 About Author:
About Author:
Ravindra Singh Tomar
M.Pharm (Medicinal & Pharmaceutical Chemistry),
B R Nahta College of Pharmacy, Mandsaur (M.P)
ravindratomar16@gmail.com
ABSTRACT-
Here a scheme has been designed for synthesis of 2-chloropyridine-5-trifluoromethyl derivatives. The probability of being antiamnesic agent has been checked by pass prediction. All compound shows antiamnesic activity as result of pass prediction. By Lipinski rule of five all compounds shows drug like activity. Also a method for pharmacological evaluation & Biochemical evaluation is described under. All synthetic procedure has been designed & described under-
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1599
1. INTRODUCTION
1.1 Alzheimer’s Disease- Alzheimer’s disease is the most common form of dementia, a group of disorders that impairs mental functioning. (Dementia literally means loss of mentation, or thinking.) At the moment, Alzheimer’s is progressive and irreversible. Abnormal changes in the brain worsen over time, eventually interfering with many aspects of brain function1. Memory loss is one of the earliest symptoms, along with a gradual decline of other intellectual and thinking abilities, called cognitive functions, and changes in personality or behavior.
Alzheimer’s advances in stages, progressing from mild forgetfulness and cognitive impairment to widespread loss of mental abilities. In advanced Alzheimer’s, people become dependent on others for every aspect of their care. The time course of the disease varies by individual, ranging from five to 20 years. The most common cause of death is infection.
It is an age-related neurodegenerative process characterized by a progressive loss of cognitive abilities, such as memory, language skills, disorientation, attention, and depression2.
Although the etiology of AD is still poorly understood, several factors such as-
* amyloid-β (Aβ) deposits,
* s-protein aggregation,
* oxidative stress or
* Low levels of acetylcholine play significant roles in the pathology of the disease.
In spite of the enormous research effort, an efficient strategy for designing new drugs for the treatment of AD is still lacking.
[adsense:468x15:2204050025]
1.2 Causes of Alzheimer’s Disease-
The causes of Alzheimer’s disease are not yet fully understood. This is one of the most exciting and most important areas of research, because understanding the causes should lead to more targeted treatments and ways to prevent the disease.
It is the result of a combination of inter-related factors, including genetic factors, which are passed along family lines of inheritance, and environmental influences, which range from previous head trauma to educational level to one’s experiences early in life.
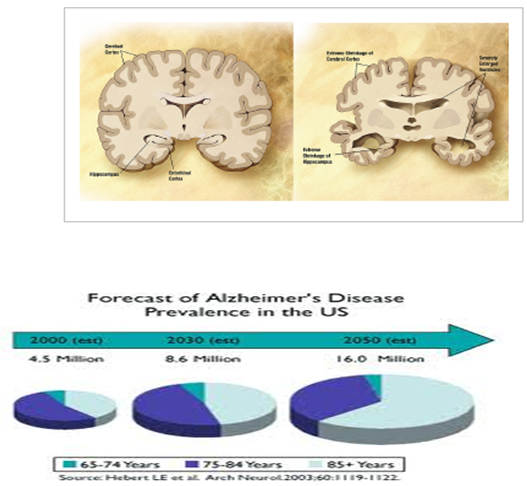
Figure 1: Comparison of a normal aged brain (left) and an Alzheimer’s patient’s brain (right).
1.3 Drugs approved for Alzheimer’s disease:
FDA-approved drugs
|
Drug Name |
Brand name |
Approved For |
FDA Approved |
|---|---|---|---|
|
1. donepezil |
Aricept |
All stages |
1996 |
|
2. galantamine |
Razadyne |
Mild to moderate |
2001 |
|
3. memantine |
Namenda |
Moderate to severe |
2003 |
|
4. rivastigmine |
Exelon |
Mild to moderate |
2000 |
|
5. tacrine |
Cognex |
Mild to moderate |
1993 |
Table 1: FDA approved drug for Alzheimer
1.4. Mechanism of action-
In the brain, neurons connect and communicate at synapses, where tiny bursts of chemicals called neurotransmitters carry information from one cell to another. Alzheimer's disrupts this process, and eventually destroys synapses and kills neurons, damaging the brain's communication network.
