vitro studies, with few animal or human studies. The existing toxicology knowledge regarding NP is almost entirely based on combustion-derived nanoparticles (CDNP) present in environmental air.
Evolving from the 'ultrafine hypothesis', this strand of research has focused on CDNP like diesel soot since this component of particulate matter (PM) is seen as a key component mediating adverse health effects.
The mechanism at the cellular level is understood in terms of the ability of particles to cause oxidative stress and inflammation and translocate from the site of deposition.
COMBUSTION-DERIVED NANOPARTICLES IN ENVIRONMENTAL AIR POLLUTION
The adverse health effects of air pollution have been recognised throughout fossil fuel combustion in towns and cities, during periods of cold weather, where there is little mixing of air have been associated with the generation of smog episodes.
These smogs consisted largely of sulphur dioxide and particles and could very high concentrations in urban air.
These adverse health effects of air pollution have been measured in hundreds of studies and there is good coherence between the acute effects seen in time series and panel studies, and the chronic effects seen in environmental studies.
Nanoparticles as the most toxic component of PM10
PM is a complex mixture of particle types that depend on season, time of day, site of sampler etc. CDNP are present in PM from conurbations and are a major toxicologically important component. CDNP originates principally from car exhausts although there are other sources.
CDNP AND THE LUNGS
The present understanding of CDNP activity in the lungs is that the surfaces, organics and metals can all produce free radicals with the potential to produce oxidative stress and contribute to inflammation.
Diesel exhaust particles (DEP) are one of the main CDNP to which individuals are exposed. DEP causes inflammation in rat and human lungs Nordenhall C et al (2000) alfollowing short-term, high level exposure.
Tumour necrosis factor-alpha (TNF-α) has been reported to be increased in macrophages exposed to DEP in vitro Yang HM et al and interleukin-6 (IL-6) is released by primed human bronchial epithelial cells exposed to DEP.
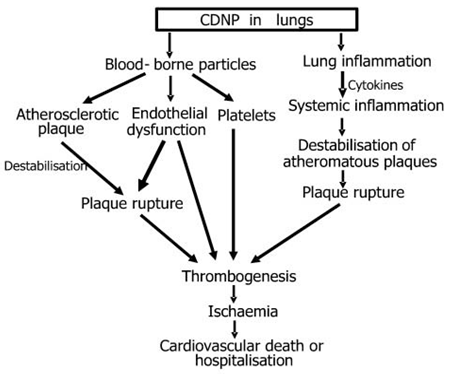
Figure no 9 : CDNP in lungs
CARDIOVASCULAR EFFECTS OF PM AND CDNP- POTENTIAL EFFECTS ON ENDOTHELIUM AND ATHEROSCLEROTIC PLAQUE STABILITY
The adverse cardiovascular events associated with increases in PM Brook RD etal (2003)could be mediated through the effects of CDNP. In a mixture of studies which have used PM, CAPS and model NP, evidence is accumulating that NP cause inflammation that could adversely affect the cardiovascular system.
There is evidence of systemic inflammation following increases in PM, as shown by elevated C-reactive protein, blood leukocytes, platelets, fibrinogen and increased plasma viscosity.
Atherothrombosis is the principle cause of cardiovascular morbidity and mortality.. Viles-Gonzalez JF et al (2004) Atherosclerosis is an inflammatory process, initiated via endothelial injury and producing systemic markers of inflammation that are risk factors for myocardial and cerebral infarction.
Normally the endothelial monolayer delicately balances regulatory pathways controlling vasomotion, thrombosis, cellular proliferation, inflammation and oxidative stress.
Loss of endothelial function results in expression of leukocyte adhesion proteins, reduced anticoagulant activity and the release of growth factors, inflammatory mediators and cytokines.
