ABOUT AUTHORS
Mohammad Sarowar Uddin*, Md. Arafat Jakir, Md. Shalahuddin Millat, Shafayet Ahmed Siddiqui
Nokhali Science and Technology University
Noakhali-3814, Bangladesh
ABSTRACT
Atorvastatin calcium is a poorly water soluble compound marketed in Bangladesh under bio-waiver conditions. The present study aimed to develop formulation and drug excipients compatibility study of Atorvastatin calcium (50 mg) sustain release tablet and optimize the final formula. The tablet were formulated by direct compression method and the results obtained were extrapolated. Solid dispersion of Atorvastatin calcium was prepared by using Hydroxypropyl methyl cellulose, Methyl cellulose, Lactose, Guar gum, Xanthan gum & Magnesium Stearate. The in vitro equivalence test was carried out in three different media. Test results were subjected to statistical analysis to compare the dissolution profiles. Other general quality parameters of these tablets such as weight variation, friability, thickness, hardness and disintegration time were also determined according to established protocols. Final formulation of solid dispersed Atorvastatin calcium revealed that successfully improvement of solubility as well as dissolution of Atorvastatin calcium in long time. This study could be very much helpful for better bioavailability of poorly water soluble drug avoiding first pass metabolism. Finally, we can claim that prepared tablets are proved to be promising dosage form for sustained drug delivery of Atorvastatin calcium by reducing dosing frequency and increasing the patient compliance.
Reference Id: PHARMATUTOR-ART-2679
INTRODUCTION
Atorvastatin calcium (ATR) is an anti-hyperlipidemic drug which is used in the management of obesity. It inhibits 3-hydroxy-3-methylglutaryl-coenzyme reductase (HMG-CoA reductase) enzyme which is responsible for conversion of HMG-CoA to mevalonate. Mevalonate is an early rate limiting step in sterol biosynthesis (Malhotra and Goa 2001).In this mechanism, ATR is administrated to decrease the cholesterol and triglycerides level in patients with hypercholesterolemia, mixed dyslipidemia and familial hypercholesterolemia (Kadu et al., 2011). ATR is very slightly soluble in distilled water, phosphate buffer and acetonitrile whereas freely soluble in methanol and slightly soluble in ethanol (Mccrindle et al., 2003). The absolute bioavailability of ATR is about 14% due to its low solubility and first-pass metabolism in liver (Cilla et al., 1996; Lennernas 2003). So the quality parameters assessment of atorvastatin sodium is an important factor for ensuring its effectiveness.
Tablet is one of the most suitable dosage form as it is easy to manufacture, convenient to administer, accurate in dosing and more stable compared to other dosage forms. Besides, it is more resistant to temperature as compared to capsules. Various factors like the disintegration and dissolution and physiological properties of drug affect the bioavailability of drugs. The process of quality control is conducted to confirm an expected level of quality in a product. It includes the necessary actions, a business conceives, essential to provide for the control and verification of various parameter of a product (Dewan et al., 2013).Different quality parameters like weight variation, hardness, friability, disintegration time, dissolution profile etc. which play a significant effect on the drug product formulation (Karmakar and Kibria, 2012).We know that atorvastatin calcium is available in market in different form except sustain release tablet. Therefore, the present study was designed to formulate and evaluate atorvastatin calcium sustain release tablet to enhance its therapeutic action.
MATERIALS AND METHODS
MATERIALS
The materials required for formulation of Atorvastatin calcium tablets are atorvastatin calcium (Active ingredient), methyl cellulose (Polymer), hydroxy propyl methyl cellulose (Polymer), lactose (polymer), guar gum, xanthan gum and magnesium stearate. Atorvastatin calcium was received as a gift sample from NIPRO JMI Pharma Ltd. while hydroxyl propyl methyl cellulose, methyl cellulose and lactose were obtained as a gift sample from BASF Bangladesh Limited, a chemical company of Bangladesh. None of the sample was brought and analysed which date of expiry has already been passed & all the samples were stored under appropriate condition. All organic solvents were of high performance liquid chromatography (HPLC grade) and all the other chemicals were of high purity being of pharmaceutical grade.