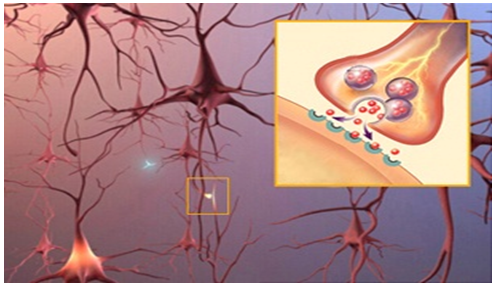
Mechanism of action
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Current FDA-approved Alzheimer drugs support this communication process through two different mechanisms:
1) Cholinesterase inhibitors work by slowing down the disease activity that breaks down a key neurotransmitter. Donepezil, galantamine, rivastigmine and tacrine are cholinesterase inhibitors.
2) Memantine, the fifth Alzheimer drug, is an NMDA (N-methyl-D-aspartate) receptor antagonist, which works by regulating the activity of glutamate, a chemical messenger involved in learning and memory.
Memantine protects brain cells against excess glutamate, a chemical messenger released in large amounts by cells damaged by Alzheimer's disease and other neurological disorders. Attachment of glutamate to cell surface "docking sites" called NMDA receptors permits calcium to flow freely into the cell. Over time, this leads to chronic over exposure to calcium, which can speed up cell damage. Memantine prevents this destructive chain of events by partially blocking the NMDA receptors.
On average, the five approved Alzheimer drugs are effective for about six to 12 months for about half of the individuals who take them.
2. REVIEW OF LITERATURE
Alzheimer’s disease (AD) is an age-related neurodegenerative process characterized by a progressive loss of cognitive abilities, such as memory, language skills, disorientation, attention, and depression.11
Although the etiology of AD is still poorly understood, several factors such as
* amyloid-β (Aβ)deposits,
* ς-protein aggregation,
* oxidative stress or
* Low levels of acetylcholine are main factor in the pathology of the disease.
In spite of the lot of research effort, an efficient strategy for designing new drugs for the treatment of AD is still lacking.
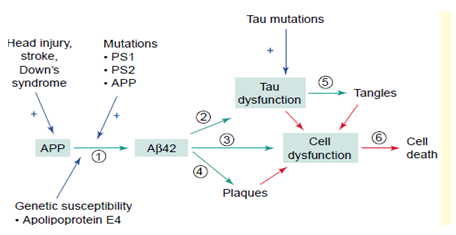
Factors involved in the pathogenesis of Alzheimer’s disease (AD). Abbreviations: Ab, b-amyloid; APP, amyloid precursor protein; PS1, presenilin 1.12
2.1 Till the research which has been done or undergoing includes-2.1.1. Acetyl cholinesterase (AChE) inhibitors13-
The cholinergic theory suggests that the selective loss of cholinergic neurons in AD results in a deficiency of acetylcholine (ACh) in specific regions of the brain that mediate learning and memory functions. Consequently, three acetyl cholinesterase (AChE) inhibitors have been approved for commercial use.
Thus, donepezil, rivastigmine, and galanthamine improve AD symptoms by inhibiting AChE, (the enzyme responsible for the hydrolysis of ACh, thereby raising the levels of ACh in the synaptic cleft.
2.1.2 β-secretase inhibitors15
The proteolytic enzyme β-secretase (BACE1) plays a central role in the synthesis of the pathogenic β-amyloid in Alzheimer’s disease.The pathological hallmarks of AD include the aggregation and extracellular deposition of β-amyloid peptide (Aβ), which leads to plaque formation, and the abnormal hyperphosphorylation of tau protein, which leads to the intracellular formation of neurofibrillary tangles.
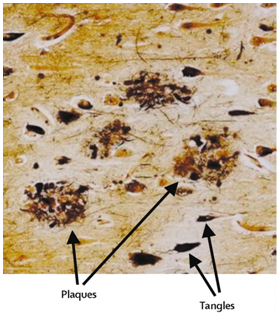
Plaques and tangles in the cerebral cortex in Alzheimer’s disease
β-amyloid deposits are predominately aggregates of the Aβ peptides (Aβ, 39– 43 residues) resulting from the endoproteolysis of the amyloid precursor protein (APP).
These peptide fragments result from the sequential cleavage of APP, first at the N-terminus by β-secretase enzyme (β-site APP cleaving enzyme, BACE1), followed at the C-terminus by one or more γ-secretase complexes (intramembrane aspartyl proteases), as part of the β-amyloidogenic pathway.
In the pathogenesis of the disease Aβ plays a central role.
Thus, processes that limit Aβ production and deposition by preventing formation, inhibiting aggregation, and/or enhancing clearance may offer effective treatments for AD.
Since β-secretase mediated cleavage of APP is the first and rate-limiting step of the amyloidogenic processing pathway, BACE1 inhibition is considered a prominent therapeutic target for treating AD by diminishing Aβ peptide formation in AD patients.
