About Author
Maneesh Banyal*, Swati joshi
Department of pharmacy, Lala Birkha Ram College of Pharmacy, Golpura, Haryana
ABSTRACT
Over the last few years pharmaceutical researcher or scientist are trying to explore transmucosal routes. Transmucosal route of drug delivery supply distant blessings over peroral administration for general result. Buccal delivery, consist of administration through the buccal mucosa, which is mainly composed of the lining of the cheeks. To overcome the deficiency associated with the other route of administration like acidic medium instability, first pass metabolism etc, buccal region of oral cavity is an alternative target for the administration of choice of drug. The administration of a drugs by buccal route, the drug are directly pass through into systemic circulation, and shows less hepatic metabolism and high bioavailability. In this regard, this review covers the areas of buccal derug delivery, structure of buccal mucosa, theories of mucoadhesion, preparation methods and evaluation of buccal films.
Introduction
Oral drug delivery system is the most generally used route of administration among the various routes of drug deliver, the oral route is perhaps the one mostly preferred by patients and clinicians. Based on our current understandings of organic chemistry and physiological aspects of absorption and metabolism, many drugs, can’t delivered effectively through the traditional oral route, as a result of when administration square measure subjected to pre-systemic clearance extensively in liver, which regularly results in a scarcity of serious correlation between membrane porousness, absorption and bioavailability [¹].
Orally administered drugs are prone to some the buccal disadvantages that’s why peptides and proteins cannot be administered orally because of hepatic first-pass metabolism and metabolism in gastrointestinal tract. So that mucoadhesion drug delivery system (buccal mucosa) is the alternative route for the drugs like protein and peptides. It avoids the presystemic first-pass effect and improves bioavailability.²
TRANSMUCOSAL ROUTES OF DRUG DELIVERY [3, 4]
Transmucosal route of drug delivery (i.e. the membrane linings of the nasal, rectal, vaginal, ocular and oral cavities) supply distant blessings over peroral administration for general result. These advantages avoid the first pass effect and avoidance of presystemic elimination within the GI tract. Among the varied transmucosal sites obtainable, membrane of the cavity was found to be most convenient and simply accessible site for the delivery of therapeutic agents for each local and general delivery, because it has expanse of smooth muscle which is relatively immobile, abundant vascularization, rapid recovery time after exposure to stress and the near absence of langerhans cells. Direct access to the circulation through the interior jugular vein by pass medicine from the viscous first pass metabolism resulting in high bioavailability.
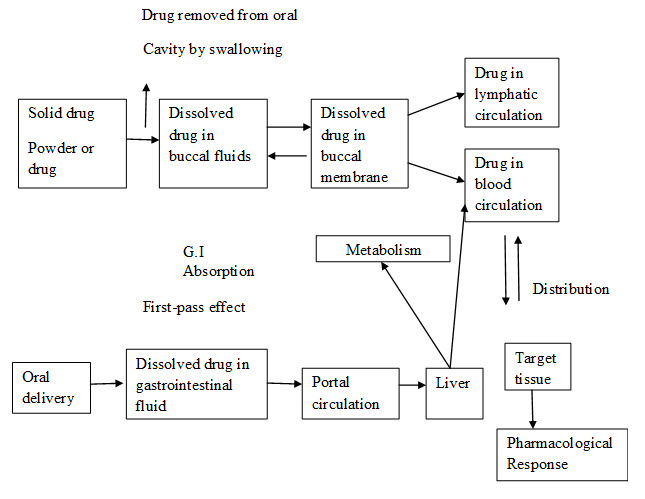
Fig. 1 : Comparative drug absorption between oral and buccal route
These dosage forms are self-controllable, reasonable and have more patient compliance. Developing a dosage form with in the optimal pharmacokinetics is a promising area for continued research as it is important and intellectual challenging. With the correct dosage form design, local nature of the mucosa can be controlled and influence in order to optimize the rate of drug dissolution and permeation.