AIRBORNE ULTRAFINE PARTICLES OVERVIEW
In recent years, interest in potential effects of exposure to airborne UFP has increased considerably, and studies have shown that they can contribute to adverse health effects in the respiratory tract as well as in extrapulmonary organs. Results on direct effects of ambient and model UFP have been reported from epidemiological studies and controlled clinical studies in humans, inhalation/instillation studies in rodents, or in vitro cell culture systems.
For example, several epidemiological studies have found associations of ambient UFP with adverse respiratory and cardiovascular effects resulting in morbidity and mortality in susceptible parts of the population (Pekkanen et al. 1997; Penttinen et al. 2001; Peters et al. 1997a, b; von Klot et al. 2002; Wichmann et al. 2002), whereas other epidemiological studies have not seen such associations (Pekkanen et al. 1997; Tiittanen et al. 1999). Controlled clinical studies evaluated deposition and effects of laboratory-generated UFP.
High deposition efficiencies in the total respiratory tract of healthy subjects were found, and deposition was even greater in asthmatic and COPD subjects. In addition, effects on the cardiovascular system including blood markers of coagulation and systemic inflammation and pulmonary diffusion capacity were observed following controlled exposures to ultrafine carbonaceous particles (Anderson et al. 1990; Wichmann et al., 2000; Brown et al. 2002; Chalupa et al. 2004; Jaques et al 2000; Pietropaoli et al. 2004; Pekkanen et al., 2002; Henneberger et al., 2005). Studies in animals using laboratory-generated model UFP or ambient UFP showed that UFP consistently induced mild yet significant pulmonary inflammatory responses as well as effects in extrapulmonary organs.
Animal inhalation studies included the use of different susceptibility models in rodents, with analysis of lung lavage parameters and lung histopathology, effects on the blood coagulation cascade and translocation studies to extrapulmonary .
In vitro studies using different cell systems showed to varying degrees pro-inflammatory and oxidative stress–related cellular responses after dosing with laboratory-generated or filter collected ambient UFP (Brown et al. 2000; Brown et al. 2001; Li etal. 2003).
Collectively, the in vitro results have identified oxidative stress related changes of gene expression and cell signalling pathways as underlying mechanisms of UFP effects, as well as a role of transition metals and certain organic compounds on combustion generated UFP.
These can alter cell signaling pathways, including Ca++ signaling and cytokine signaling (e.g., IL-8) (Donaldson et al., 2002; Donaldson et al, 2003). Effects were on a mass basis greater for ultrafine model particles than for those of fine particles, whereas for ambient UFP cellular responses sometimes were greater and sometimes less than those of fine and coarse particles.
The interpretation of the in vitro studies is of tentimes difficult because particles of different chemical compositions were used, target cells were different, duration, endpoints, and generally high dose levels also differed. Results from high doses in particular should be viewed with caution if they are orders of magnitude higher than predicted from relevant ambient exposures.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
COPPER NANOPARTICLE
Nanosized copper particles (herein after refer to as “nano-copper”), one of the manufactured nanoparticles, are now industrially produced and available commercially. Recently, nano-copper particles are used as the additive in lubricants, polymers/plastics, metallic coating and inks, etc. Due to excellent mending effects of nano-copper particles nano-copperparticles are homogeneously deposited on the surface of graphite to improve the charge–discharge property significantly, such as coulombic efficiency, cycle characteristics,
The copper-fluoropolymer nano-composite is employed as bioactive coatings that are capable of inhibiting the growth of target microorganisms such as Saccharomycescerevisiae, Escherichia coli, Staphylococcus aureus.
Nano-copper particles, similar to any of other nanomaterials, are likely to enter the environment and human body via different paths such as effluent, spillage during shipping and handling, consumer products and disposal, etc.
In human body, copper is maintained in homeostasis. If the intake of copper exceeds the range of the human tolerance, it would cause toxic effects such as hemolysis, jaundice and even death.
Most recently, the study indicates that the overload of common copper in vivo can induce a set of toxicological activities such as hepatocirrhosis , changes in lipid profile, oxidative stress, renal dysfunction and stimulation of mucous membrane of alimentary canal, etc.