Example- pyrrolyl 2- aminopyridines as BACE1 inhibitors.
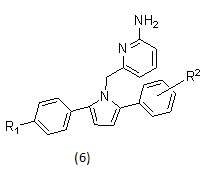
2.1.3. γ-secratase inhibitor17-
In AD protein aggregates in the brain composed of the amyloid-β-peptide (Aβ), which is called amyloid plaques.
The amyloid-β-peptide is generated by the subsequent degradation of the amyloid precursor protein (APP), a type I transmembrane protein, by two aspartyl proteases, the β-secretase and the γ-secretase. The γ-secretase is a promising target for therapeutic intervention as it liberates various Aβ-peptides with a length of 38, 40, or 42 amino acids. The toxicity depends on the length:Aβ42 is the most toxic species while Aβ38 is regarded to be nontoxic as increased production of Aβ38 does not diminish cellular viability. Several γ-secretase inhibitors (GSI), which decrease total Aβ levels, are developed.
Example- casein kinase 1e (CK1e) inhibitor.
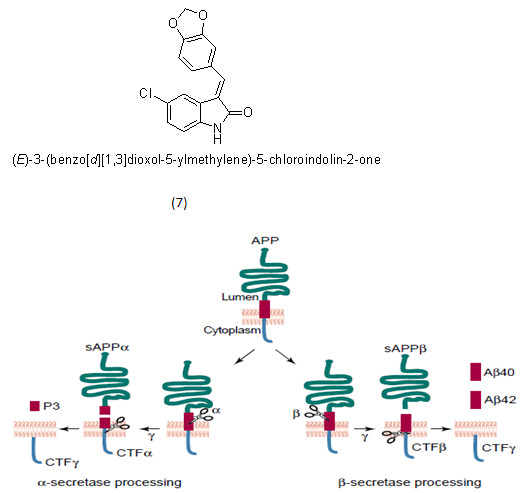
APP processing pathways
2.1.4. γ-secretase modulators18-
Unfortunately, GSIs also inhibit cleavage of other secretase substrates, including Notch. It is this inhibition of Notch processing which is responsible for adverse events in clinical trials, particularly gastrointestinal and immunological toxicities.In contrast to GSIs, compounds known as γ-secretase modulators (GSMs) inhibit Aβ42 production without interfering in the processing of Notch and other substrates.
They are thought to intervene at an allosteric site on γ-secretase and shift the APP cleavage site so that shorter, soluble, non-toxic peptides (e.g., Aβ38) are produced instead of the highly insoluble and neurotoxic Aβ42.
Derivatives of COX inhibitors functioned as modulators of γ-secretase independent of their COX activity. However, Flurizan is a weak GSM that failed in clinical trials testing its ability to treat Alzheimer’s disease.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Example- an imidazyl-phenyl series compounds
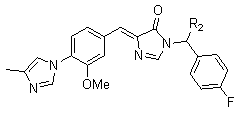
2.1.5. Huprine derivatives22
Huprines, Tacrine–Huperzine A hybrids have been described a few years ago as highly potent AChE inhibitors
2.1.6. Muscarinic receptor 1 agonist23
The cholinergic hypothesis of aging and of dementia suggests that the loss of central forebrain cholinergic neurons contributes to the decline in cognitive abilities associated with AD. The presynaptic cholinergic deficits in AD indicate that a cholinergic replacement therapy might be beneficial in alleviating some of the cognitive dysfunctions in this disorder.
The loss of presynaptic marker enzyme choline acetyltransferase and the muscarinic receptors of the M2 subtype are mainly responsible in causing deficits in central cholinergic transmission in Alzheimer’s patients.
M1 muscarinic receptors play a role in an apparent linkage of three major hallmarks of AD: β-amyloid (Aβ) peptide; tau hyperphosphorylation and paired helical filaments (PHFs); and loss of cholinergic function conducive to cognitive impairments.
Muscarinic acetylcholine (mACh) receptor is a growing number of G protein-coupled receptors; each specifically regulates a different physiological and biochemical function in the body24. Four types of muscarinic receptors are known, named M1–M4 and five subtypes of muscarinic receptors have been cloned and designated m1–m5.
M1 selective muscarinic agonists, are capable of crossing the blood–brain barrier has active pharmacological application.
Most of the potent muscarinic agonists, including those which were evaluated in AD patients, show adverse central and peripheral side effects, and are either non-selective or M2> M1 selective. Thus, they may also activate inhibitory M2 autoreceptors resulting in decreased acetylcholine (ACh) release.