Within the oral membrane cavity, delivery of medication is assessed into 3 categories: (a) sublingual delivery, that is general delivery of medication through the membranes lining the floor of the mouth, (b) buccal delivery, which is drug administration through the mucosal membranes lining the cheeks (buccal mucosa), and (c) local delivery, which is drug delivery into the oral cavity.¹¹
BUCCAL DRUG DELIVERY [4]
Buccal delivery, consist of administration through the buccal mucosa, which is mainly composed of the lining of the cheeks. The buccal membrane lines the inner cheeks and buccal formulations are placed between the higher gingivae (gums) and cheeks to treat native and general conditions. The buccal route provides potential routes for usually giant, hydrophilic and unstable proteins and polysaccharides, moreover as typical little drug molecules. The oral cavity has been used as a site for local as well systemic drug delivery.
1) Advantages of drug delivery via the buccal lining [5]
• Site specific or local delivery of drugs.
• Circumventing the hepatic first pass metabolism.
• Increased contact time permitting localization improves the residence time of the drug.
• Those drugs that have bioavailability problem, enhances the bioavailability
• Improve Drug stability
• Ease of drug administration increased.
• To inhibit metabolizing enzymes in a localized area.
• Sustained drug delivery system.
• Transmucosal systems possess a rapid initiation and decline of delivery than do Transdermal patches.
• The large contact surface of the oral cavity provides rapid and extensive drug absorption.
Limitations of buccal drug delivery [5, 6]
• For local action the faster elimination of drugs due to the flushing action of saliva or the ingestion of foods stuffs may lead to the requirement for frequent dosing.
• The uneven distribution of drug within saliva on release from a solid or semisolid delivery system could mean that some areas of the oral cavity may not receive effective levels.
• For local as well as systemic action, patient acceptability in terms of taste, irritancy and mouth feel is an issue.
• Once placed at the absorption website the patch shouldn’t be disturbed.
• Eating and drinking are restricted till complete absorption has taken place.
BUCCAL ADHESIVE DRUG DELIVERY [5]
Retentive buccal mucoadhesive formulation proves to be an alternative to the conventional oral medications as they can be easily attached to the buccal cavity retained over for an extended period of time and withdraw at any time. Bioadhesion formulations designed for the buccal application ought to exhibit appropriate physical science and mechanical properties, as well as pseudo plastic or plastic flow with thixotrophy, easy application, sensible spreadability, applicable hardness and prolonged duration within the oral fissure. These properties may affect the ultimate performance of the preparations and their acceptance by the patients.
An ideal buccal adhesive system must have the following properties [7, 8]
• Should attach to the site of attachment for a few hours.
• Should release the drug in a controlled release manner.
• Should promote the rate and extent of drug absorption.
• Should not cause any irritation to the patient.
• Should not interfere with the normal activities such as talking, drinking etc.
STRUCTURE OF ORAL MUCOSA
The oral mucosa is consists of an outermost layer of stratified squamous epithelium. Below this present a basement membrane, a lamina propria followed by the sub mucosa as the innermost layer (Fig.1.2). The epithelium is alike to stratified squamous epithelia found in the rest of the body in that it has a mitotically active basal cell layer, advancing through a number of differentially intermediate layers to the superficial layers, where cells are shed from the surface of the epithelium.¹⁵ The epithelium of the buccal mucosa is near about 40-50 cell layers thick, while that of the sublingual epithelium contains somewhat fewer. The cell size of epithelial increases and become flatter when they travel from the basal layers to the superficial layers.
The turnover time for the buccal epithelium has been about at 5-6 days and this is probably representative of the oral mucosa as a whole. The oral mucosa thickness varies depends on the site: the buccal mucosa measures at 500-800 µm, while the tissue layer thickness of the exhausting and soft palates, the ground of the mouth, the ventral tongue, and therefore the gingivae live at regarding 100-200 µm. The composition of the epithelium also varies depends on the site in the oral cavity. The mucosa of areas subjected to mechanical stress (the gingivae and hard palate) are keratinized similar to the epidermis. The mucosae of the soft palate, the sublingual, and the buccal regions, however, are not keratinized [9]. The keratinized epithelia contain neutral lipids like ceramide and acylceramides which have been associated with the barrier function. These epithelia are mostly impermeable to water. In contrast, non-keratinized epithelia, such as the floor of the mouth and the buccal epithelia do not contain acylceramides and only have less amounts of ceramide [10]. They also contain less amounts of neutral but polar lipids, mainly cholesterol sulfate and glucosyl ceramide. These epithelia have been found to be considerably high permeable to water than keratinized epithelial [11]. (Fig. 2)
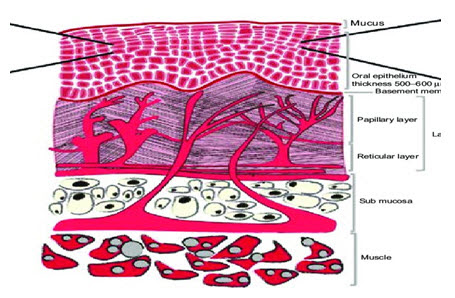
Fig. 2: Schematic diagram of the oral mucosa
Environment of Buccal mucosa [12]
The oral cavity is marked by presence of saliva which is produced by salivary glands and mucus which is secreted by major and minor salivary glands as part of saliva.