In mice exposed to micro-copper at almost all dose levels, necropsy and pathological examinations of the experimental animals do not show observably pathological changes with viscera. Only in the highest dose group (M7), 1 male and 1 female mice died and whose intestines show ileus. Unlike these observations, viscera (e.g., kidney, spleen and liver) of all mice (N1–N7) exposed to nano-copper particles were gravely harmed.
SILICA NANOPARTICLE
Until recently, toxicological research into silica particles focused mainly on "natural" crystalline silica particles of 0.5 to 10 μm (coarse or fine particles). This research was/is fed by the clear association of occupational inhalation exposure and severe health effects, mainly on the respiratory system.
The typical lung reaction induced by chronic inhalation of crystalline silICLica is silicosis.
Calvert et al. recently reported an association of crystalline silica (mainly quartz) exposure and silicosis, as well as lung cancer, chronic obstructive pulmonary disease (COPD), and pulmonary tuberculosis.Hnizdo and Vallyathan suggested that chronic exposure to levels of crystalline silica dust, which does not cause disabling silicosis, may cause chronic bronchitis, emphysema, and/or small airway disease leading to airflow obstruction, even in the absence of radiological evidence of silicosis.
Silica nanoparticles
Ultrafine particles (< 0.1 μm) have been demonstrated to cause greater inflammatory responses and particle-mediated lung diseases than have fine particles (< 2.5 μm) per given mass. Also, experiments involving silica have shown that nanoparticles, both ultrafine colloidal silica and crystalline silica , have a greater ability to cause lung injury as compared with fine particles.

Figure no 10 :Silica Whiskers
In vitro studies of nanosilica toxicity
Chen and von Mikecz investigated the effects of nanoparticles on structure, function, and proteasomal proteolysis in the cell nucleus by incubating differentcell lines with unlabeled and fluorescently labeled amorphous silica particles of different sizes.
The cytotoxicity of amorphous (colloidal) SNPs (15 and 46 nm) in cultured human alveolar epithelial cells.
Crystalline nanosilica
Cytotoxicity (by MTT assay) and genotoxicity of ultrafinecrystalline SiO2 particulates (UF-SiO2) in cultured human lymphoblastoid cells.
Mesoporous silica
The cytoxicity of amorphous mesoporous SNPs (MSNs) was recently studied intensively because they are promising materials for drug delivery systems and cell markers . Several studies have demonstrated that efficient cellular uptake of MSNs could be achieved at concentrations < 50 μg/ml, with no cytotoxic effects observed up to 100 μg/ml in different mammalian cells . Lu et al. reported on the optimal size of ~50 nm MSNs for cell uptake. Slowing et al.reported that, contrary to the known cytotoxicity of amorphous SNPs toward red blood cells, mesoporous SNPs exhibit high biocompatibility at concentrations adequate for potential pharmacological applications.
Surface-modified/functionalized silica
Evaluate the role of shape in particle toxicity in the lung; the authors compared the response of rod-shaped and spherical amorphous silica particles (Stöber), not coated or coated with fibronectin or polyethylene glycol (PEG), under stretched and static conditions.
In vivo studies of nanosilica toxicity
Along with particle size, surface area and particle number appear to be integral components contributing to the mechanisms of lung toxicity induced by nano-sized particles. The high deposition rate of ultrafine particulates is a result of a small aerodynamic diameter and is assumed to be important in the lung inflammatory process. Some evidence suggests that inhaled nanoparticles, after deposition in the lung, largely escape from alveolar macrophage clearance and gain greater access to the pulmonary interstitium via translocation from alveolar.