The following probes for mAChRs were suggested as a rational treatment strategy in AD;
(a) M1 agonists;
(b) M2 antagonists;
(c) Mixed M1 agonist and M2 antagonist in the same compound.
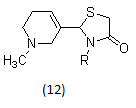
In research, many structurally novel arecoline-based muscarinic agonists have been synthesized which potentially may overcome the limited oral activity, short duration of action, or lack of separation between central and peripheral effects of classical agonists.
Example-
Arecoline oximes or oxadiazoles, arecoline thiadiazoles, arecoline oxazoles, arecoline amides are the new generation muscarinic agonists, arecoline thiazolidinones, N-arylsulphonamide substituted 3-morpholino arecoline analogues and N-arylthiourea substituted 3-morpholino arecoline analogues as muscarinic receptor 1 agonist.
2.1.7. Neuronal nitric oxide synthase inhibitors25
Neuronal nitric oxide synthase (nNOS) has been involved in various neurodegenerative diseases, including Parkinson’s disease and neuronal damage resulting from stroke.
Inhibition of nNOS could have therapeutic benefit in these and other diseases, but this inhibition must be achieved without effect on the other isoforms of NOS, endothelial (eNOS) and inducible (iNOS); inhibition of eNOS could lead to side effects such as hypertension, and inhibition of iNOS could result in a higher probability of Alzheimer’s disease.
Example-
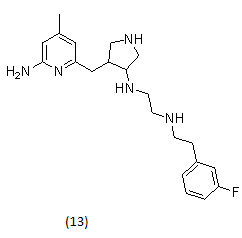
2.1.8. Met kinase inhibitors26
Met is a receptor tyrosine kinase protein that has a high binding affinity for hepatocyte growth factor/scatter factor (HGF/SF). Upon activation with its endogenous ligand HGF/SF, Met mediates various cellular responses, such as epithelial cell dissociation (scattering), invasion, tubular morphogenesis, and angiogenesis.
While Met/ HGF signaling is essential for normal physiological events, such as placental development and liver regeneration, activation of this pathway is reported to lead to tumorigenicity and metastasis.
Due to the prevalence of Met amplification/over expression and mutations in a variety of human malignancies, inhibition of Met kinase activity by small molecules or biologics would likely have broad therapeutic utility.
Example-
Met kinase inhibitors with malonamide and acylurea groups substituted on the pyrrolo[2,1-f][1,2,4]triazines.
pyrrolopyridine- and aminopyridine- based Met kinase inhibitors.
3.1 SCHEME-
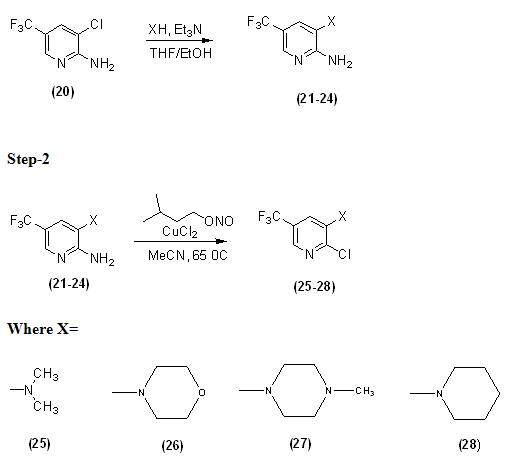
3.1.1 GENERAL PROCEDURE-
2-amino-3-chloro-5-trifluoromethyl pyridine was suspended in 3:1 mixture of THF/EtOH (15 ml/mmol of starting pyridine). An excess of secondary amine was then added, followed by triethylamine (1.0equiv). The mixture was heated under reflux. After cooling the solvent was removed in vacuum and resulted crude submitted to recrystllisation to give final compound.
3.2 DETERMINATION OF LIPINSKI DESCRIPTORS-
Fundamental physicochemical features of CNS drugs are related to their ability to penetrate the blood-brain barrier affinity and exhibit CNS activity. Lipinski et al looked for a generalized rule that is a “Rule of five” that govern drug like properties. The “Rule of five” is so named because all the essential physical properties are parameters of five. According to this rule, a good absorption and permeability is likely if:
1) Molecular weight is ≤ 500.
2) Oil/water distribution coefficient (Log P) is ≤ 5.
3) Hydrogen bond donor ≤ 5 (expressed as the sum of OHs and NHs).
4) Hydrogen bond acceptor ≤ 10 (expressed as the sum of Os and Ns). A fifth rule was added later:
5) Number of rotatable bonds ≤ 10.