TABLE 1: Role of Saliva ad Mucus
Function of Oral Cavity [13]
• It is helps in chewing mastication and mixing of food stuff.
• It is Helps to lubricate the food material and bolus.
• To identify the ingested material by taste buds of tongue.
• To initiate the carbohydrate and fat metabolism.
• As a portal for intake of food material and water.
• To aid in speech and breathing process.
Pathways of drug Absorption from buccal mucosa [12, 14]
Two major routes are involved:
1. Transcellular (intracellular, passing through the cell).
2. Paracellular (intercellular, passing around the cell).
The transcellular route may include permeation across the apical cell membrane, intracellular space and basolateral membrane either by passive transport (diffusion, pH partition) or by active transport (facilitated and carrier-mediated diffusion, endocytosis). The transcellular pathway of drug is a complex function of various physicochemical properties including size, lipophilicity, hydrogen bond potential, charge and conformation. Transportation through aqueous pores in cell membranes of epithelium is also possible for substance with low molar volume (80 cm3 / mol).
Within the paracellular route (intercellular space) hydrophobic molecules pass through the narrow aqueous regions which is adjacent to the polar head groups of the lipids.
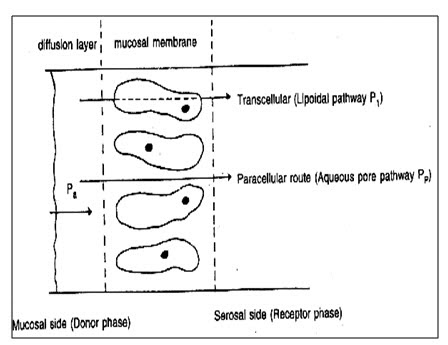
Fig. 3: Mechanism of transmucosal permeation
BIOADHESION / MUCOADHESION
Bioadhesion may be defined as the state in which two materials, at least one of which is biological in nature, are held together for longer periods of time by interfacial forces. When the adhesive attachment is to mucus or a mucous membrane, the phenomenon is referred to as mucoadhesion [15]
Bioadhesion Mechanism [16, 17]
Mechanism of bioadhesion can be described in following steps:
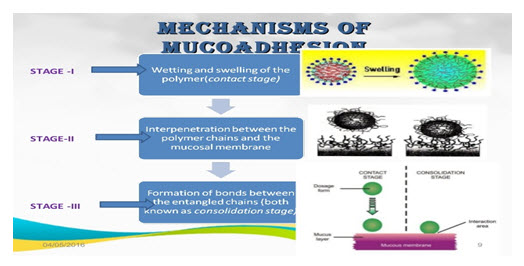
Fig. 4 : Stages of Mucoadhesion
The bonds that form in bioadhesion can be either mechanical or chemical in origin.
Mechanical or physical bonds: The bonding occurs chiefly through both physical and mechanical bonds results from entanglement of the adhesive material and the extended mucus chains.
Chemical bonds: Chemical bond may be due to electrostatic interaction, hydrophobic interactions, hydrogen bonding and dispersion forces. Electrostatic interaction and hydrogen bonding appear to be important as a result of the large number of charged and hydrophilic species, e.g. hydroxylic (-OH), carboxylic (-COOH), sulfate (SO3H) and amino (-NH2) groups.
THEORIES OF BIOADHESION [15, 18]
There are six general theories of adhesion, which have been adapted to study bioadhesion.