In vivo versus in vitro; amorphous versus crystalline
In vitro and in vivo studies to investigate oxidative stress and pro-inflammatory responses induced by amorphous SNPs (average primary size 12 nm). RAW 264.7 cells derived from mouse peritoneal macrophages were exposed to SNPs (5-40 ppm) in vitro and showed ROS generation and decreased intracellular GSH levels, as well as increased levels of nitric oxide released from the cultured macrophage cell line. In vivo, mice were treated with a single intraperitoneal dose of 50 mg/kg of nanosilica. The treatment produced activated peritoneal macrophages, increased blood level of IL-1β and TNF-α, and increased level of nitric oxide released from peritoneal macrophages. Ex vivo, cultured peritoneal macrophages harvested from the treated mice showed the expression of inflammation-related genes (IL-1, IL-6, TNF- α, inducible nitric oxide synthase, cyclooxygenase 2). In the spleen, the relative distribution of natural killer cells and T cells was increased 184.8% and 115.1%, respectively, as compared with control animals, and that of B cells was decreased to 87.7%.
Established and validated co-culture systems may provide a tool to better mimic thein vivo system. Using recently developed 3-D cell cultures and improving the exposure system (likewise exposure at the air-liquid interface of a human epithelial airway model reported by Brandenberger et al. could substantially improve the outcome from in vitro studies with nanomaterials.
III. ZINC OXIDE NANOPARTICLE
This document is a summary of the literature review on the toxicity and ecotoxicology of nanoparticles in general and nano-zinc oxide in particular. It consists of a thorough literature review, evaluation exercise, and identification of gaps in the current state of knowledge of the physico-chemical and (eco)toxicological properties of both cerium oxide and zinc oxide nanomaterials.
Identification:
Zinc oxide is an inorganic compound with the formula ZnO. It usually appears as a white powder and is nearly insoluble in water (water solubility of zinc oxide ranges from 1.6 mg/L to 5 mg/L).
ZnO is present in the Earth crust as a mineral zincite; however most ZnO used commercially is produced synthetically.
Aquatic toxicity
Short-term toxicity to fish
Zhu et al. compared the toxicity of several metal oxide aqueous suspensions to Zebrafish (Dani rerio) early developmental stage; of the substances tested, ZnO was the most toxic material to zebrafish embryos and larvae.
Metal oxide nanoparticles with different chemical compositions have different zebrafish developmental toxicity.
Short-term toxicity to aquatic invertebrates
Wiench et al. reported the acute and chronic effects of nano- and non-nanoscale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate, Daphnia magna.
It was concluded that the acute effects of ZnO on the mobility of Daphnia magna are probably due to the ion toxicity of Zn and are not an effect of exposure to the metal oxide ZnO.
Toxicity to aquatic algae and cyanobacteria
Franklin et al. compared the toxicity of various metal oxide nanoparticles and zinc chloride to a Freshwater Microalga.
Toxicity experiments using the freshwater alga Pseudokirchneriella subcapitata revealed comparable toxicity for nanoparticulate ZnO, bulk ZnO, and ZnCl2, with a 72-h IC50 value near 60 μg Zn/L, attributable solely to dissolved zinc.
Toxicity to microorganisms
Adams et al. compared the eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions.
Antibacterial activity was reported to increase with dose. The results showed that the Gram-negative E.coli was less sensitive to the addition of ZnO nanoparticles than Gram-positive B. subtilis, which was also tested.
Toxicity to terrestrial plants
Lin studied the effect of phytotoxicity of nanoparticles and its effect on seed germination and root growth.
Seed germinations were not affected by the nanoparticles except for seeds of ryegrass and corn. Seed germination of ryegrass and corn was inhibited by nano-Zn and nano-ZnO, respectively. Lin also reported the phytotoxicity effect of ZnO nanoparticle on ryegrass growth. The growth of seedlings was greatly inhibited under the nano-ZnO treatments. Root uptake and phytotoxicity of was also reported.
Toxicokinetics, metabolism and distribution
Dermal absorption
Gamer et al. reported on the dermal adsorption of zinc oxide particles.
The results show that microfine ZnO particles were not able to penetrate the porcine dermatomed skin preparations.
Cross et al. reported on human skin penetration of sunscreen nanoparticles.