Lipinski also laid down rule for CNS penetration30
· Molecular weight ≤ 400.
· Log P ≤ 5.
· Hydrogen bond donor ≤ 3.
· Hydrogen bond acceptor ≤ 10.
· Number of rotatable bonds ≤ 8.
Log P as the descriptor of lipophilicity has been observed very important for CNS penetration. For the optimal penetration of blood brain barrier log P values should be in range of 1.5 – 2.7. CNS drugs have significantly reduced molecular weight compared with other therapeutics that is less than 400. The total polar surface area (TPSA), count of hydrogen bond donors (nOHNH) and hydrogen bond acceptors (nON) are all correlated with polarity. Standard values are TPSA 60-70 ?, nOHNH ≤ 3 and nON ≤ 8.
These compounds were subjected to ‘Lipinski rules of five’. The MIPC (Mol Inspiration Property Calculator) program has been utilized (www.molinspiration.com) for calculating Lipinski descriptors30. The Lipinski descriptors for the four compounds under consideration have been provided in table 3.
|
Compound Code & No. |
Mlog p |
PSA (?2) |
Mw |
No. of rotatable bonds |
Hydrogen bond |
|
|
Acceptor(s) |
Donor(s) |
|||||
|
RST-1, (25) |
2.646 |
16.13 |
224.613 |
2 |
2 |
0 |
|
RST-2, (26) |
2.492 |
25.364 |
266.65 | 2 |
3 |
0 |
|
RST-3, (27) |
2.583 |
19.368 |
279.693 |
2 |
3 |
0 |
|
RST-4, (28) |
3.554 |
16.13 |
264.678 |
2 |
2 |
0 |
Table 3: Lipinski descriptors for the compounds (25-28).
On the basis of above-mentioned criterion and with a goal to provide the diversity of potent cognition enhancers, the chosen compounds were experimentally tested as nootropics.
3.3 PASS PREDICTION
In order to accelerate our search for potent New Chemical Entities (NCEs), the assistance of Computer Aided Drug Discovery Program PASS (Prediction of Biological Activity Spectra for Substances) was used to predict the cognition enhancing action for 2-chloropyridine-5-trifluoromethyl derivatives (25-28).
Contrary to many other existing methods of SAR/ QSAR/ QSPR/ molecular modeling methods focused on predicting a single type of biological activity within the same chemical series, computer-aided program PASS predicts not only for the desirable pharmacological effect but also for molecular mechanisms of action and different unwanted side effects like mutagenicity, carcinogenicity, teratogenicity and embryotoxicity. Such analysis of heterogeneous sets increases considerably the chance of discovering NCEs (e.g., cognition enhancers). The technique of PASS is based on the analysis of SARs for the training set currently including about 46,000 drugs, drug candidates and lead compounds whose biological activities are determined experimentally. The set of MNA (Multilevel Neighbourhood atoms) descriptors is generated on the basis of structural formulas presented in the MOL-file (SDF-file) form. Since MNA descriptors are generated for each compound de novo, new descriptors can be obtained upon presentation of a novel structural feature in the compound under study. Based on the statistics of MNA descriptors for active and inactive compounds from the training set, two probabilities are calculated for each activity: Pa probability of compound being active and Pi—probability of compound being inactive. Being probabilities, the Pa and Pi values vary from 0.000 to 1.000 and in general Pa + Pi < 1, since these probabilities are calculated independently31.
Recently, an Internet version of PASShas been made available at the PASS developer’s web site. The user can submit the MOL-file of the molecule under study and obtain the predicted biological activity spectrum on their computer immediately. This new internet version of PASS provides access to prediction of 783 kinds of biological activity, in contrast to an earlier version that predicted 319 activities32. MOL-files of compounds 25-28 were submitted and predicted biological activity spectra were obtained.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3.3.1 PASS PREDICTION-RESULTS-
The PASS predictions can be interpreted and used in a flexible manner—(i) only activities with Pa > Pi are considered as possible for a particular compound. (ii) If Pa > 0.7—the chance to find the activity experimentally is high. But, in many cases the compound may occur to be a close analogue of known pharmaceutical agents. (iii) If 0.5 < Pa < 0.7—the chance to find the activity experimentally is less, but the compound is probably not so similar to known pharmaceutical agents.(iv) If Pa < 0.5—the chance to find the activity experimentally is even less, but the chance to find a structurally new compound, that is, NCEs increases33.
|
COMPOUND CODE & No. |
Pa |
Pi |
|
RST-1, (25) |
0.478 |
0.015 |
|
RST-2, (26) |
0.482 |
0.106 |
|
RST-3, (27) |
0.600 |
0.055 |
|
RST-4, (28) |
0.592 |
0.058 |
Table 4:The probabilities of being active for nootropic activity (Pa) of the synthesized compounds (25-28) on the basis of PASS prediction.