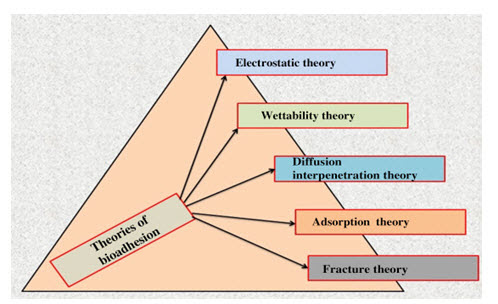
Fig. 5: Theories of bioadhesion
FACTORS AFFECTING MUCOADHESION [19]
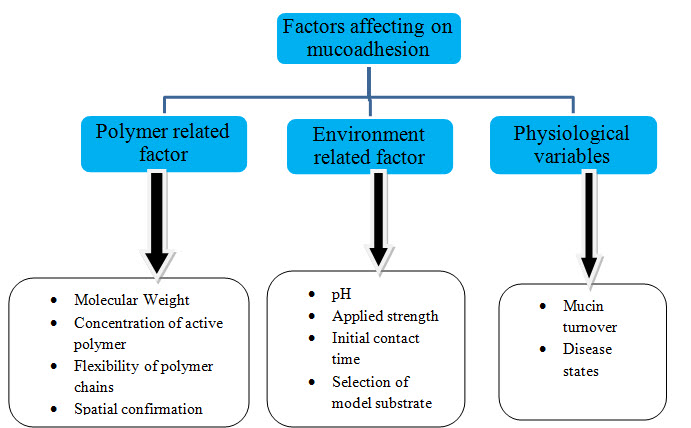
Fig. 6: Factors affecting mucoadhesion
BIOADHESIVE DOSAGE FORM
Buccal mucoadhesive dosage forms can also be categorized into three types on the basic geometry [20]
Type 1: It`s one layer device with multidirectional drug unharnessed. This kind of indefinite quantity type suffers from important drug loss because of swallowing.
Type 2: It’s a tool during which associate rubberised backing layer is superimposed on prime of the drug loaded bioadhesive layer, making a double- layered device and preventing drug loss from the highest surface into oral fissure.
Type 3: It`s a one –way drug unharnessed device, from that drug loss is smallest, since the drug is discharged solely from the aspect adjacent to the buccal mucous membrane. This may be achieved by coating each face of the indefinite quantity type, expect the one that`s in reality with the buccal mucous membrane.
They are broadly classified into [21, 22]
A. Solid buccal adhesive dosage forms.
B. Semi- solid buccal adhesive dosage forms.
C. Liquid buccal adhesive dosage forms.
A. Solid buccal adhesive indefinite quantity type
1. Bioadhesive tablets:
Buccal tablets are small, flat and oval, with a diameter approximately 5-8 mm. Buccal mucoadhesive tablet allow for drinking and speaking without major discomfort. They soften, attach to the mucosa, and are retained in position until dissolution and/or release is complete. These tablets can be applied to different sites in the oral cavity, including the palate, the mucosa lining the check, as well as between the lip and the gum.
Adhesive microparticles:
Bioadhesive micro particles offer the same advantages as tablets but their physical properties enable them to make intimate contact with a large mucosal surface area. The small size of microparticles compared with the tablets means they are less likely to cause local irritation at the site of adhesion and the uncomfortable sensation of a foreign object within the oral cavity is reduced.
Adhesive lozenges:
Bioadhesive lozenges may be used for the delivery of drugs that act topically within the mouth including antimicrobials, corticoids, local anesthetics, antibiotics and antifungal. Conventions lozenges produce a high initial release of drug in the oral cavity, which rapidly declines to sub therapeutic levels, thus multiple daily dosing is required.
B. Semi-solid buccal adhesive dosage form
Bioadhesive patches:
Patches are laminates consisting of an impermeable backing layer, a drug- containing reservoir layer from which the drug is released in a controlled manner, and a bioadhesive surface for mucosal attachment.
Bioadhesive films:
Films are the most recently developed dosage form for buccal drug delivery system. Buccal films may be preferable over adhesive tablets in terms of flexibility and comfort. They can circumvent short residence time of oral gels on the mucosa, which can easily be washed away and removed by saliva.