METHODS
Study design
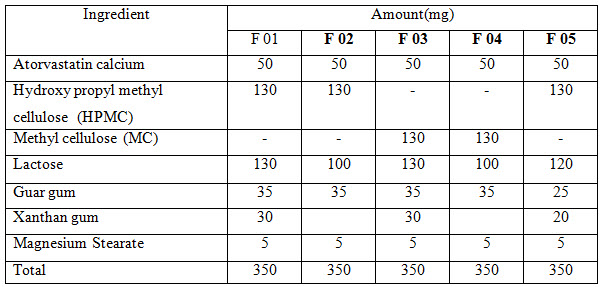
The study was designed for formulating five formulations of 350 mg atorvastatin calcium tablet (F 01-F 05) and each of the formulation contains 10 tablets. The tablet prepared was evaluated for weight variation, hardness, friability, disintegration time as procedure given in Indian pharmacopoeia.
Preparation of Atorvastatin calcium tablet
Various methods are available for producing controlled release atorvastatin calcium tablet preparation. In our experiment, direct compression technique was followed to prepare atorvastatin calcium tablet from the following formulations:
Weight variation test
Weight variation for tablets should not exceed 10% or more having average weight of 80 mg or less (British Pharmacopoeia, 2005). Weight variation test is the test by which variation of weight from tablet to tablet may be determined. Weight variation was conducted to ensure that, each of tablets contains the right amount of drug. The test was performed by weighing the 10 tablets individually using analytical balance, then calculating the average weight, and comparing the individual tablet weights to the average. The deviation of the weight of the tablets from the average weight was calculated as the weight variation (Sarwar et al. 2013). The following formula is used:
Weight variation =
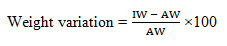
Where,
IW= Individual weight of tablet
AW= Avarage weight of tablet
Hardness test
Hardness of formulated tablets was determined using ‘Monsanto’ type hardness tester (Intech, Korea). Finally the mean crushing strengths were determined using the following formula.

Friability test
In this investigation, friability was determined by using Electrolab EF-2 Friabilator (USP) and the values of friability were presented as percentage (%). Ten tablets from each formulation were individually weighed and transferred into friabilator which was operated at 25 rpm and continued up to 4 minutes (100 revolutions). Then, the tablets weights were measured again and the percent (%) of friability was determined using following formula (Kalakuntla et al., 2010).
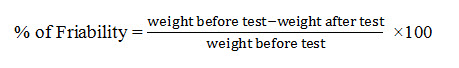
Disintegration time test
The disintegration test was carried out using disintegration tester –USP; (Electro lab EF 2L; United Kingdom) with disc in distilled water medium. To determine disintegration time, three tablets of formulation were placed in each tube and the basket rack is positioned in a 1 liter beaker of water at 37 ± 0.50c. The time required to break of each tablet into small particles and move through the mesh was recorded. Then, the mean disintegration time was determined for each formulation of tablet (Gangwar et al., 2010).
Dissolution test
The dissolution test was conducted using Dissolution Tester – USP Apparatus-1 (Basket type). Three tablets of each formulation were placed in 3 different beakers in dissolution medium containing 900 ml of 0.1N HCl buffer (pH 7.4). The apparatus was rotated at a speed of 100 rpm by maintaining temperature at 37±1ºC in each test. Samples were withdrawn at regular interval of 5 minutes as 5 ml which was predetermined and this was continued up to 40 minutes by replacing equal quantity of fresh dissolution medium. The filtered samples were analyzed by using UV Spectrophotometer (SHIMADZU UV Spectrophotometer: UV-1800-240V) and percentage (%) of drug release was determined by measuring the absorbance (Kumar et al., 2011).
Statistical analysis
The data were analyzed by using the above mentioned mathematical formula and MS Excel®, 2007.
RESULTS AND DISCUSSION
The weights of different formulations of atorvastatin tablets shown in Table 2. According to the BP (British Pharmacopeia) and IP (Indian Pharmacopeia) specifications, it was established that weight variation should not be more than ± 5% deviation for tablets above 250 mg. All formulations of atorvastatin tablets showed no deviation by ± 5% of the average tablet weight according to IP/BP specifications.
Table 2: Measurement of weight variation of different formulation of atorvastatin tablet

In hardness study, all the formulation exhibited satisfactory hardness (table 3) which is required for safe handling and transportation. Again, according to BP/IP the friability should be less than 1%. All formulation of atorvastatin tablets showed friability less than 1% (table 3). Moreover, the results of disintegration time were exhibited in table 3. In our study, all formulations of atorvastatin calcium disintegrated within the BP and USP mentioned time boundary. The disintegration time lies between 1-2 minute where F03 showed minimum disintegration time and F02 showed maximum disintegration time.
Table 3: Results of hardness, friability, disintegration tests of different formulation of atorvastatin tablet.