PASS prediction results showed that for all the synthesized compounds (25-28) the probability of being active was observed in the range of 0.600 > Pa > 0.478 for nootropic activity (table 4). This showed that for compounds (27,28) with values 0.7 < Pa > 0.5 the chance of finding the activity experimentally is less but the chance of finding a structurally new compound was greater. Compound (25, 26) is having Pa value less than 0.5, so the chance of finding the activity experimentally is even lesser but the chance of finding a structurally new compound is maximum.
3.4 PHARMACOLOGICAL EVALUATION
3.4.1 ANTIAMNESIC ACTIVITY34
ELEVATED PLUS MAZE
The test measures the transfer latency (TL) i.e. the time in which the mouse moves from open arm to the enclosed arm.TL was recorded on the first day. If the animal did not enter into one of the covered arms within 90 s, it was gently pushed into one of the two covered arms and the TL was assigned as 90 s. The mouse was allowed to explore the maze for 10 s and then returned to its home cage. Immediately after the training, the animals were administered the test compounds intraperitonially (i.p.). On the second day i.e. 24 h after the first exposure, TL was again noted. The decrease in TL was taken as index of antiamnesic activity.The results are expressed as % retention (Mean ±S.E.M) calculated as:
TL on 1st day - TL on 2nd day Χ 100
-----------------------------------
TL on 1st day
3.5 BIOCHEMICAL EVALUATION
The biochemical study to be carried out in two phases, the estimation of acetylcholinesterase activity and IC50 and second is the estimation of protein Content.
3.5.1 ASSAY OF ACETYLCHOLINESTERASE
The activity of acetylcholinesterase to be determined in the brain homogenate according to the Ellman et al (1961) method35.
Principle
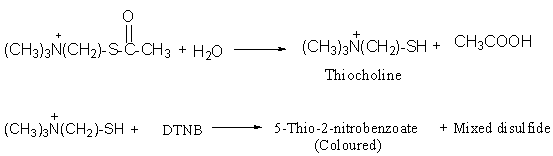
The substrate used in the assay system is acetylthiocholine iodide, the ester of thiocholine and acetic acid. The substrate, acetylthiocholine is hydrolyzed into thiocholine and acetate by the enzyme AChE. Thiocholine forms mercaptan, which reacts with the oxidizing agent 5, 5’-dithio-bis-2-nitrobenzoic acid (DTNB) to form 5-thio-2-nitrobenzoate, which has a maximum absorption at 412 nm. Thus the activity of AChE can be measured by following an increase in absorbance at 412 nm.
Reagents
· 0.1 M Sodium phosphate buffer (pH 8.0)
· 10 mM DTNB (Ellman’s reagent)
· 14.9 mM Acetylthiocholine iodide
Calculations
AChE activity to be calculated using molar extinction coefficient of 5-thio-2-nitrobenzoic acid (14.15 ´103 M-1cm-1). The results to be expressed as nmols of acetylthiocholine iodide hydrolyzed/min/mg protein.
Change in absorbance per minute Volume of assay
------------------------ ? ------------------------
14150 mg of protein by protein estimation
3.5.2 ESTIMATION OF PROTEIN CONTENT
The protein content to be estimated according to the method of Lowry36 et al, 1951.
Principle37, 38
This method is based on the colour reactions of amino acids tryptophan and tyrosine with the Folin’s phenol reagent. These amino acids react with phosphomolybdic acid and phosphotungstic acid (present in Folin’s reagent) to give blue colour, which is estimated colourimetrically. This colour is the result of reduction of phosphomolybdic acid and phosphotungstic acid and biuret reaction of proteins with Cu2+ ions in alkaline medium.
Reagents
· Reagent A: 1 % (w/v) Copper sulphate solution.
· Reagent B: 2 % (w/v) Sodium potassium tartarate.
· Reagent C: 2 % (w/v) Sodium carbonate in 0.1 N Sodium hydroxide.
· Lowry’s reagent: It was prepared just before use by mixing reagents A, B and C in the ratio of 1:1:98.
· Folin-Ciocalteau reagent: It was prepared fresh by diluting the commercial 2N Folin’s reagent with double distilled water (1:1, v/v).
· Standard bovine serum albumin (BSA) solution (1 mg/ml).
Calculations
The protein concentration was calculated from the standard curve made by taking different concentrations of the BSA standard.