Buccal gels and ointments
Dosage forms may not be as accurate as from tablets, patches and films. Poor retention of buccal gel and ointment has the advantages of easy dispersion throughout the oral mucosa. However, drug dosing from semisolid the gels at the site of application has been overcome by using bioadhesive formulations. Certain bioadhesive polymers e.g., polyxamer 407, sodium carboxymethyl cellulose, Carbopol, etc. undergo a phase change from a liquid phase to a semisolid phase. This increase the viscosity, which results in sustained and controlled release of drugs.
C. Liquid Buccal adhesive dosage form
Viscous liquids:
Viscous liquids containing bioadhesive polymers such as carboxymethyl cellulose may be utilized to protect mucosal membranes from damage and irritation. They can also be used to provide drug to specific sites.
Gel forming liquids:
These formulations are introduced as liquids but undergo a change in their form in response to conditions such as temperature and pH. Such formulations are used for the controlled – release of drugs in to the eye.
FORMULATION DESIGN OF MUCOADHESIVE BUCCAL FILM
1. Active pharmaceutical ingredient.
2. Mucoadhesive polymers.
3. Plasticizers.
4. Penetration enhancers.
1). Active pharmaceutical ingredients [22]
For buccal drug delivery, it is important to increase the contact between API and mucosa to obtain the desired therapeutic effect. The important drug properties that affect its diffusion through the patch as well as the buccal mucosa are molecular weight, chemical function and melting point. The selection of a suitable drug for design of buccal drug delivery system should be based on following characteristics:
• The conventional single dose of the drug should be low.Medicine having biological half- life between 2-8 hours is smart candidates for controlled drug delivery.
• The drug absorption ought to be passive once given orally.
• It should not produce any irritancy, allergy and discoloration or erosion of teeth.
2). Bioadhesive polymer [23, 24, 25]
Bio adhesive polymers play a major role in mucoadhesive drug delivery systems of drugs. Polymers also are utilized in matrix devices during which the drug is embedded within the chemical compound matrix, that management the length of release of medication. Bio adhesive polymers are from the most diverse class and they have considerable benefits upon patient health care and membrane by means of rate controlling layer or core layer. Bio adhesive polymers that adhere to the mucin/ animal tissue surface are effective and result in important improvement within the oral drug delivery.
An ideal polymer for mucoadhesive drug delivery systems should have following characteristics [26, 27]
• It ought to be inert and compatible with the atmosphere. The polymer and its degradation merchandise ought to be non- toxic absorbed from the mucosa layer.
• It ought to adhere quickly to moist tissue surface and may possess some site specificity.
• The polymer should not decompose on storage or throughout the period of the dose kind.
• It ought to enable simple incorporation of drug in to the formulation.
Criteria followed in polymer selection:
• It ought to kind a robust non covalent bond with the mucin/epithelial surface.
• It should have high relative molecular mass and slim distribution.
• It should be compatible with the biological membrane.
3. Plasticizers [27]
These are the materials which are used to achieve softness and flexibility of thin films of polymer or blend of polymers. Examples of common plasticizers includes glycerol, propylene glycol, PEG 400, castor oil etc. the plasticizers helps in releasing of the drug substances from the polymer base as well as acting as penetration enhancers. The choice of the plasticizer depends upon the ability of plasticizers material to solvate the polymer and alters the polymer-polymer interactions. When utilized in correct proportion to the chemical compound, these materials impart flexibility by relieving the molecular rigidity.
4. Penetration enhancers [12]
Penetration enhancer’s are used in mucoadhesive formulations to improve the release of the drug. They aid is the systemic delivery of the drug by allowing the drug to penetrate more readily into the viable tissue. Substances that promote the permeation through buccal mucosa are referred as permeation enhancers [33]. As most of the penetration enhancers were originally designed for purpose other than absorption enhancement, a systemic search for safe and effective penetration enhancers must be a priority in drug delivery.