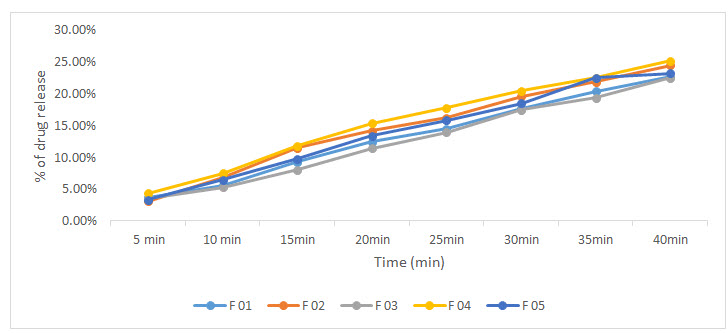
The results of dissolution were noted in figure 1. The dissolution test is used to determine the amount of the drug released into the dissolution medium with time. All formulation release more than 20% drug in 1st 40 minute. These results support the analytical criteria mentioned in BP and USP which encourages the formulation of atorvastatin calcium sustain release tablet in large volume to address respective illness patterns.
Figure 1: Drug release curve of different formulation atorvastatin tablet.
CONCLUSION
Pre-formulation and drug excipient compatibility study indicate the way and method of drug formulation. Formulation of Atorvastatin calcium tablet fulfil different analytical test like weight variation, hardness, friability, disintegration and dissolution. Besides, method of preparation is simple, cost effective and scalable. So, it can be concluded that formulation of atorvastatin tablet will be beneficial for delivering the drug which will meet the therapeutic action.
REFERENCES
1. British Pharmacopoeia (2000); Volume-I; British Pharmacopoeia Commission; London; 79-36-39. 3
2. British Pharmacopoeia (2013); British Pharmacopoeia Commission; London.
3. Cilla Jr, D.D., Whitfield, L.R., Gibson, D.M., Sedman, A.J. and Posvar, E.L.,(1996); Multiple‐dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG‐CoA reductase, in healthy subjects; Clinical Pharmacology & Therapeutics; 60(6); 687-695.
4. Dewan, A., Pacifico, R., Zhan, R., Rinberg, D. and Bozza, T., (2013); Non-redundant coding of aversive odours in the main olfactory pathway; Nature; 497(7450); 486
5. Gangwar, S., Singh, S., Garg, G., Garg, V. and Sharma, P.K., (2010); To compare the disintegrating property of papaya starch and sago starch in paracetamol tablets; Int J Pharmacy Pharm Sci; 2(Suppl 2); 148-51.
6. Indian Pharmacopoeia (2007); The Indian Pharmacopoeia Commission, Ghaziabad; 1; 141-142. 7.
7. Indian Pharmacopoeia (2010); The Indian Pharmacopoeia Commission, Ghaziabad; 2; 1479.
8. Kalakuntla, R., Veerlapati, U., Chepuri, M. and Raparla, R., (2010); Effect of various super disintegrants on hardness, disintegration and dissolution of drug from dosage form; J. Adv. Sci. Res; 1(1); 15-19.
9. Kumar, K.A., Mohanakrishna, A., Sudheer, M., Rajesh, K.S. and Ramalingam, P., (2011); UV spectrophotometric method for the estimation of alprazolam in tablet dosage form;Int. J. Chem. Tech. Res; 3(1); 161-164.
10. Kadu, P.J., Kushare, S.S., Thacker, D.D. and Gattani, S.G., (2011); Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS); Pharmaceutical Development and Technology; 16(1); 65-74.
11. Kale, V.V., Kasliwal, R. and Parida, S.K., 2004. Formulation and release characteristics of guar gum matrix tablet containing metformin HCl. Int. J. Pharm. Expt, pp.75-80.
12.Lennernäs, H., (2003); Clinical pharmacokinetics of atorvastatin; Clinical pharmacokinetics; 42(13); 1141-1160.
13. Malhotra, H.S. and Goa, K.L., (2001); Atorvastatin; Drugs; 61(12); 1835-1881.
14. McCrindle, B.W., Ose, L. and Marais, A.D., (2003); Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial; The Journal of pediatrics; 143(1); 74-80.
15. Sarwar, G., Das, A., Kibria, M.G., Karmakar, P. and Sattar, M.M., (2013); In vitro Quality Analysis of Different Brands of Alprazolam Tablet Available in Bangladesh. Bangladesh Pharmaceutical Journal; 16(2); 185-188.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