Volume of homogenate X sample OD X
------------------------------ ------- = mg/ml protein
0.372 (Standard) 1000
Referances-
1. Anonymous, “alzinfo.org” accessed on 21/09/10.
2. Abdelouahid Samadi, Jose Marco-Contelles, Elena Soriano, Monica Alvarez-Perez, Mourad Chioua, Neuroprotective properties for the treatment of Alzheimer and neuronal vascular diseases. Bioorganic & Medicinal Chemistry, 2010, 5861-5872.
3. Alan M. Palmer, Pharmacotherapy for Alzheimer’s disease: progress and prospects, trends in Pharmacological Sciences Vol.23 No.9, September 2002.
4. Anonymous “pubs.acs.org.” accessed on 29/09/10.
5. Mark A. Smith and George , Causes and Consequences of Oxidative Stress in Alzheimer’s Disease, Free Radical Biology & Medicine, Vol. 32, No. 11, 2002,1061–1070.
6. Anonymous,“whoindia.org/en/section3/ section128/ section 235_426.html” accessed on 11.10.2010.
7. Anonymous, “alz.org” accessed on 21/10/10.
8. Tripathi, K. D. Essentials of Medical pharmacology. 6th ed.; Jaypee Brothers, New Delhi, 2008.
9. Kaj Blennow, Mony Leon, Henrik Zetterberg, Alzheimer’s disease thelancet.com Vol 368, 2006 387-406.
10. Hardy, J Higgins, G. A. Amyloid deposition as the central event in the etiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1992, 12, 383–388
11. Abdelouahid Samadi, Jose Marco-Contelles, Elena Soriano, Monica Alvarez-Perez, Mourad Chioua, Neuroprotective properties for the treatment of Alzheimer and neuronal vascular diseases. Bioorganic & Medicinal Chemistry, 2010, 5861-5872.
12. Todd E. Golde and Steven G. Younkin, Presenilins as therapeutic targets for the treatment of Alzheimer’s disease, trends in Molecular Medicine Vol.7 June 2001.
13. Aldo Andreani, Alberto Leoni, Alessandra Locatelli, Rita Morigi, Mirella Rambaldi, 4-Aminopyridine derivatives with antiamnesic activity, Eur. J. Med. Chem. 35 2000 77−82.
14. Alan M. Palmer, Pharmacotherapy for Alzheimer’s disease: progress and prospects, trends in Pharmacological Sciences Vol.23 No.9, September 2002.
15. Michael S. Malamas, Keith Barnes , Yu Hui , Matthew Johnson , Frank Lovering , Jeff Condon , William Fobare , William Solvibile , Novel pyrrolyl 2-aminopyridines as potent and selective human b-secretase (BACE1) inhibitors, Bioorganic & Medicinal Chemistry Letters, 20, 2010, 2068–2073.
16. Kaj Blennow, Mony Leon, Henrik Zetterberg, Alzheimer’s disease www.thelancet.com Vol 368, 2006 387-406.
17. Nicole Hottecke , Miriam Liebeck , Karlheinz Baumann , Robert Schubenel , Edith Winkler ,Harald Steiner , Inhibition of c-secretase by the CK1 inhibitor IC261 does not depend on CK1d, Bioorganic & Medicinal Chemistry Letters, 20, 2010, 2958–2963.
18. John P. Caldwell , Chad E. Bennett, Troy M. McCracken, Robert D. Mazzola, Thomas Bara, Alexei Buevich, Iminoheterocycles as c-secretase modulators, Bioorganic & Medicinal Chemistry Letters, 20, 2010, 5380–5384.
19. Alexey Rivkin , Sean P. Ahearn , Stephanie M. Chichetti , Yoona R. Kim , Chaomin Li , Andrew Rosenau , Piperazinyl pyrimidine derivatives as potent c-secretase modulators, Bioorganic & Medicinal Chemistry Letters, 20, 2010,1269–1271.
20. Heiko Zettl1, Sascha Weggen, Petra Schneider and Gisbert Schneider, Exploring the chemical space of γ-secretase modulators, Trends in Pharmacological Sciences, Vol.31, No.9., 2010
21. Johan Lundkvist and Jan Na slund, γ-Secretase: a complex target for Alzheimer’s disease Current Opinion in Pharmacology, 2007, 7,112–118.
22. Cyril Ronco, Geoffroy Sorin, Florian Nachon , Richard Foucault , Ludovic Jean, Anthony Romieu , Synthesis and structure–activity relationship of Huprine derivatives as human acetylcholinesterase inhibitors, Bioorganic & Medicinal Chemistry, 17, 2009 ,4523–4536.