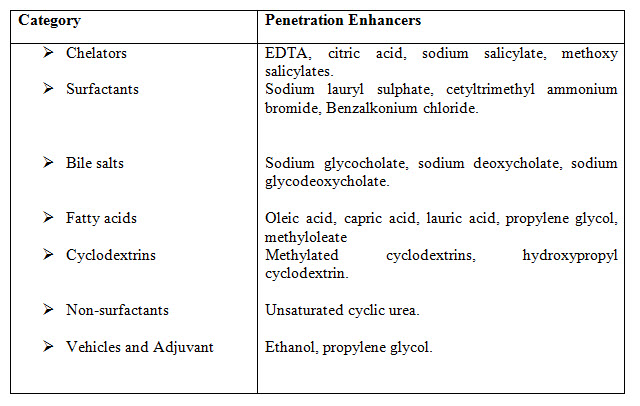
TABLE 2 : Penetration enhancers
METHODS OF PREPARATION OF BUCCAL FILMS [28, 29, 30]
The manufacturing process of buccal films includes the following techniques:
1. Solvent casting technique.
2. Hot melt extrusion technique.
1. Solvent casting method
The solvent casting method is widely used method for the manufacture of buccal films. In this process water soluble ingredients (polymers) are dissolved in water to form homogeneous viscous solution. API and other excipients are dissolved in suitable solvent to form homogenous viscous solution. Both the solutions are mixed and the resulting solution is casted as a film and then the casted film is dried suitably.
2. Hot melt extrusion technique
Hot melt extruder is used in this process. This technique includes shaping a polymer into a film via the heating process. An admixture of pharmaceutical ingredients including API in dry state is filled in the hopper, conveyed, mixed and subjected to the heating process, and then extruded out in molten state melted by the extruder. The liquefied mass thus formed is used to cast the film. A critical step in this process is casting and drying process.
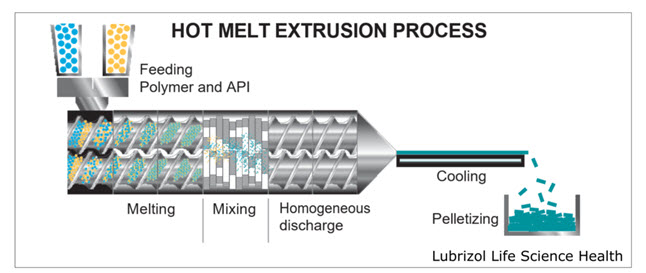
Fig. 7: Hot Melt Extrusion Process
EVALUATION OF THE BUCCAL FILMS [30, 31, 32]
The buccal films are evaluated by following methods:
1. Weight and thickness of the film
For evaluation of film weight, three films of every formulation are taken and weighted individually on a digital balance. The average weights are calculated similarly, three films of each formulation were taken and film thickness is to be measured using micrometer screw gauge at three different places, and the mean value is to be calculated [34].
2. Surface pH of films
Surface pH determination, three films of each formulation is allowed to swell for 2h on the surface of agar plate. The surface pH is to be measured by using a pH paper on to the surface of the swollen patch. A mean value of three readings is to be recorded.
3. Swelling index
After determination of the original film weight and diameter, the samples is allowed to swell on the surface of agar plate kept in an incubator maintain at 37°C. Weight of the three films of each formulation is determined at different time intervals (1-5h). The percent swelling (%S) is to be determined by using the following equation:
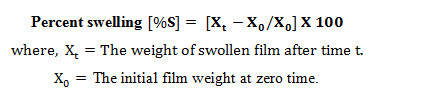
4. Folding endurance
Three films of each formulation are cut in required shape by using sharp blade. Folding endurance value is to be obtained by repeatedly folding the films at the same place, till it is broken. The number of times, the film could be folded at the same place without breaking provides the value of folding endurance [33, 34].
5. Moisture contents
The prepared buccal films are to be weighed individually and kept in a desiccators containing calcium chloride at room temperature for 24 h. The films are to weighed again after a specified time, until they show a constant weight. The percent moisture content is to be obtained by using following formula [33, 34].

6. Moisture uptake
Weighed films are placed in desiccators at `room temperature for 24h. These films are then taken out and exposed to 84% relative humidity using saturated solution of potassium chloride in desiccators, until a constant weight is achieved. % moisture uptake is calculated as,
%Moisture uptake = [Final weight–Initial weight/Initial weight] ×100
7. In-Vitro residence time
In-Vivo residence time is determined using IP disintegration apparatus using 900 ml of the disintegration medium maintaining at 37°C. The rat intestinal mucosa segments, each of 3 cm length, are to be glued to the surface of a glass slab, which is then vertically fixed to the apparatus. Three Mucoadhesive films of each prepared formulation are hydrated on one surface and the hydrated surface is brought into contact with the mucosal membrane. The glass slab is vertically fixed to immerse in the buffer solution at the lowest point, and is out at the highest point. The time required for complete detachment of the films from the mucosal surface is to be recorded.