Less than 0.03% of the applied zinc content penetrated the epidermis. No particles could be detected in the lower stratum corneum or viable epidermis by electron microscopy, suggesting that minimal nanoparticle penetration occurs through the human epidermis.
Zvyagin et al. investigated the distribution of topically applied ZnO in excised and in vivo human skin, using multi photon microscopy (MPM) imaging with a combination of scanning electron microscopy (SEM) and an energy-dispersive x-ray (EDX) technique to determine the level of penetration of nanoparticles into the sub-dermal layers of the skin.
The overall outcome from MPM, SEM and EDX studies was that, in humans in vivo ZnO nanoparticles stayed in the stratum corneum and accumulated into skin folds and/or hair follicle roots of human skin.
Acute toxicity: oral
Wang et al. studied the acute toxicological impact of nano- and submicro-scaled zinc oxide powder. The biochemical and pathological investigation shows that the toxic effects between the 20 nm and 120 nm ZnO particles are a little different. For example, the blood viscosity could be induced by low and median dose of 20 nm ZnO but high dose of fine ZnO after oral administration.
Acute toxicity: inhalation
Sayes et al. assessed the toxicity of fine and nano-scale particles.The study concluded the following.
In vivo Bronchoalveolar lavage (BAL) Fluid LDH Response:
Exposure to nano-ZnO or fine-ZnO particles suspensions produce enhanced cytotoxic responses at 24 h and 1 week post instillation exposure (pe) time periods.
In vivo Pulmonary Inflammation:
Intratracheal instillation exposure to high-dose nano-ZnO (5 mg/kg) or fine-ZnO particles produced substantial lung inflammatory responses measured at 24 h pe followed by a minimal, recruitment of neutrophils through 1 week pe (i.e. 15 -20 % polymorphnuclear leukocytes). These effects were not measured at the 1 and 3 month pe time points, indicating resolution of the inflammatory responses.
Acute toxicity: other routes
Liu et al. reported on the Acute Toxicity of Nano-sized Zinc Oxide (N-ZnO) in ICR Mice via Intratracheal Instillation. The intratracheal instillation of N-ZnO induced significant pulmonary inflammation and marked body weight loss accompanied with anemia.
Repeated dose toxicity
Ma-Hock et al. reported on the repeated dose toxicity of nano-scale zinc oxide and pigmentary zinc oxide by inhalation. Minimal to moderate necrosis of the olfactory epithelium was noted. The effects were in a concentration-related manner and were reversible within the recovery period. Only a multifocal increase in alveolar macrophages was still present at the end of the recovery period. Similar effects were also observed in the animals exposed to ZnO powder.
Genetic toxicity in vitro
Salmonella typhimurium reverse mutation assays according to OECD TG 471 were conducted by BASF on the Z-COTE HP1 and Z-COTE MAX material , showing no mutagenic effect in this testsystem.
Genetic toxicity in vivo
BASF also studied the effect of Z-COTE HP1 in the in vivo micronucleus test in bone marrow cells of mouse (OECD TG 474). Under the experimental conditions chosen, the test substance Z-COTE HP1 does not have any chromosome damaging (clastogenic) effect, and there were no indications of any 16 impairment of chromosome distribution in the course of mitosis (aneugenic activity) in bone marrow cells in vivo.
Specific investigations
A couple of specific studies were done on the phototoxicity of zinc oxide. Diambeck studied the effect of Zinc Oxide (H&R, PN 104702) using the 3T3 Neutral Red Uptake Phototoxicity Test. The studies concluded that both ZnO irradiated and unirradiated had similar cytotoxic concentration response curves.
Exposure related observations in humans
There is some inhalation data reported by Kuschner et al. and the European Chemicals Bureau. Metal fume fever is a flu-like illness caused by zinc oxide fume inhalation and mediated by unknown mechanisms. It is one of a group of work-related febrile inhalational syndromes.