23. C.T. Sadashiva, J.N. Narendra Sharath Chandra, C.V. Kavitha, A. Thimmegowda, M.N. Subhash, Synthesis and pharmacological evaluation of novel N-alkyl/aryl substituted thiazolidinone arecoline analogues as muscarinic receptor 1 agonist in Alzheimer’s dementia models, European Journal of Medicinal Chemistry, 44, 2009, 4848–4854.
24. Y. C. Sunil Kumar, Manish Malviya, J. N. Narendra Sharath Chandra,C. T. Sadashiva, M. N. Subhashb, Effect of novel N-aryl sulfonamide substituted 3-morpholino arecoline derivatives as muscarinic receptor 1 agonists in Alzheimer’s dementia models, Bioorganic & Medicinal Chemistry, 16 ,2008,5157–5163.
25. Graham R. Lawton, Hantamalala Ralay Ranaivo, Laura K. Chico, Haitao Ji, Fengtian Xue, Pavel Martásek, Silverman, Analogues of 2-aminopyridine-based selective inhibitors of neuronal nitric oxide synthase with increased bioavailability, Bioorganic & Medicinal Chemistry, 17, 2009, 2371–2380.
26. Zhen-Wei Cai, Donna Wei, Gretchen M. Schroeder, Lyndon A. M. Cornelius, Kyoung Kim, Xiao-Tao Chen, Discovery of orally active pyrrolopyridine- and aminopyridine-based Met kinase inhibitors, Bioorganic & Medicinal Chemistry Letters, 18, 2008, 3224–3229.
27. Bo Zhang, Aifang Nie, Wei Bai, Ziqiang Meng , Effects of aluminum chloride on sodium current, transient outward potassium current and delayed rectifier potassium current in acutely isolated rat hippocampal CA1 neurons, Food and Chemical Toxicology, 42, 2004, 1453–1462.
28. Luigi Scipione, Daniela De Vita, Alessandra Musella, Lisa Flammini, Simona Bertonib, 4-Aminopyridine derivatives with anticholinesterase and antiamnesic activity, Bioorganic & Medicinal Chemistry Letters, 18, 2008, 309–312.
29. Daniel T. Smith, Riyi Shi, Richard B. Borgens, Jennifer M. McBride, Kevin Jackson, Development of novel 4-aminopyridine derivatives as potential treatments for neurological injury and disease, European Journal of Medicinal Chemistry, 40 ,2005 ,908–917.
30. Pajouhesh, H.; Lenz, G. R. Medicinal chemical properties of successful central nervoussystem drugs, J. American Society Exp. Neuro. Ther. 2005, 2, 541-553.
31. Geronikaki, A. A.; Dearden, J. C.; Filimonov D.; Galaeva, I.; Garibova, T. L. Design of new cognition enhancers: from computer prediction to synthesis and biological evaluation. J. Med. Chem. 2004, 47, 2870-2876.
32. Marwaha, A.; Goel, R. K.; Mahajan, M. P. PASS-predicted design, synthesis and biological evaluation of cyclic nitrones as nootropics. Bio. Med. Chem. Letters 2007, 17, 5251-5255.
33. Anzali S.; Barnickel G.; Cezanne B.; Krug M.; Filimonov D.; Poroikov V. Discriminating between drugs and nondrugs by Prediction of Activity Spectra for Substances (PASS). J. Med. Chem. 2001, 44, 2432-2437.
34. Manetti, D.; Martini, E.; Ghelardini, C.; Dei, S.; Galeotti, N.;Guandalini, L.; Romanelli, M. N.; Scapecchi, S.; Teodori, E.; Bartolini, A.; Gualtieri, F. 4-Aminopiperidine derivatives as a new class of potent cognition enhancing drugs. Bioorg. Med. Chem. Lett. 2003, 13, 2303-2306.
35. Andreani, A.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Pietra, C.; Villetti, G. 4-Aminopyridine derivatives with antiamnesic activity. Eur. J. Med. Chem. 2000, 35, 77−82.
36. Gerhardt, P.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. (1994) “Methods for General and Molecular Bacteriology”, ASM, Washington DC, ISBN 1-55581-048-9, p 518.
37. Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. (1996). “Extraction of extracellular polymers from activated sludge using a cation exchange resin”, Water Res 30(8), pp. 1749-1758.
38. Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. (1951) “Protein measurement with the Folin Phenol Reagent”, J Biol Chem 193, pp. 265-275.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