8. Drug content Uniformity
Three film units (each of 22nm diameters) of each formulation have to be taken in separate 100ml volumetric flasks, 100ml of solvent has to be added and continuously stirred for 24 h. The solution have to be filtered, diluted suitably and analyzed at specified nm in UV spectrophotometer. The average drug content of three films has to be taken as final reading [33, 34].
9. Surface characterization studies
The SEM photograph of the film is taken at 6000 X magnification. The drug containing prepared film is examined for clear and colorless surface. The photomicrographs of the films with the drug and the blank film are compared, and are examined whether the drug is distributed uniformly throughout the film in an amorphous form.
10. In- Vitro dissolution studies
Dissolution studies are carried out for all the formulations, employing USP dissolution apparatus at 37°C, rotated at constant speed of 50 rpm using 900 ml of dissolution medium. A drug film sample is used in ach test. An aliquot of the sample is withdrawn at suitable time interval and the volume is replaced with fresh dissolution medium. The sample is analyzed spectrophotometrically at specified nm [33, 34, 35].
CONCLUSION:
This review about the mucoadhesive/ bioadhesive dosage form might be useful tool for the powerful design of novel mucoadhesive drug delivery system. For the various drugs, mucoadhesion can be used as a model for the controlled drug delivery approaches. Oral route is the most recommended and probably most complex route of drug delivery. For extended period of time and for controlled delivery of buccal mucosa offers specific advantages like avoidance of first pass metabolism, pre-systemic elimination in GI tract. Retentive buccal mucoadhesive formulation proves to be an alternative to the conventional oral medications as they can be easily attached to the buccal cavity retained over for an extended period of time and withdraw at any time. Bioadhesion formulations with the various buccal applications like appropriate physical science and mechanical properties, as well as pseudo plastic or plastic flow with thixotrophy, sensible spreadability, applicable hardness and prolonged duration within the oral fissure may affect the ultimate performance of the preparations and their acceptance by the patients.
References
1. Ummadi S, Rao NG, Reddy MS. Overview on Controlled Release Dosage Form, International Journal of Pharma Sciences (2013); 3(4):258-269.
2. Shojaei, A.H., R.K. Chang, X. Guo, B.A. Burnsid and R.A Couch, 2001 systemic drug delivery via the buccal mucosa route. Pharm Tech, 6: 70-81.
3. Shojaei AH, Chang RK, Guo X, Couch RA. Systemic Drug Delivery via the Buccal Mucosal Route, Pharmaceutical Technology (2001); 70-81.
4. Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery-A promising option for orally less efficient drugs, Journal of Controlled Release (2006); 114:15-40.
5. Prajapati V, Bansal M, Sharma PK. Mucoadhesive buccal patches and use of natural polymer in its preparation- A Review, International Journal of PharmTech Research (2012); 4(2):582-589.
6. Roy S, Pal K, Anis A, Prabhakar B. Polymer in mucoadhesive drug delivery system: A brief note. Design monomers and polymers. 2009; 12:483-95.
7. Mathew M, Sharma MS. Controlled Drug Delivery system, International Journal of Pharmaceutical and Chemical Sciences (2014); 3(3):636-641.
8. Patel N, Chaudhary A. Controlled Drug Delivery System: A Review, Indo American Journal of Pharmaceutical Sciences (2016); 3(3):227-233.
9. Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery-A promising option for orally less efficient drugs, Journal of Controlled Release (2006); 114:15-40.
10. Gandhi RE, Robinson JR. Bioadhesion in Drug Delivery, Indian Journal of Pharmaceutical Sciences (1988); 50:145-152.
11. Harris D, Robinson JR. Drug Delivery via the mucous membranes of the oral cavity, Journal of Pharmaceutical Sciences (1992); 81:1-10.