The bronchoalveolar lavage (BAL) obtained from cigarette smoking and non smoking human volunteers was examined after controlled exposure to purified zinc oxide fume to explore the possible roles of proinflammatory cytokines in this condition. Purified zinc oxide fume inhalation caused an exposure-dependent increase in proinflammatory cytokines and Polymorphonuclear leukocytes (PMNs) in the lung.
Photoirritation
Several studies have also reported data on the photoirritation caused by zinc oxide particles. The studies conclude that no phototirritant skin reaction in any volunteer was observed, thus ZnO as tested was not photo-irritant.
FIBER
- Many naturally occurring and man-made fibers can induce mesothelioma, lung cancer and/or pulmonary fibrosis.
- Fibrous erionite & zeolite: High rate of mesothelioma in the Anatoly region of Turkey where they occur naturally–more potent than asbestos made vitreous fibers
- Man Man made refractory ceramic fibers
- Silicon carbide whiskers: Similar potency to asbestos
- Aluminum oxide, attapulgite, dawsonite, potassium titanate

Figure no 11: Fibres
CONCLUSION
The pulmonary toxicity study findings of multifocal granulomas that we have reported herein may not have physiological relevance and may be related to the instillation of a bolus of agglomerated nanotubes (I.e., nanoropes). If nanotubes reach the lungs, they are much more toxic than carbon black and can be more toxic than quartz.If workers are exposed to respirable SWCNT particles at the current PEL (for graphite particles) they may be at risk of developing some lung lesions.If multiwalled carbon nanotubes reach the lung they are biopersistent…and induce lung inflammation and fibrosis.The precautionary principle should be applied and adequate industrial hygiene measures implemented.CNTs should be considered a serious occupational health hazard.SWCNTs do not cause lung inflammation and yet induce the formation of small, focal interstitial fibrotic lesions in the alveolar regions of the lungs of rats. Low levels of contaminating metals coupledwith high surface area determine the toxicity and fibrogenic potential of SWCNT.. In this paradigm, oxidative stress and inflammation are identified as key processes in the local effects in the lungs. In addition, inflammatory effects and blood translocation could explain adverse cardiovascular effects observed in epidemiology studies NP and CDNP, where adverse cardiovascular effects such as clotting, plaque development and endothelial dysfunction are enhanced after NP exposures in a number of different models. with air pollution particles. In the limited studies so far published, engineered NP, such as the CNT are also reported to induce oxidative stress, cell death and inflammation.
REFERENCE
-
Aitken RJ, Creely KS, Tran CL, 2004. Nanoparticles: an occupational hygiene review. Sudbury,Suffolk,G.-B. HSE, 100p.
-
Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. 2002. Nanocrystal targeting in vivo. Proc Natl Acad Sci U SA 99 (20) : 12617-21.
-
Aprahamian M, Michel C, Humbert W, Devissaguet JP, Damge C, 1987. Transmucosal passage of polyalkylcyanoacrylate nanocapsules as a new drug carrier in the small intestine. Biol Cell 61 (1-2) : 69-7.
-
Brook RD, Brook JR, Rajagopalan S. Air pollution: the "Heart" of the problem. Curr Hypertens Rep. 2003;5:32
-
Cahouet A, Denizot B, Hindre F, Passirani C, Heurtault B, Moreau M, Le Jeune J, Benoit J, 2002. Biodistribution of dual radiolabeled lipidic nanocapsules in the rat using scintigraphy and gamma counting. Int J Pharm 242 (1-2) : 367-71.
-
Chen HH, Yu C, Ueng TH, Chen S, Chen BJ, Huang KJ, Chiang LY, 1998. Acute and subacute toxicity study of water-soluble polyalkylsulfonated C60 in rats. Toxicol Pathol 26 (1) : 143-51.
-
Chou, C.-C. et al., Nano Lett., 2008, 8, 437–445.
-
Cui D, Tian F, Ozkan CS, Wang M, Gao H, 2005. Effect of single wall carbon nanotubes on human
-
Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. 2002. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298 (5599) : 1759-62.
-
Fernandez-Urrusuno R, Fattal E, Feger J, Couvreur P, Therond P, 1997. Evaluation of hepatic antioxydant systems after intravenous administration of polymeric nanoparticles. Biomaterials 18 : 511-517.
-
Gatti AM, 2004. Biocompatibility of micro- and nano-particles in the colon. Part II. Biomaterials 25 (3) :385-92.
-
Hett A, 2004. Nanotechnology: small matter, many unknowns. Zurich, Suisse , Swiss Re.
-
Hillyer JF, Albrecht RM, 2001. Gastrointestinal persorption and tissue distribution of differently sizedcolloidal gold nanoparticles. J Pharm Sci 90 (12) : 1927-36.
-
Holister P, Roman V, Harper T, 2003. Fullerenes. Technology White Papers #7. [S.l.] Cientifica, 2003, 12 p. Page d'accueil visionnée le 26/04/2004.
-
Huczko A, Lange H, 2001a. Carbon nanotubes: experimental evidence for a null risk of skin irritation and allergy. Fullerene Sci Technol 9 (2) : 247-250.
-
Iwata N, Mukai T, Yamakoshi TN, Hara S, Yanase T, Shoji M, Endo T, Miyata N, 1998. Effects of C60, a fullerene, on the activities of glutathione s-transferase and glutathione-related enzymes in rodent and human livers. Fullerene Science and Technology 6 (2) : 213-226.
-
Jani P, Halbert GW, Langridge J, Florence AT, 1990. Nanoparticle uptake by rat gastrointestinal mucosa : quantification and particle size dependancy. J Pharm Pharmacol 42 : 821-826.
-
Kamat JP, Devasagayam TPA, Privadarsini KI, 1998. Oxydative damage induced by the fullerene C60 on photosensitization in rat liver microsomes. Chemico-Biological Interactions. Vol. 114, p. 145-159.
-
Kante B, Couvreur P, Dubois-Krack G, De Meester C, Guiot P, Roland M, Mercier M, Speiser P, 1982.Toxicity of polyalkylcyanoacrylate nanoparticles I: free nanoparticles. Journal of Pharmaceutical Sciences 71 (7) : 786-790.
-
Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schultz H et al. 2002. Translocation of ultrafine insoluble iridium particles from lungs epithelium to extrapulmonary organs in size dependent but very low. J Tox Environ Health 65 (20) : 1513-1530.
-
Kroto HW, Heath JR, O’Brian SC, 1985. C60: Buckminsterfullerene. Nature 318: 162-163.
-
Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W, 1999. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol 12 (5) : 247-56.
-
Lucarelli M, Gatti AM, Savarino G, Quattroni P, Martinelli L, Monari E, Boraschi D, Woolley DE, Tetlow LC, 2004. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Mast cell activation and its relation to proinflammatory cytokine production in the rheumatoid lesion. Eur Cytokine Netw 15 (4) : 339-46.
-
Meng., C. Y. et al. (2004). Biocompatibility and security of new type nano-hydroapatite crystals and polyamide for bone reconstruction and repair after implanting in vivo. Chinese Journal of Clinical Rehabilitation (China). Vol. 8, No. 29, p. 6330-6333.
-
Moussa F, Pressac M, Genin E, Roux S, Trivin F, Rassat A, Ceolin R, Szwarc H, 1997. Quantitative analysis of C60 fullerene in blood and tissues by high-performance liquid chromatography witt photodiode-array and mass spectrometric detection. J Chromatogr B Biomed Sci Appl 696 (1) :153-9.
-
Nelson MA, Domann FE, Bowden GT, Hooser SB, Fernando Q, Carter DE, 1993. Effects of acute and subchronic exposure of topically applied fullerene extracts on the mouse skin. Toxicol Ind Health 9 (4) : 623-30.
-
Nordenhall C, Pourazar J, Blomberg A, Levin JO, Sandstrom T, Adelroth E. Airway inflammation following exposure to diesel exhaust: a study of time kinetics using induced sputum. Eur Respir J.2000;15:1046–1051.
-
Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L, 2004. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv 11 (3) : 169-83
-
Pekkanen J, Timonen KL, Ruuskanen J, Reponen A, Mirme A. 1997. Effects of ultrafine and fine particles in urban air on peak expiratory flow among children with asthmatic symptoms. Environ Res 74(Art. No. ER973750):24-33.
-
Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. 2001. Ultrafine particles in urban air and respiratory health among adult asthmatics. Eur Resp J 17(No. 3):428-435.
-
Peters A, Doring A, Wichmann H-E, Koenig W. 1997a. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet 349(No. 9065):1582-1587.
-
Rajagopalan P, Wudl F, Schinazi RF, Boudinot FD, 1996. Pharmacokinetics of a water-soluble fullerene in rats. Antimicrob Agents Chemother 40 (10) : 2262-5.
-
Royal Society and Royal Academy of Engineering. Nanoscience and nanotechnologies: opportunities and uncertainties. The Royal Society; 2004.
-
Sayes C, Fortner J, Guo W, Lyon D, Boyd AM, Ausman KD, et al., 2004. The differential cytoxicity ofwater-solule fullerenes. Nano letters 4 (10) : 1881-1887.
-
Sera N et al., 1996. Mutagenicity of the fullerene C60-generated singlet oxygen dependent formation of lipid peroxides. Carcinogenesis 17 (10) : 2163-9.
-
Smith AM, Gao X, Nie S, 2004. Quantum-Dot Nanocrystals for In-vivo Molecular and Cellular Imaging. Photochem Photobiol 80 : 377-385.
-
Torres-Lugo M, Garcia M, Record R, Peppas NA, 2002. Physicochemical behavior and cytotoxic effects of p(methacrylic acid-g-ethylene glycol) nanospheres for oral delivery of proteins. J Control Release 80 (1-3) : 197-205.
-
Tsuchiya T, Oguri I, Nakajima Yamakoshi YN, Miyata N, 1996. Novel harmful effects of [60] fullerene on mouse embryos in vitro and in vivo. FEBS Lett. 393 (9 September 1996) : 139-145.
-
Viles-Gonzalez JF, Anand SX, Valdiviezo C, Zafar MU, Hutter R, Sanz J, et al. Update in atherothrombotic disease. Mt Sinai J Med. 2004;71:197–208.
-
Von Klot S, Wolke G, Tuch t, et al. 2002. Increased asthma medication use in association with ambient fine and ultrafine particles. Eur RespirJ 20:691-702.
-
Wang H, Wang J, Deng X; Sun H, Shi Z, Gu Z, Liu Y, Zhaoc Y, 2004. Biodistribution of carbon singlewall carbon nanotubes in mice. J Nanosci Nanotech 4 (8) : 1019-1024.
-
Yang XL, Fan CH, Zhu HS, 2002. Photo-induced cytotoxicity of malonic acid [C60]fullerene derivatives and its mechanism. Toxicol in vitro 16 : 41-46.
-
Zakharenko LP et al, 1997. [Determination of the genotoxicity of fullerene C60 and fullerol using the method of somatic mosaics on cells of Drosophila melanogaster wing and SOS-chromotest] Genetika. 33 (3) : 405-9.
-
Zhang Z, Kleinstreuer C, Donohue JF, Kim CS, 2005b. Comparison of micro- and nano-size particle depositions in a human upper airway model. J Aerosol Sci 36 (2).
-
Zhou J, Zeng FQ, Li C, Tong QS, Gao X, Xie SS, Yu LZ, 2005. Preparation of arsenic trioxide-loaded albuminutes immuno-nanospheres and its specific killing effect on bladder cancer cell in vitro. Chin Med J (Engl) 118 (1) : 50-5.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