12. Venkatalakshmi R, Yajman S, Verma M. Buccal Drug Delivery Using Adhesive polymeric Patches, International Journal of Pharmaceutical Sciences and Research (2012); 3(1):35-41.
13. Patel M, karigarasif, Savaliya Pratikra, Ramma MV. Buccal drug delivery system.International research journal of pharmacy, 2011; 2(2): 4-11.
14. Gandhi PA, Patel K.R, Patel MR. A Review Article on Mucoadhesive Buccal Drug Delivery Systems, International Journal of Pharmaceutical Research and Development (2011); 3(5):159-173
15. Smart JD. The basics and underlying mechanisms of mucoadhesion, Advanced Drug Delivery Reviews (2005); 57:1556-1558.
16. J.M. Gu, J.R. Robinson, S.H.S. Leung, Binding of acrylic polymers to mucin/epithelial surfaces: structure property relationships. Crit. Rev. Ther. Drug Carr. Syst. 5 (1998) 21-67zsde
17. Serra L, Domenech J, Nicholas AP. Engineering design and molecular dyanamics of mucoadhesive drug delivery system as targeting agents. European journal of pharmaceutics and biopharmaceutics. 2009; 71: 519-528.
18. Ahuja RP, Khar J. Mucoadhesive drug delivery systems, Drug Development and Industrial Pharmacy (1997); 23:489-515.
19. Mitul Patel, Asif Karigar Buccal drug delivery system .International research journal of pharmacy X(X), (2011)
20. Helfand E, Tagami Y. Theory of the intern between immiscible polymers. J Chem Phys 1972; 57: 1812-13.
21. Rathbone MJ, Drummond BK, Tucker LG. The oral cavity as a site for systemic drug delivery, Advanced Drug Delivery Review (1994); 13:1-22.
22. Desai KGH, Kumar TMP. Development and evaluation of noval buccal adhesive core-in-cup tablets of propranolol hydrochloride. Indian J Pharm Sci. 2004; 66 (4):438.
23. Steward A et al, The Effect of Enhancers on the Buccal Absorption of Hybrid (BDBB) Alpha Interferon, Int. J. Pharm, 104, 1994, 145–149
24. Deirdre Faye Vaughan, Pharmacokinetics of Albuterol and Butorphanol Administered Intravenously and via a Buccal Patch, A Thesis Submitted to the office of Graduate Studies of Texas A&M University In Partial Fulfillment of the requirements for the Degree of Master of Science, May 2003
25. Wise Donald L, Handbook of Pharmaceutical controlled release technology: 255-265
26. Yajaman S., Bandyopadhyay A.K., Buccal bioadhesive drug delivery- A promising option for orally less efficient drugs, Journal of Controlled Release, 2006;114:15–40
27. Jain N.K., Controlled and novel drug delivery; 65-75; 371-377.
28. Sindhu RK, Narasimha R, Swathi E. Formulation and Evaluation of Rapidly Dissolving Buccal Patches, International Journal of Pharmaceutical and Biopharmaceutical Sciences (2001); 1(3):145-159
29. Chaudhary R, Patel J, Giri IC. Formulation, Development and In-Vitro evaluation of mucoadhesive buccal patches of methotrexate, International Journal of Pharma Sciences and Research (2010); 1(9):357-365.
30. Semalty M, Semalty A, Kumar G. Development of mucoadhesive buccal films of glipizide, International Journal of Pharmaceutical Sciences and Nanotechnology (2008); 1(2):185-190.
31. Vishnu YV, Chandrasekharan K, Rao YM. Development of mucoadhesive patches for buccal administration of carvedilol, Current Drug Delivery (2007); 4(1):27-39.
32. Cilurzo F. Fast dissolving film of maltdodextrins, European Journal of Pharmaceutics and Biopharmaceutics (2008); 70:895-900.
33. Banyal M, Joshi S. Transdermal patch: an innovative technique for transdermal drug delivery system, IJPPR (2021); 20(3):109-131.
34. Joshi S, Banyal M, Faruk A. Formulation and evaluation of clotrimazole loaded transdermal patch, WJPR (2020); 9(10):790-803.
35. Banyal M, Joshi S, Arya A, Faruk A. Formulation and evaluation of fluconazole emulgel by using various polymers, WJPR (2020); 9(8):2084-2098.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE